Endo M., Iijima S., Dresselhaus M.S. (eds.) Carbon nanotubes
Подождите немного. Документ загружается.

178
U.
ZIMMERMAN
et
at.
ber of electrons bonded by
SO2
and
0.
What effect
does C60 as an impurity have on the electronic shell
structure? Will it merely shift the shell closings by
6
(the number of electrons possibly transferred to the
c60
molecule)? We will investigate this in the follow-
ing paragraphs.
Up to this point, we have always studied the clus-
ters using brute force (i.e., heating them
so
strongly
that they evaporate atoms). But the electronic shell
structure of clusters can also be investigated more gen-
tly by keeping the photon flux low enough to prevent
the clusters from being heated and using photon en-
ergies in the vicinity of the ionization energy
of
the
clusters.
The ionization energy
of
alkali metal clusters
os-
cillates with increasing cluster size. These oscillations
are caused by the fact that the s-electrons move almost
freely inside the cluster and are organized into
so-
called shells. In this respect, the clusters behave like
giant atoms.
If
the cluster contains just the right num-
ber of electrons to fill a shell, the cluster behaves like
an inert gas atom (Le., it has a high ionization energy).
Howeve?, by adding just one more atom (and, there-
fore, an additional s-electron), a new electronic shell
must be opened, causing a sharp drop in the ioniza-
tion energy. It is a tedious task to measure the ioniza-
tion energy of each of hundreds of differently sized
clusters. Fortunately, shell oscillations in the ioniza-
tion energy can be observed in a much simpler exper-
iment. By choosing the wavelength
of
the ionizing light
so
that the photon energy is not sufficient
to
ionize
closed-shell clusters, but is high enough
to
ionize open-
shell clusters, shell oscillations can be observed in a
single mass spectrum. Just as in the periodic table
of
elements, the sharpest change in the ionization energy
occurs between a completely filled shell and
a
shell
containing just one electron. In a threshold-ionization
mass spectrum this will be reflected
as
a
mass peak
of
zero intensity (closed shell) followed by
a
mass peak
at high intensity (one electron in
a
new shell). This be-
