Elsevier Encyclopedia of Geology - vol I A-E
Подождите немного. Документ загружается.

This reaction was the major control on the amount
of carbon dioxide in the atmosphere before the onset
of photosynthesis, respiration, and organic-matter se-
questration. During subduction, carbonate minerals
carried in the descending oceanic crust are heated,
releasing carbon dioxide and water as volatile com-
ponents in arc magmas, completing the cycle back to
the atmosphere. In hydrothermal vents within basaltic
crust, water oxidizes Fe
2þ
in olivine, yielding hydro-
gen, magnetite, and serpentine. In gas-phase high-
temperature reactions, hydrogen can reduce carbon
dioxide to methane, but it is not likely that much
hydrogen was maintained in the atmosphere, owing
to its high rate of escape from the top of the atmos-
phere. The currently held view is that the early sec-
ondary atmosphere was, at most, weakly reducing,
with the major components – carbon dioxide, nitro-
gen, water vapour, and carbon monoxide – supple-
mented by minor mixing fractions of hydrogen and
methane. Estimates have been made of the rate of
decline of carbon dioxide over geological time that
provided the major greenhouse forcing to the atmos-
phere, while tracking secular changes in solar lumi-
nosity. Initially high partial pressures of carbon
dioxide, possibly supplemented by biogenic methane,
would have kept surface temperatures warm enough
to avoid freezing of the planet until the crisis condi-
tions of the snowball Earth in the Late Proterozoic.
Since the era of severe Proterozoic glaciations, there
has been a generally gentle decline in levels of carbon
dioxide, maintaining the stability of the biosphere in
the face of rising solar luminosity (of the order of
about 6% per billion years).
Atmospheric Evolution
Early Anoxic Atmospheres
Free oxygen was rare or absent on the early Earth, so
that the atmosphere was effectively anoxic. Con-
straints on ancient levels of atmospheric oxygen
have been sought for many years, and most models
of the early Earth consider oxygen levels to have been
very low (less than 10
2
of present atmospheric level
(PAL)) before about 2.3 Ga. Evidence to support this
comes from detrital gold, uraninite, and pyrite grains
in sediments older than 2.3 Ga, which it has been
argued could not have been preserved in a high-
oxygen atmosphere. Other geochemical indications,
such as Fe
2þ
/Fe
3þ
in palaeosols and discussions of the
possible origins of banded iron formations (see Sedi-
mentary Rocks: Banded Iron Formations) and of
carbon isotopic signatures in Archaean and Palaeo-
proterozoic organic matter and carbonates, have also
been used to support the contention that the early
atmosphere was essentially devoid of oxygen. In the
absence of a ready source of free oxygen and, there-
fore, no effective ozone (O
3
) screen from solar ultra-
violet, atmospheric water vapour would have
photodissociated into hydrogen and oxygen. Since
the oxygen formed by this reaction produces just
enough ozone to inhibit the reaction, accumulation
of oxygen by this means is limited. Oxygen combines
readily with hydrogen to form water vapour, so little
oxygen is lost from the atmosphere to space. The
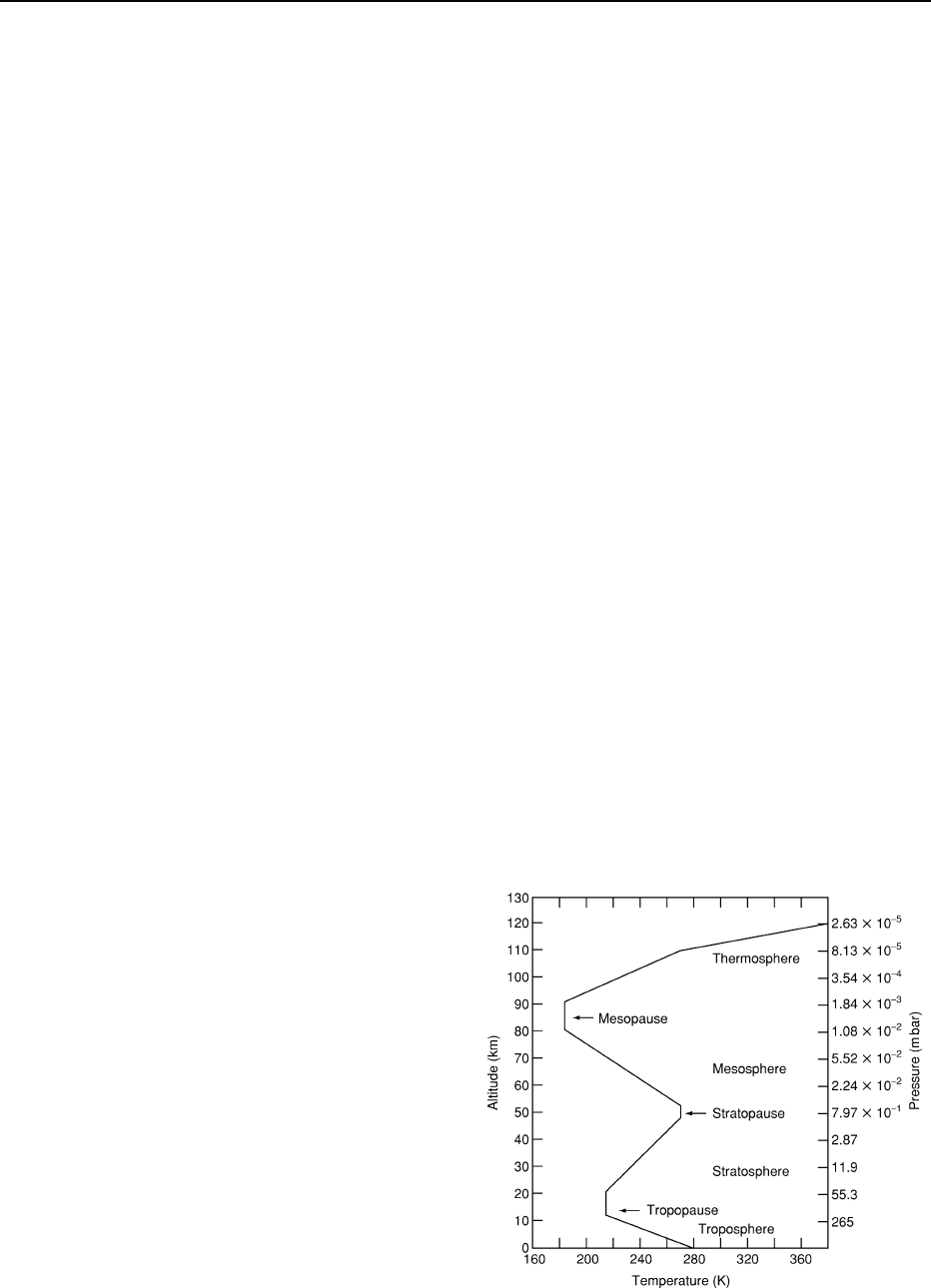
bottom of the stratosphere acts as a cold trap and
maintains a constant temperature of 56
C, keeping
water from escaping from the top of the atmosphere
(Figure 5).