havior is often seen. However, it is
not
unusual to find
that this step in the mass spectrum is ‘washed out’ for
large clusters due to the fact that the ionization thresh-
old of a single cluster is not perfectly sharp.
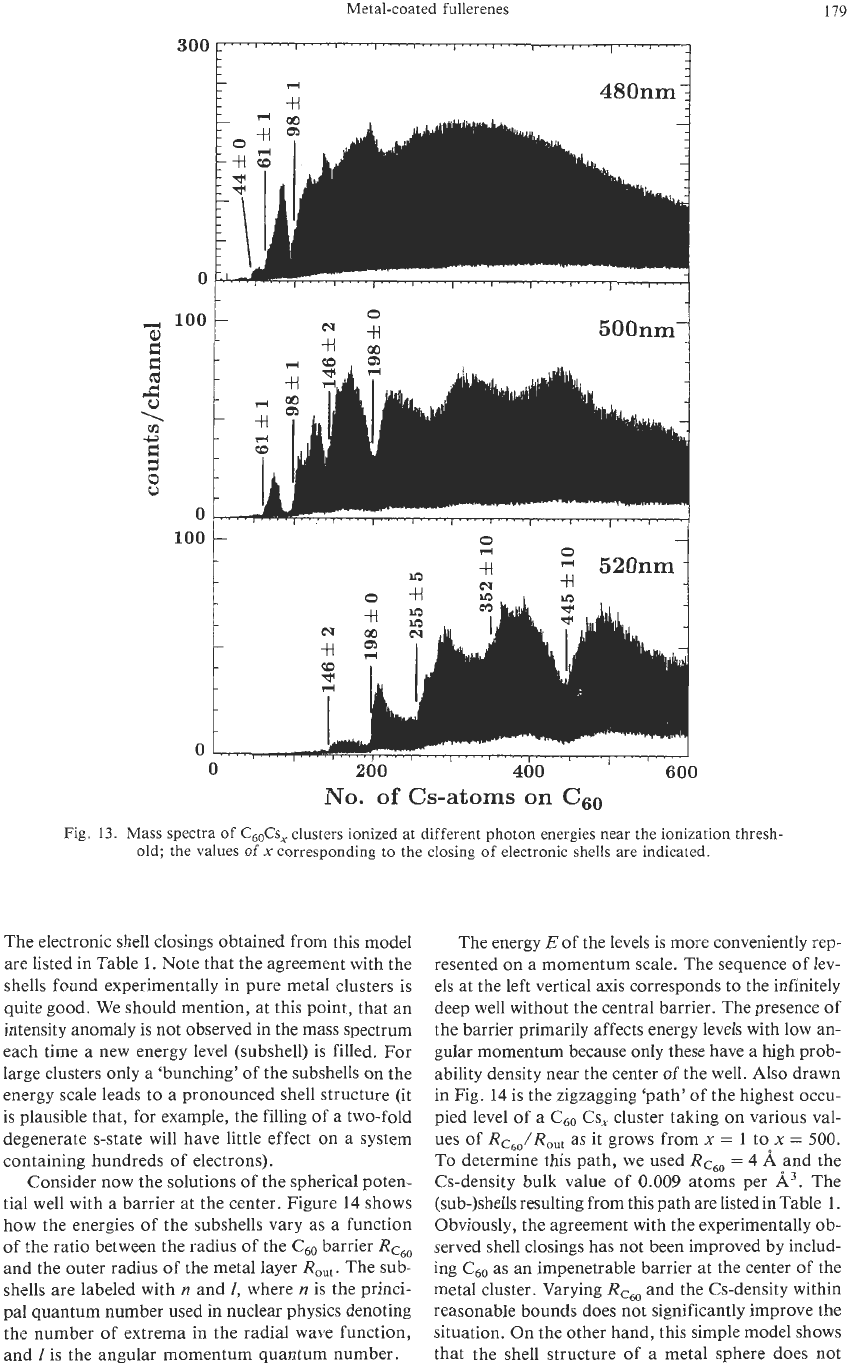
Figure
13
shows a set of spectra of C60Cs, clusters
for three different wavelengths of the ionizing laser.
Note the strong oscillations in the spectra. Plotted on
a
n1’3
scale, these oscillations occur with an equal
spacing. This is a first hint that we are dealing with
a shell structure. Because this spacing is almost iden-
tical to the one observed in pure alkali metal clusters,
these oscillations are most certainly due to electronic
rather than geometric shells. The number
of
atoms at
which the shell closings occur are labeled in Fig.
13
and listed in Table
1.
Note that these values do not
correspond to the minima in the spectra as long as
these have not reached zero signal.
Also listed in Table
1
are the shell closings observed
in pure alkali metal clusters[9,21,23]. These values and
the ones observed for the Cs-covered
Cm
have been
arranged in the table in such
a
way as to show that
there is some correlation between the two sets of num-
bers, but no exact agreement. If we make the simpli-
fying assumption that six Cs atoms transfer their
valence electrons to the
c60
molecule and that these
electrons will no longer contribute to tne sea of quasi-
free electrons within the metal portion of the cluster,
the number
6
should be subtracted from the shell clos-
ings observed for metal-coated
c60.
This improves
the agreement between the two sets of shells. How-
ever, it is really not surprising that the agreement is
still not perfect, because
a
c60
molecule present in
a
metal cluster will not only bond
a
fixed number
of
electrons but will also act as
a
barrier for the remain-
ing quasi-free metal electrons. Using the bulk density
of
Cs,
a spherical cluster Cs,,, has
a
radius
of
ap-
proximately
24
A.
A
Cm
molecule with
a
radius of
approximately
4
A
should, therefore, constitute
a
bar-
rier of noticeable size.
To
get some idea
of
the effect
such a barrier has
on
the shell closings, let us consider
the following simple model.
The metal cluster will be modeled as an infinitely
deep spherical potential well with the C60 represented
by an infinitely high spherical barrier. Let us place this
barrier in the center of the spherical cluster to simplify
the calculations. The simple Schrodinger equation,
containing only the interaction of the electrons with
the static potential and the kinetic energy term and ne-
glecting any electron-electron interaction, can then be
solved analytically, the solutions for the radial wave
functions being linear combinations
of
spherical Bessel
and Neumann functions.
Such
a
simple model, without the barrier due to the
c60
at
the center, has been used
to
calculate the elec-
tronic shell structure
of
pure alkali metal clusters[9].
Table
1.
Comparison
of
experimentally observed electronic
shell closings with model calculations*
-
Experiment Potential well
c6@,
M,
[21,23]
With barrier Without barrier
12
f
0
8
8
8
27
f
1
20
20 20
33
f
1
34 32 34
44
f
0
40 40
61
f
1
58
50
58
98
*
1
92 90 92
146
f
2
138 130 138
178 186
198
i
0
198
i2
196
255
f
5
263
f
5
252
254
352
f
10 341
f
5
330 338
445
f
10
443
a
5
428
440
80
*See text. The first two columns give the numbers
of
metal atoms
at
which electronic shell closings have been ob-
served in experiment
for
Cscovered C,, and for pure alkali
metal clusters, respectively. The columns on the right list the
number
of
electrons
required
for
shell closings in
an
infinitely
deep potential well with and without a central barrier. The
numbers in the different columns are mainly arranged in a
manner to show correlations.
Metal-coated fullerenes
179
i
500nm
1
0
rl
0
rl
520nm
ii
-ti
N
v)
200
400
600
No.
of
Cs-atoms
on
C60
0
0
Fig.
13.
Mass spectra of
C&s,
clusters ionized at different photon energies near the ionization thresh-
old; the values
of
x
corresponding to the closing
of
electronic shells are indicated.
The electronic shell closings obtained from this model
are listed in Table
I.
Note that the agreement with the
shells found experimentally in pure metal clusters is
quite good. We should mention, at this point, that an