Sulphur isotopes: a unique window onto early atmos-
pheric chemistry Further evidence in support of an
early anoxic atmosphere comes from the unusual
chemistry of sulphur isotopes. Most physical processes
fractionate the isotopes of an element because of
the relative mass differences between the isotopic
species. For instance, because the mass difference bet-
ween
33
Sand
32
S is half that between
34
Sand
32
S, any
mass-dependent (defined by D
33
S ¼ 0%;where
D
33
S ¼ 0.515d
34
S – d
33
S) physical effects on the ratio
of
33
Sto
32
S are expected to be about half those on
the ratio of
34
Sto
32
S. However, certain unusual gas-
phase chemical reactions in the absence of free oxygen
are capable of producing anomalous sulphur isotopic
values that do not follow the mass-dependent relation-
ship described above. The recent discovery of the signal
of the mass-independent fractionation effect (where
D
33
S 6¼ 0%) in geological materials has important im-
plications for understanding the chemical evolution of
Figure 5 Temperature and pressure profile of the Earth’s
atmosphere.
ATMOSPHERE EVOLUTION 201
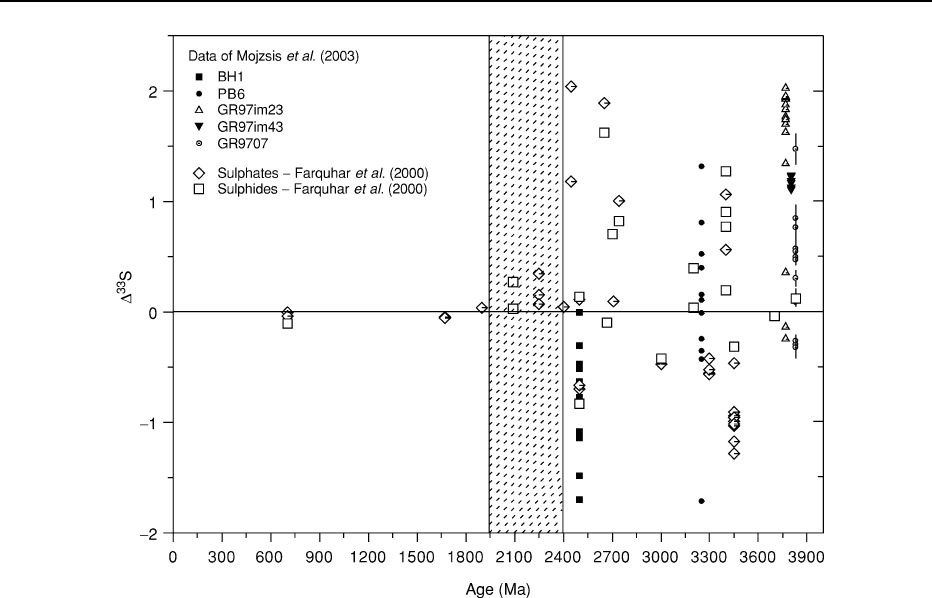
the atmosphere. It has been found that sedimentary
sulphide and sulphate minerals in Archaean rocks con-
tain sulphur where the levels of the minor isotope (
33
S)
deviate from the mass-fractionation line with other
post-2.3 Ga terrestrial sulphur minerals. It appears
that the signal of this chemistry can be transferred
from the atmosphere to surface materials and there-
after to the geological record. The mass-independent
fractionation effect of sulphur in ancient sulphides
records chemical reactions in early anoxic terrestrial
atmospheres, which were destroyed in the Early Pro-
terozoic (2.4–2.3 Ga), and tracks the rise in levels of
atmospheric oxygen (Figure 6).
Metabolic Energy and the Rise of Oxygen
Changes in atmospheric composition have had a pro-
found effect on the evolution of life. Sequence analyses
used to compare genomes have been used to infer the
phylogenetic relatedness of living organisms and their
evolutionary history in terms of molecular biology and
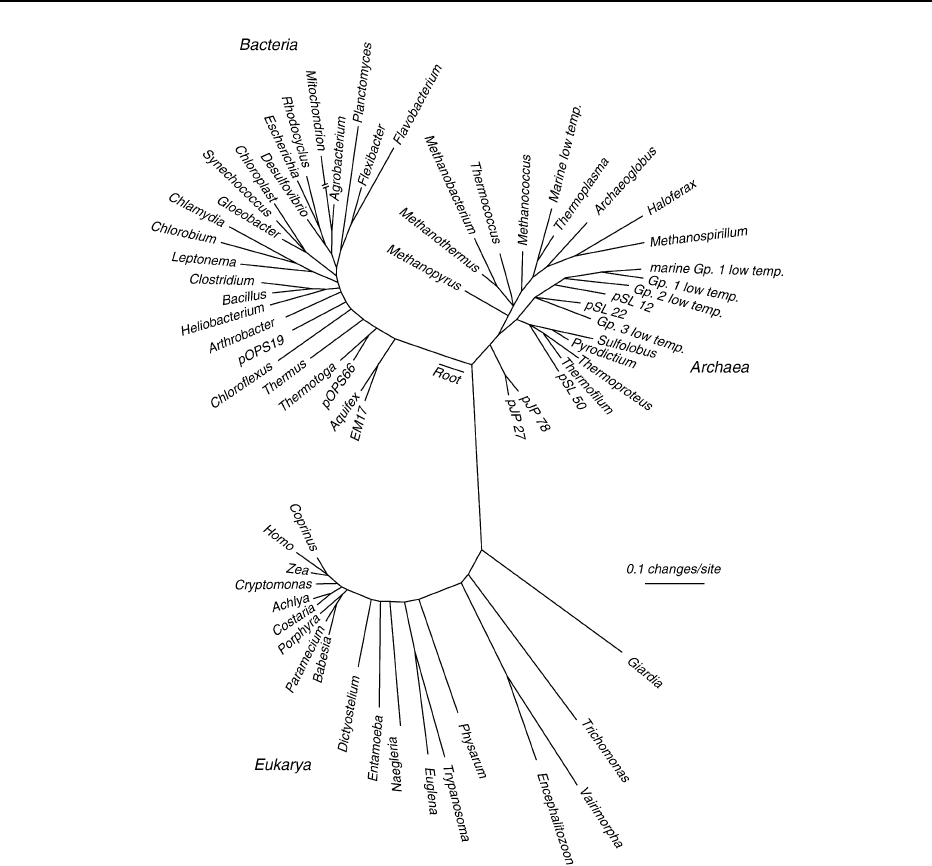
metabolic style. It has been found that three great
domains exist within the ribosomal RNA tree of life:
Bacteria, Archaea, and Eukarya (Figure 7)(see Precam-
brian: Prokaryote Fossils). The organisms with the
deepest branches in the tree, corresponding to the
most ancient pedigrees, close to the root in Figure 7,
are hyperthermophilic (heat-loving) chemoautotrophs
(organisms that obtain their metabolic energy from
chemical disequilibria). It appears that at least some
of the earliest organisms lived off chemical energy, in
much the same way as contemporary hot-spring micro-
bial communities. Such environments are widespread
in volcanic centres on land and on the seafloor, and they
wouldhavebeenevenmorewidespreadonageologic-
ally restive early Earth. However, hyperthermophilic
organisms are restricted to zones around hydrothermal
vents where optimal chemical and temperature condi-
tions persist. Photosynthesizers, on the other hand, are
less restricted and have evolved to inhabit environ-
ments away from those of their chemosynthetic ances-
tors into the open ocean.
Light from the Sun provides a readily accessible,
stable, and inexhaustible energy source to drive meta-
bolic reactions. Photosynthesis uses light energy to cap-
ture electrons and move low-energy-state ions to higher
energy states. The energy released can then be used
to power metabolic reactions such as growth and
reproduction. Photosystem I was the earliest photosyn-
thetic pathway and used light-activated chlorophylls
and electron donors such as H
2
SandFe
2þ
to yield S
0
and Fe
3þ
as oxidative products. At a later stage, photo-
system II developed; this uses water as the electron
Figure 6 Mass-independent sulphur isotopic compositions track changes in atmospheric composition with time. (Reproduced from
Mojzsis
et al. (2003).)
202 ATMOSPHERE EVOLUTION
donor to reduce carbon dioxide to biologically useful
organic compounds such as carbohydrates. Liquid
water and carbon dioxide are available to life in prac-
tically unlimited quantities; sunlight drives the reac-
tion, and cyanobacteria that use it, although relative
late-comers in phylogenetic terms, are arguably the
most successful organisms in the history of life on
Earth. It is photosystem II that releases free oxygen as
a by-product in the reversible reaction:
6CO
2
þ 6H
2
O þ light energy $ C
6
H
12
O
6
þ 6O
2
The gradual build-up of oxygen in the atmosphere
was accomplished by the slow toilsome efforts of cy-
anobacteria over many hundreds of millions of years.
The immense importance of this metabolic reaction
to the history of all life beyond the microbial level
cannot be overstated (see Earth System Science).
Rise in atmospheric oxygen Initially, the vast
amounts of reduced iron (Fe
2þ
) and other chemical
species dissolved in the oceans, and perhaps
some reduced gases in the atmosphere (such as me-
thane), provided a sink for the oxygen produced by
photosynthesis. As these sinks were exhausted, local
concentrations of oxygen would have appeared, cre-
ating a dilemma for the early anaerobic microbial
biosphere. High levels of oxygen produce toxic rad-
icals in the environment, which cause extensive
damage to cell components. This would have been
a strong driving force for natural selection leading
to adaptation: microbes that did not immediately
Figure 7 The phylogenetic tree of life based on comparative 16s rRNA sequences. (Reproduced from Pace NR (2001) The universal
nature of biochemistry. Proceedings of the National Academy of Sciences USA 98: 805–808.)
ATMOSPHERE EVOLUTION 203

go extinct survived by retreating, for example, to
anaerobic environments, such as deep in crustal
rocks, organic-rich muds, and animal digestive tracts,
where they continue to thrive today. New forms of
life emerged with superoxide dysmutase and catalase
enzymes, which catalyze the reduction of oxic free
radicals to water. Finally, another group (the
Eukarya; Figure 7) developed aerobic metabolism,
providing energy yields approximately twenty times
greater than those of anaerobic metabolism (see Pre-
cambrian: Eukaryote Fossils). The mitochondria –
cell organelles contained in most Eukarya, which fa-
cilitate oxidative metabolism – arose from the endo-
symbiosis of proteobacteria. These organelles
actually contain their own subunits of DNA that
further implicate a eubacterial heritage.