intensity anomaly is not observed in the mass spectrum
each time a new energy level (subshell) is filled. For
large clusters only a ‘bunching’
of
the subshells on the
energy scale leads to a pronounced shell structure (it
is plausible that, for example, the filling of a two-fold
degenerate s-state will have little effect on a system
containing hundreds of electrons).
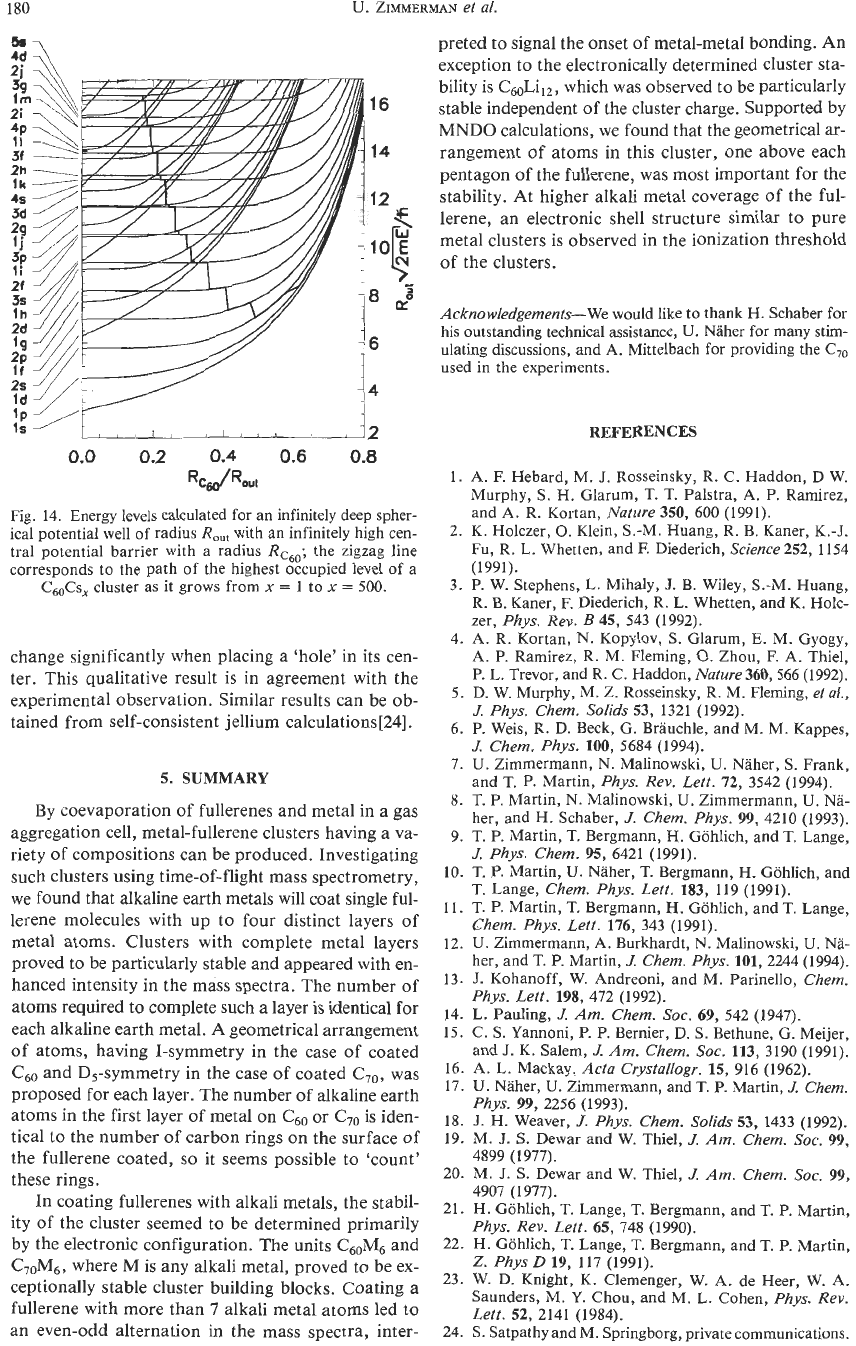
Consider now the solutions
of
the spherical poten-
tial well with a barrier at the center. Figure
14
shows
how the energies of the subshells vary as a function
of the ratio between the radius of the
C60
barrier
RC60
and the outer radius
of
the metal layer
R,,,.
The sub-
shells are labeled with
n
and
1,
where
n
is the princi-
pal quantum number used in nuclear physics denoting
the number
of
extrema
in
the radial wave function,
and
I
is
the angular momentum quantum number.
The energy
E
of
the levels is more conveniently rep-
resented on a momentum scale. The sequence
of
Iev-
els at the left vertical axis corresponds to the infinitely
deep well without the central barrier. The presence
of
the barrier primarily affects energy levels with low an-
gular momentum because only these have a high prob-
ability density near the center of the well. Also drawn
in Fig.
14
is the zigzagging ‘path’ of the highest occu-
pied level of
a
C60
Cs,
cluster taking on various val-
ues of
Rc,/R,,,
as it grows from
x
=
1
to
x
=
500.
To determine this path, we used
RC6,,
=
4
A
and the
Cs-density bulk value
of
0.009
atoms per
A3.
The
(sub-)shells resulting from this path are listed in Table
1.
Obviously, the agreement with the experimentally
ob-
served shell closings has not been improved by includ-
ing
C60
as an impenetrable barrier at the center of the
metal cluster. Varying
RCW
and the Cs-density within
reasonable bounds does not significantly improve the
situation. On the other hand, this simple model shows
that the shell structure
of
a metal sphere does not
180
U.
ZIMMERMAN
et ai.
IS’
I,,
,
,
,
,
,
,
, ,
,
0.0
0.2
0.4
0.6
0.8
RCJR0.t
Fig.
14.