The increased energy yield of aerobic metabolism
set the stage for the evolution of all life above the
unicellular level. The energy source that supports
the aerobic biosphere is the Sun – thus established
oxygenic photosynthesis and aerobic respiration
governed the flow of carbon and oxygen through
the atmosphere–hydrosphere system. During the Ar-
chaean the atmosphere and hydrosphere initially con-
tained small amounts of free oxygen from photolysis
of water vapour and then increasing amounts from
oxygenic photosynthesis. By the end of the Archaean–
Early Proterozoic, oxygen levels had begun to creep
upwards, balanced by oxygen-consuming reactions
such as weathering, hydrothermal activity, respir-
ation, oxidation of organic matter, and differential
rates of organic-matter burial. By about 2 Ga, levels
of oxygen were about 1% of PAL. A transition era
ensued, with oxygen levels fluctuating around the 1%
PAL value and the oceans remaining reducing and
sulphidic. After about 1.8 Ga, oxygen-consuming re-
actions were generally exhausted and free-oxygen
concentrations reached about 10% of PAL. Over
time, these levels stabilized at near ‘normal’ Phaner-
ozoic atmospheric concentrations. It remains unquan-
tified how rapidly oxygen levels increased during the
Proterozoic.
The rise in atmospheric oxygen had an acute effect
on the surface environment, not only because of its
toxicity to many microbial organisms (providing the
motive force to drive the evolution of more efficient
aerobic metabolisms) but also by establishing an ef-
fective ultraviolet screen. Ozone is far more effective
than diatomic oxygen at absorbing ultraviolet. The
ozone screen formed in the stratosphere from accu-
mulating O
2
that photodissociated to produce free
oxygen radicals (O
), which then recombined with
O
2
to make ozone. This ozone screen effectively
made dry land habitable for plants and animals
by the Palaeozoic. Industrial pollutants such as
chlorofluorocarbons are now severely damaging the
ozone layer.
A Neoproterozoic Snowball Earth
The increased oxidation of the surface zone in the
Middle Proterozoic was probably a consequence of
the sequestration of large quantities of organic carbon
in sediments. From the oxygenic photosynthesis reac-
tion, it can be seen how this scenario leads to a net loss
of carbon dioxide and a net increase of oxygen in the
atmospheric reservoir. The boosted greenhouse effect
of heightened levels of carbon dioxide before about
2.5 Ga was lost to the Earthat a most inopportune time.
Levels of solar luminosity were about 10%–15%
lower at 2.2 Ga than at present (Figure 1). Loss of
insolation from the carbon dioxide greenhouse
spelled disaster for the Proterozoic Earth. The planet
froze over, locking the oceans in ice and creating a
high-albedo feedback loop that kept the planet frozen
for extended periods, until levels of carbon dioxide
increased due to passive volcanism and outgassing.
Heightened levels of carbon dioxide warmed the at-
mosphere and dark dusty ice reduced the albedo,
causing catastrophic melting and massive planetary
warming. Subsequent weathering in a carbon diox-
ide-rich atmosphere, combined with massive algal
blooms, led to enhanced burial of organic carbon,
drawdown of carbon dioxide levels, and a renewed
snowball Earth. The cycle is thought to have been
broken by the secular increase in solar luminosity
and a steady redistribution of the continents via
plate motions. The survivors of these repeated ‘ice-
house’ and ‘hothouse’ Earths were multicellular or-
ganisms that inherited a more stable environment
high in oxygen (see Palaeoclimates).
The Phanerozoic Atmosphere
Phanerozoic Atmospheric Changes
Geochemical evidence from the study of palaeosols,
coupled with data from carbonate and organic
carbon in sediments as well as sedimentary pyrite
and the chemistry of sedimentary silicate minerals,
has been used to improve models of the carbon cycle
of palaeoenvironments. These models have been used
to document and explain fluctuations in levels of
oxygen and carbon dioxide over Phanerozoic time.
Long-term changes in the carbon dioxide and oxygen
concentrations in the Phanerozoic atmosphere are
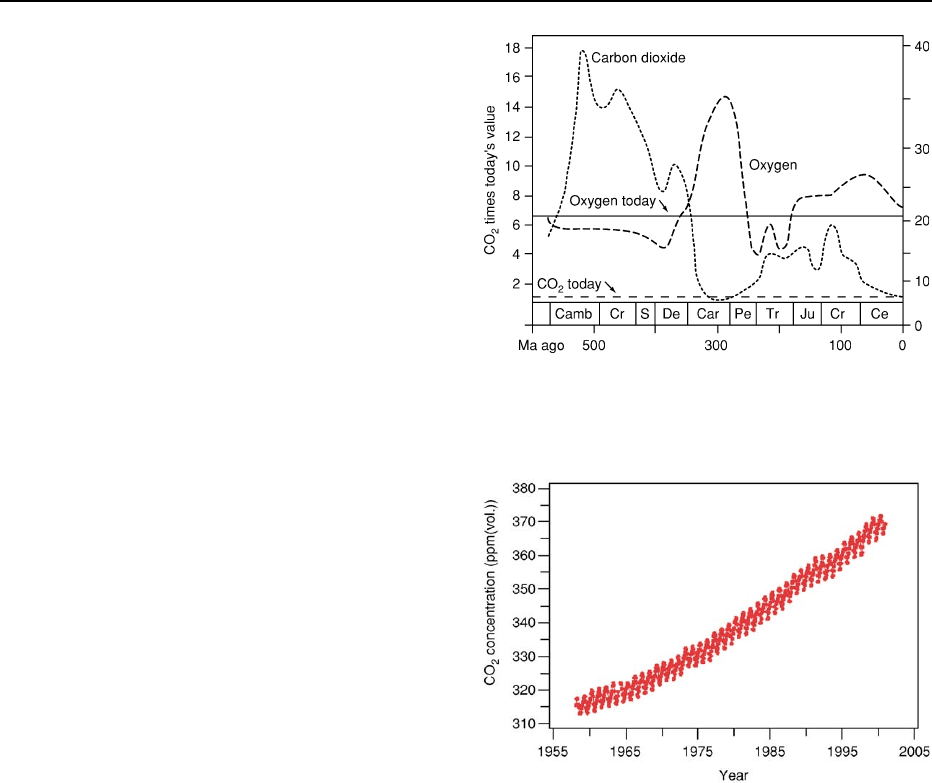
summarized in Figure 8.
In the first part of the Phanerozoic, the Early
Palaeozoic (Cambrian–Ordovician), evidence indi-
cates that levels of carbon dioxide were about fifteen
times PAL. These declined to within a few percent of
204 ATMOSPHERE EVOLUTION
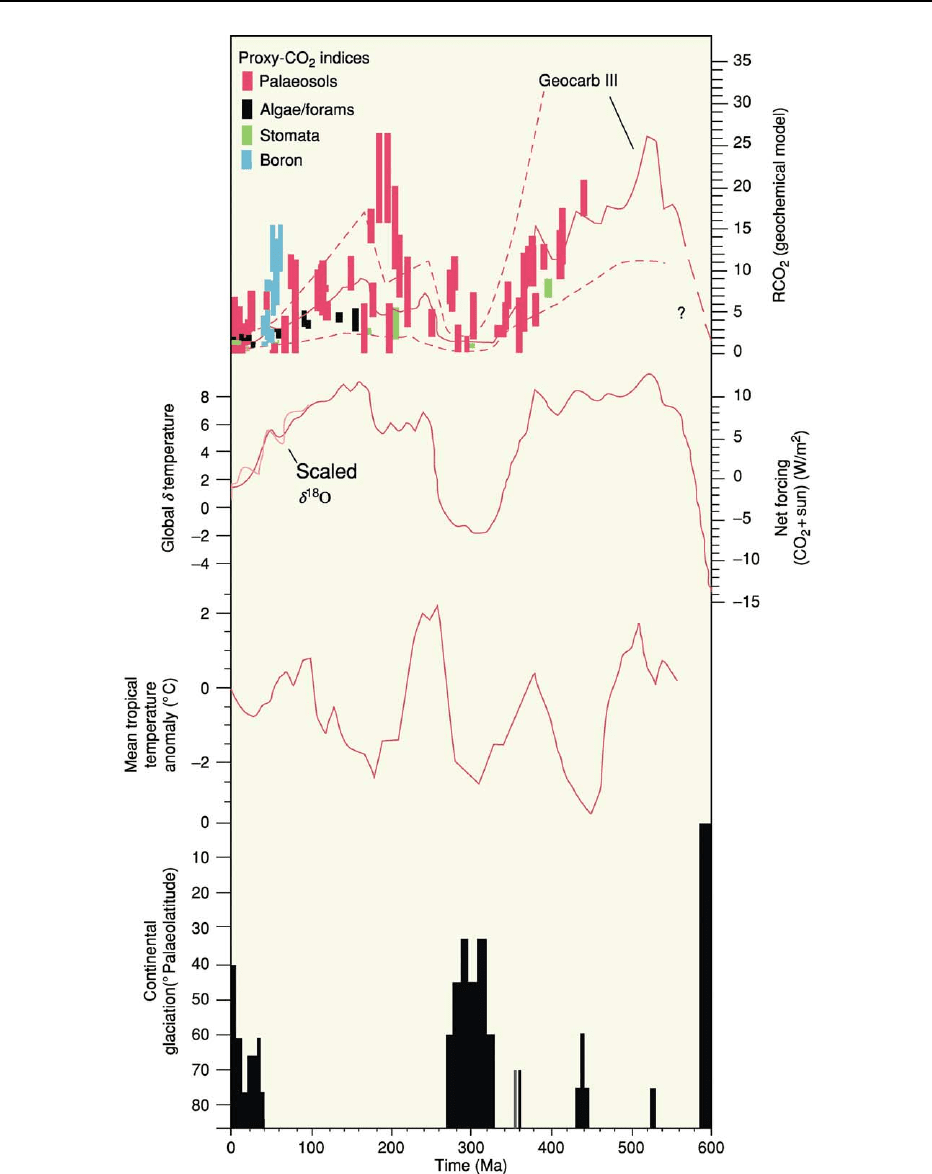
PAL at the end of the Carboniferous. Massive carbon
burial and increased solar radiation by the Carbon-
iferous meant that atmospheric oxygen concentra-
tions were greater than at any other time in Earth
history (see Palaeozoic: Carboniferous). This may
have allowed the existence of the huge Carboniferous
insects observed in the fossil record (see Fossil Inverte-
brates: Insects). Insects rely on diffusion-limited flow
Figure 8 Phanerozoic temperature history. (Reproduced from with permission from Berner RA. Geocarb II: A revised model of
atmospheric CO
2
over phanerozoic time, Am. Jour. Sci., 294: 56–91)
ATMOSPHERE EVOLUTION 205
of oxygen through their exoskeletons: to be big they
need to live in conditions of high atmospheric oxygen,
so that sufficient oxygen passively diffuses into their
blood to power muscles for flight.