Energy levels calculated for an infinitely deep spher-
ical potential well of radius
R,,,
with an infinitely high cen-
tral potential barrier with a radius
Rc6,;
the zigzag line
corresponds to the path
of
the highest occupied level
of
a
C,Cs,
cluster as it grows from
x
=
1
to
x
=
500.
change significantly when placing a ‘hole’ in its cen-
ter. This qualitative result is in agreement with the
experimental observation. Similar results can be ob-
tained from self-consistent jellium calculations[%].
5.
SUMMARY
By
coevaporation of fullerenes and metal in a gas
aggregation cell, metal-fullerene clusters having a va-
riety of compositions can
be
produced. Investigating
such clusters using time-of-flight mass spectrometry,
we found that alkaline earth metals will coat single ful-
lerene molecules with up to four distinct layers of
metal atoms. Clusters with complete metal layers
proved to be particularly stable and appeared with en-
hanced intensity in the mass spectra. The number
of
atoms required to complete such
a
layer
is
identical for
each alkaline earth metal.
A
geometrical arrangement
of
atoms, having I-symmetry in the case
of
coated
c60
and D,-symmetry
in
the case of coated
C,,,
was
proposed for each layer. The number
of
alkaline earth
atoms
in
the first layer
of
metal on
c60
or
C70
is iden-
tical
to
the number
of
carbon rings
on
the surface
of
the fullerene coated,
so
it
seems possible
to
‘count’
these rings.
In coating fullerenes with alkali metals, the stabil-
ity of the cluster seemed to be determined primarily
by the electronic configuration. The units C&i6 and
C7oM6, where M is any alkali metal, proved
to
be ex-
ceptionally stable cluster building blocks. Coating
a
fullerene with more than
7
alkali metal atoms led to
an even-odd alternation in the mass spectra, inter-
preted to signal the onset
of
metal-metal bonding.
An
exception to the electronically determined cluster sta-
bility is C60Li12, which was observed to be particularly
stable independent of the cluster charge. Supported by
MNDO calculations, we found that the geometrical
ar-
rangement of atoms in this cluster, one above each
pentagon of the fullerene, was most important for the
stability. At higher alkali metal coverage of the ful-
lerene, an electronic shell structure similar
to
pure
metal clusters
is
observed in the ionization threshold
of
the clusters.
Acknowledgements-We
would like to thank H. Schaber for
his
outstanding
technical
assistance, U. Naher for many
stim-
ulating discussions, and A. Mittelbach
for
providing the
C,,
used in the experiments.
REFERENCES
1.
A.
E
Hebard, M.
J.
Rosseinsky, R. C. Haddon, D W.
Murphy,
S.
H.
Glarum,
T.
T.
Palstra,
A.
P.
Ramirez,
and
A.
R. Kortan,
Nature
350,
600
(1991).
2.
K. Holczer,
0.
Klein, S.-M. Huang, R. E. Kaner,
K.4.
Fu, R.
L.
Whetten, and
E
Diederich,
Science
252,
1154
(1991).
3.
P.
W. Stephens,
L.
Mihaly,
J.
B. Wiley,
S.-M.
Huang,
R. B. Kaner,
E
Diederich, R.
L.
Whetten, and K. Holc-
zer,
Phys. Rev. B
45,
543 (1992).
4.
A.
R. Kortan, N. Kopylov,
S.
Glarum,
E.
M. Gyogy,
A.
P. Ramirez, R. M. Fleming,
0.
Zhou,
E
A. Thiel,
P.
L.
Trevor, and R.
C.
Haddon,
Nature
360,566 (1992).
5.
D.
W. Murphy, M.
Z.
Rosseinsky, R. M. Fleming,
eta/.,
J.
Phys. Chem. Solids
53,
1321 (1992).
6.
P.
Weis, R. D. Beck, G. Brauchle, and
M.
M.
Kappes,
J.
Chem. Phys.
100,
5684 (1994).
7.
U.
Zimmermann,
N.
Malinowski,
U.
Naher, S. Frank,
and
T.
P.
Martin,
Phys. Rev. Lett.
72, 3542 (1994).