Reduced greenhouse forcing and glaciation at the
end of the Palaeozoic with the assembly of Pangaea
reduced organic-matter burial, and there was a slow
rise in carbon dioxide levels to around six times PAL
in the Permian and Triassic. Levels of carbon dioxide
gradually decreased, and stabilized near present-day
levels in the Mesozoic. Oxygen tends to track carbon
dioxide inversely; geological evidence and palaeocli-
mate models suggest a maximum of near 35% oxygen
in the atmosphere at the beginning of the Permian.
During the Permian, the oceans were highly stratified,
with carbonate-rich water at depth that was depleted
in oxygen. This system was unstable: ocean hypoxia
could occur if ocean circulation intensified enough
to mix deep-water carbon dioxide and hydrogen
sulphide into surface waters. A protracted (20 Ma)
whole-ocean hypoxia event is considered to be a
major mechanism in the Permian–Triassic extinction
event, which wiped out 90%–95% of all marine
species (see Palaeozoic: End Permian Extinctions).
Carbon Dioxide and Climate Changes
High-resolution information about changes in atmos-
pheric chemistry over the past 160 000 years can be
obtained by studying the record of trapped gases in ice
cores from the Greenland and Antarctic ice-caps. Fur-
thermore, oxygen isotopic values from marine sedi-
ments, marine planktonic and benthonic fossils, cave
deposits, and other sources can be used to estimate
marine palaeotemperatures. Direct measurements of
carbon dioxide and methane concentrations in ice
cores permit assessment of past atmospheric levels of
these gases, providing factors to incorporate into
models of past air temperatures and sea-levels.
Data from deep ice cores taken in polar re-
gions, coupled with complex palaeoclimate models
(Figure 9), show large fluctuations in atmospheric
carbon dioxide, oxygen and methane levels, leading
to long-term temperature changes of the order of
6
C or more. There is a strong correlation between
levels of atmospheric greenhouse gases and palaeo-
temperature. The periodicities in these data provide
clear evidence of the role of Milankovitch forcing by
changes in the Earth’s orbital parameters. The two
strongest Milankovitch cycles observed correspond
to the 26 000 year precession of the equinoxes and
the 100 000 year period of rotation of the Earth’s
orbital axis (see Earth: Orbital Variation (Including
Milankovitch Cycles)). Changes in greenhouse-gas
concentrations appear to follow rather than guide
long-term climate, suggesting that Milankovitch
cycles are the prime mechanism bringing the Earth
into a greenhouse or icehouse condition. Greenhouse
gases do provide positive feedback at the beginning of
temperature changes by boosting insolation. Anthro-
pogenic emissions of greenhouse gases are not
governed by Milankovitch cycles and represent a sep-
arate and increasingly important climate-forcing
mechanism. Figure 9 shows that carbon dioxide con-
centrations changed by almost 100 ppm(vol.) towards
the end of the last glaciation. Modern levels of carbon
dioxide are near 370 ppm(vol.) and rising. Almost all
of this change has occurred since the Industrial Revo-
lution, and high-resolution monitoring at the Mauna
Loa observatory shows clear diurnal and annual
cycles in carbon dioxide levels (Figure 10), with a
mean annual increase of 1.16 ppm(vol.) year
1
. This
is over one hundred times the rate of increase of
carbon dioxide levels inferred from all available
Figure 9 Phanerozoic carbon dioxide and oxygen concentra-
tions.
Figure 10 Changes in the concentration of atmospheric carbon
dioxide over the last 60 years as measured at Mauna Loa, Hawaii.
Data provided by D. Keeling and T. Whorf.
206 ATMOSPHERE EVOLUTION

geological data, with no clear stabilization of these
levels in sight.
Other greenhouse gases Neglecting the small contri-
bution from internal heating driven by radioactive
decay, planetary surface temperatures are governed
by the amount of solar radiation received and its
interaction with the atmosphere. Re-radiation to
the surface of infrared radiation by greenhouse
gases, chiefly carbon dioxide but also water vapour,
methane, nitrous oxide species (NO
x
), and chloro-
fluorocarbons (CFCs), keeps the present average sur-
face temperature some 33 K above the black-body
temperature of 255 K. Although they are present in
small amounts relative to carbon dioxide and water
vapour, methane
,
nitrous oxide species, and CFCs
(which can form only from industrial processes) are
very effective greenhouse gases. Methane in the at-
mosphere (concentration of 1.7 ppm(vol.)) is domin-
antly formed by biological processes and absorbs
infrared radiation approximately 21 times more ef-
fectively than carbon dioxide; its levels have increased
rapidly in the last several centuries. Gaseous nitrous
oxide compounds are present at low concentrations
(ca. 0.3 ppm(vol.)), are produced by biological
nitrification, and have a long residence time in the
atmosphere. The concentration of CFCs is very low
(less than 0.003 ppm(vol.)), but they have a green-
house effect ten thousand times greater than that of
carbon dioxide and likewise have long residence
times in the atmosphere.
Conclusions
The splendour of contemporary life on Earth, as re-
vealed by the geochemical, palaeontological, and mo-
lecular phylogenetic records, took billions of years to
achieve, and its evolution occurred for the most part
within the envelope of the atmosphere and hydro-
sphere. In the context of astrophysical changes to
the Sun and geophysical changes to the solid Earth,
the atmosphere has evolved through a complex set of
interrelated cycles, within which biology has been of
central importance. Life has affected the planetary
atmosphere, and biological evolution was in turn
affected by it. The gaseous envelope of the planet
evolved in response to changing geophysical condi-
tions, such as mantle heat flow and solar luminosity.
Early in Solar System history, Venus and Mars appar-
ently had geochemical paths that paralleled that of
the Earth, probably including liquid water at their
surfaces. However, long ago they diverged from a
physicochemical regime that promoted habitability.
The nascent biosphere on these planets, if it ever
existed, was either burned to a crisp (on Venus) or
freeze-dried (on Mars). In the far future, as the lumi-
nosity of the Sun continues to increase, Earth will go
the way of Venus.
See Also
Earth: Mantle; Crust; Orbital Variation (Including Milan-
kovitch Cycles). Earth Structure and Origins. Earth
System Science. Fossil Invertebrates: Insects. Palaeo-
climates. Palaeozoic: Carboniferous; End Permian Ex-
tinctions. Precambrian: Overview; Eukaryote Fossils;
Prokaryote Fossils. Sedimentary Rocks: Banded Iron
Formations. Solar System: The Sun; Asteroids, Comets
and Space Dust; Meteorites; Mercury; Venus; Moon;
Mars; Jupiter, Saturn and Their Moons; Neptune, Pluto
and Uranus.
Further Reading
Hoffman PF and Schrag DP (2000) Snowball Earth.
Scientific American 282: 68–75.
Holland HD (1984) The Chemical Evolution of the Atmos-
phere and Oceans. Princeton: Princeton University Press.
Margulis L (1984) Early Life. Boston: Jones and Bartlett.
Mojzsis SJ and Harrison TM (2000) Vestiges of a
beginning: clues to the emergent biosphere recorded in
the oldest known sedimentary rocks. GSAToday 10: 1–6.
Pace NR (2001) The universal nature of biochemistry.
Proceedings of the National Academy of Sciences USA
98: 805–808.
Royer DL, Berner RA, Montan
˜
ez IP, Tabor NJ, and Beerling
DJ (2004) CO
2
as a primary driver of Phanerozoic
climate. GSA Today 14: 4–10.
Sagan C and Mullen G (1972) Earth and Mars: evolution
of atmospheres and surface temperatures. Science 177:
52–56.
ATMOSPHERE EVOLUTION 207

AUSTRALIA
Contents
Proterozoic
Phanerozoic
Tasman Orogenic Belt
Proterozoic
I M Tyler, Geological Survey of Western Australia,
East Perth, WA, Australia
ß 2005, Elsevier Ltd. All Rights Reserved.
Introduction
The Proterozoic is the period of geological time
extending from the end of the Archaean, 2500 million
years ago (Ma), to the start of the Phanerozoic (the
base of the Cambrian System), 545 Ma. The Protero-
zoic is divided into the Palaeoproterozoic (2500–
1600 Ma), the Mesoproterozoic (1600–1000 Ma),
and the Neoproterozoic (1000–545 Ma). In Austra-
lia, Proterozoic rocks are present to the west of the
‘Tasman Line’ that separates ‘Proterozoic Australia’,
where geophysical datasets show that Precambrian
basement is continuous beneath Phanerozoic sedi-
mentary basins, from the Tasmanides, where predom-
inantly Palaeozoic basement is overlain by Mesozoic
and younger sedimentary basins. Extensive exposures
of Proterozoic rocks are present in western Australia,
in northern, central, and north-eastern Australia, and
in southern Australia and Tasmania. Proterozoic
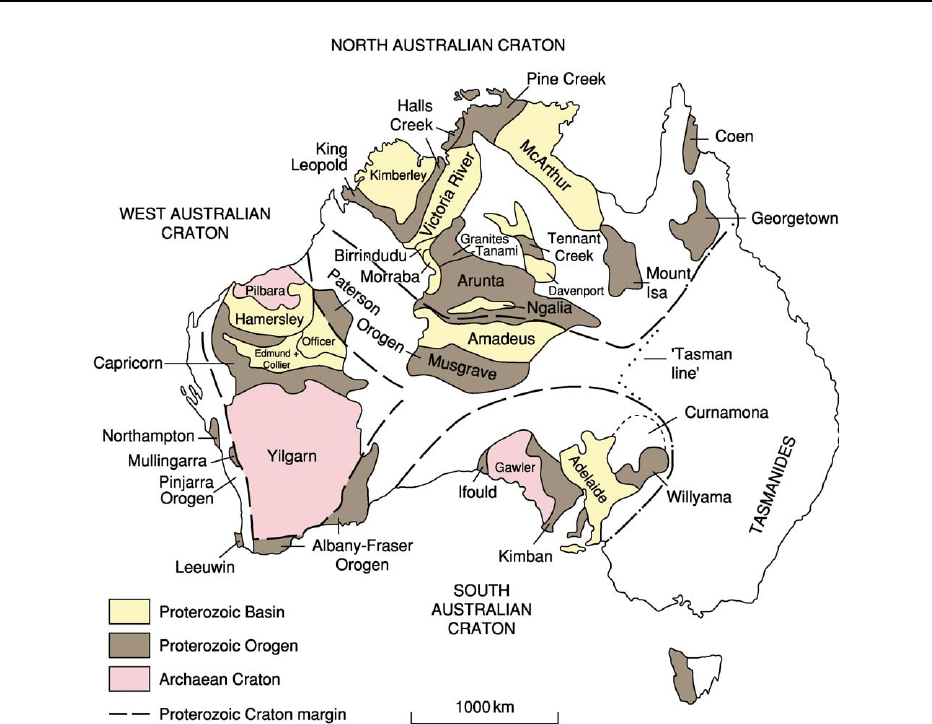
Australia is made up of three distinct cratons, the
West Australian Craton, the North Australian
Craton, and the South Australian Craton (Figure 1).
These probably formed originally as parts of larger
cratons or continental blocks (the South Australian
Craton together with the previously adjacent part of
Antarctica formed the Mawson Craton) and are
dominated by Archaean and Palaeoproterozoic to
Mesoproterozoic crust. The three cratons are separ-
ated by two predominantly Mesoproterozoic to
Neoproterozoic orogenic belts, the Paterson Orogen
and the Albany–Fraser Orogen. The Palaeoprotero-
zoic to Neoproterozoic Pinjarra Orogen is present
along the western margin of Australia.