T.
P. Martin,
N.
Malinowski,
U.
Zimmermann,
U.
Na-
her, and H. Schaber,
J.
Chem. Phw.
99.
4210 (1993).
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
T.
P.
Martin,
T.
Bergmann, H. Gohlich, and T. Lange,
J.
Phys. Chem.
95,
6421 (1991).
T.
P.
Martin, U. Naher, T. Bergmann, H. Gohlich, and
T.
Lange,
Chem. Phys. Lett.
183,
119 (1991).
T. P.
Martin,
T.
Bergmann, H. Gohlich, and
T.
Lange,
Chem. Phys. Lett.
176, 343 (1991).
U.
Zimmermann,
A.
Burkhardt,
N.
Malinowski,
U.
Na-
her, and
T.
P. Martin,
J.
Chem. Phys.
101, 2244 (1994).
J.
Kohanoff,
W.
Andreoni, and M. Parinello,
Chem.
Phys. Lett.
198,
472 (1992).
L. Pauling,
J.
Am. Chem.
SOC.
69,
542 (1947).
C.
S.
Yannoni,
P. P.
Bernier, D.
S.
Bethune, G. Meijer,
and
J.
K. Salem,
J.
Am.
Chem. Soc.
113, 3190 (1991).
A.
L. Mackay,
Acta Crystallogr.
15, 916 (1962).
U. Naher,
U.
Zimmermann, and T.
P.
Martin,
J.
Chem.
Phys.
99,
2256
(1993).
J.
H.
Weaver,
J.
Phys. Chem.
Solids
53,
1433 (1992).
M.
J.
S. Dewar and W. Thiel,
J.
Am. Chem.
SOC.
99,
4899 (1977).
M.
J.
S.
Dewar and W. Thiel,
J.
Am. Ckem.
SOC.
99,
4907 (1977).
H.
Gohlich,
T.
Lange, T. Bergmann, and
T.
P. Martin,
Phys. Rev. Lett.
65, 748 (1990).
H. Gohlich,
T.
Lange,
T.
Bergmann, and
T.
P.
Martin,
Z.
Phys
D
19, 117 (1991).
W.
D.
Knight,
K.
Clemenger,
W.
A. de Heer, W.
A.
Saunders, M.
Y.
Chou,
and
M.
L.
Cohen,
Phys. Rev.
Lett.
52,
2141 (1984).
S.
Satpathy and M. Springborg, private communications.
SUBJECT
INDEX
acetylene source
15
AFRVI:
see
atomic force microscopy
alkalUalkali earth metals
169
arc plasma, nanotube growth
11
atomic force microscopy
(AFM)
65
band gap
37
band structure
37
buckybundles
see
buclqtubes
buckytubes
149
properties
111
cage forms 77
cahon fibers
87
carbon nanotubes
see
nanotubes; pyrolitic carbon
carbon-carbon intralayer distance
59
catalysis
vapo-grown
1
nanotubes
growth
mechanism
87
nanotubule production
15
single-layer nanotubes
47
chiral nanotubes
27
clusters, metal-fullerenes
169
cobalt nanocrystals
153
cobalt particles
47
coiled carbon nanotubes
87
diameters, Mlerene-scale
15
dekcts
7
1
disordered carbons
129
electric field, nanotube growth
11
electrical properties
47
electrical resistivity
12
1
electron irradiation
163
electronic bands
27
electronic properties
11 1
carbon nanotubes
121
structure
37
electronic shells
169
fiber-reinforced composites
143
fibers
47
structures
65
fullerenes
87
growth pathway
65
metal-coated
169
multi-shell, synthesis
153
nanotubes comparison
15
fundamental parameters
27
geometry
carbon nanotubes
59
metal-coated fullerenes
169
glow discharge, buckybundles
11
1
graphene layers, flexibility
149
graphene model
37
graphite structure
1
graphitic carbon 77
graphitic particles, onion-like
153
helical forms
77
helix angle
59
hemi-toroidal nanostructures
105
high-resolution transmission electron microsopy
(HREM)
1,37, 111, 163
icosahedral layers
169
incomplete bonding defects
7
1
infrared studies
129
interlayer distance
59
iron
nanocrystals
153
knee structures
87
magnetic properties, buckytubes
11
1
magnetoresistance
121
magnetic susceptibility
12 1
mass spectroscopy, metal-coated fullerenes
169
mechanical properties
47,
143
metal particles
47
metal-coated fullerenes
169
molecular dynamics 77
multi-shell fullerenes
163
multi-shell
tubes
65
multi-wall nanotubes 27
nanocapsules
153
nanocones, STM
65

182
Subject Index
nanofibers
87
nanoparticles 153
nanostructures 65, 163
nanotubes
bundles
47
catalytic production 15
coiled
carbon
87
defect structures
7
1
electric effects
11
electronic properties
37,
121
fullerene-scale diameters 15
fullerenes, comparison 15
geometry 59
growth mechanisms
1,
11,65,87
hemi-toroidal networks 105
mechanical properties 143
nanoparticles 153
single-layer
47, 187
STM
65
structural properties
37
thermal properties 143
vibrations, theory of 129
natural resonance
143
nickel filled nanoparticles 153
normal modes 129
onion-like graphitic particles 163
open tips 11
PCNTs
see
pyrolyk carbon nanotubes
pitch angle
59
pyrolitic carbon nanotubes
(PCNTs)
hemi-toroidal networks 105
vapor grown
1
Raman scattering studies 129
rare-earth elements 153
rehybridization defects
7
1
scanning
tunneling
microscopy
(STM)
65,
121
single-layer walls 47
single-wall nanotubes 27
spectroscopy
121
stiffness constant 143
STM
see
scanning tunneling microscopy
strain energy
37
structural properties
37
thermal properties 143
topological defects
7
1
toroidal cage forms
77
toroidal network
1
torus form 77
transport properties, buckytubes
1
11
tubes,
growth
pathways 65
tubule arrays
27
topology
77
vapor growth 65
vapor-grown carbon
fibers
1
vibrational modes 27, 129

AUTHOR
INDEX
Bernaerts,
D.
15
Bethune,
D.
S.
47
Beyers,
R.
47
Burkhardt,
A.
169
Chang,
R.
P.
H.
111
Colbert,
D.
T.
11
Daguerre,
E.
149
Despres,
J.
F.
149
Dravid,
V.
P.
111
Dresselhaus, 6.27
Dresselhaus,
M.
S.
vii,
ix
27
Ebbeson,
T.
W.
71
Eklund,
P.
C.
129
Endo,
M.
vii
1:
105
Fonseca,
A.
IS,
87
Goddard
III,
W.
A.
47
Heremans,
J.
121
Hernadi,
K.
87
Holden,
J.
M.
129
Ihara,
S.
77
Iijimla,
S.
vii
Issi,
J.-P.
121
Itoh,
S.
77
Ivanov,
V.
15
Jishi,
R.
A.
129
Ketterson,
J.
B.
111
Kiang,
C.-H.
47
Kobori,
K.
1
Kroto,
H.
W.
1,
105
Lafdi,
K.
149
Lambin,
P.
15,87
Langer,L.
121
Lin,
X.
W.
111
Lorents,
D.
C.
143
Lucas,
A.
A.
15,87
Malinowski,
N.
169
Martin,
T.
P.
169
Mintmire,
J.
W.
37
Nagy,
J.
B. 15,87
Olk,
C.
H.
121
Ruoff,
R
S.
143
Saito,
R.
27
Saito,
Y.
153
Sarkar,
A.
1, 105
SattIer,
K.
65
Setton,
R.
59
Smalley,
R.
E.
11
Song,
S.
N.
11
1
Takada,
T.
71
Takahashi,
K.
I
Takeuchi,
K.
1
Ugarte,
D.
163
Wang,
X.
K.
11
1
White,
C.
T.
37
Xhang,
X.
B.
15
Zimmerman,
U.
169
183