Plate tectonic models can be applied to Proterozoic
Australia. The increasing availability of high-quality
geochronological data has highlighted the presence of
distinct tectonostratigraphic terranes with differing
geological histories within orogenic belts, and geo-
physical datasets reveal the heterogeneous nature of
the crust throughout Proterozoic Australia. However,
Proterozoic plate-tectonic processes may differ from
modern processes, and the real lack of accretionary
complexes and ophiolites, and significant differences
in the geochemical and isotopic compositions of igne-
ous rocks, may reflect changes in the nature and
composition of the oceanic lithosphere through
time. Palaeomagnetic evidence is placing greater con-
trols on the movement and relative positions of the
constituent crustal components. Diverse cratons and
continental blocks aggregated during the Palaeopro-
terozoic and Early Mesoproterozoic to form Protero-
zoic Australia, which then played an integral part
in the formation and breakup of the Meso- to Neo-
proterozoic supercontinent, Rodinia (Figures 2–7;
summarized in Table 1). Proterozoic Australia is
host to a wide variety of minerals , including world-
class deposits of iron, uranium, gold, copper–gold,
lead–zinc–silver, and diamond orebodies (Figure 8).
Neoarchaean to Palaeoproterozoic
Assembling Proterozoic Australia:
(2770–1600 Ma)
West Australian Craton
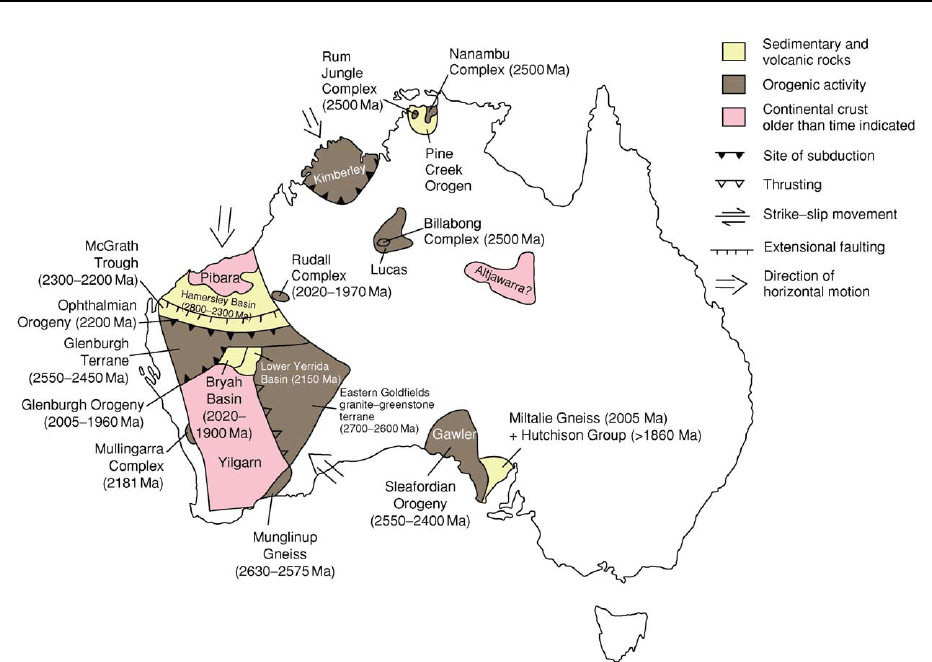
Within the West Australian Craton, large areas of
Archaean rocks are exposed in the geologically dis-
tinct Pilbara and Yilgarn cratons (Figures 1 and 2).
The Hamersley Basin was initiated on the southern
part of the Pilbara Craton at the start of the Neoarch-
aean. West- to south-westerly directed rifting within
cratonized granite–greenstone basement represents a
distinct change to a Proterozoic and Phanerozoic tec-
tonic style. The flood basalts (2770–2690 Ma) of the
Fortescue Group dominate the rift-related lower part
of the basin. These were buried beneath a breakup
unconformity overlain by a passive margin sequence
characterized by cherts and banded iron formations
208 AUSTRALIA/Proterozoic
(BIFs) of the uppermost Fortescue Group and the
Hamersley Group (2600–2440 Ma), which were
deposited on a shelf or platform open to an ocean
(see Sedimentary Rocks: Banded Iron Formations).
A collisional, intracontinental back-arc setting has
been proposed for the BIFs and mafic and felsic mag-
matic rocks of the upper part (2470–2440 Ma) of the
Hamersley Group. The overlying Turee Creek Group,
which includes probable glacial deposits, and the
lower Wyloo Group were deposited in the McGrath
Trough, a foreland basin developed in front of a
northward-verging fold-and-thrust belt during the
Ophthalmian Orogeny (2200 Ma) (Figure 2). The
Glenburgh Terrane in the southern part of the Gas-
coyne Complex within the Capricorn Orogen to the
south contains basement from 2550–2450 Ma and
may represent a remnant of the colliding continent
that drove this orogeny.
In the Yilgarn Craton, tectonic processes typical of
the Archaean developed the Eastern Goldfields gran-
ite–greenstone terrane 2700–2600 Ma, at the same
time as the upper Fortescue Group on the Pilbara
Craton. In the southern Capricorn Orogen, rifting
and continental breakup took place in the gneiss ter-
ranes and granite–greenstones along the northern
margin of the Yilgarn Craton. The 2150-My-old
rocks of the basal Yerrida Basin (Figure 2) formed
initially in a sag basin followed by an abrupt change
to a rift setting. The development of the Bryah and
Padbury basins 2020–1900 Ma at the north-western
margin of the Yilgarn Craton may reflect the de-
velopment of a back-arc basin, which was then over-
lain by a foreland basin during the Glenburgh
Orogeny 2005–1960 Ma (Figure 2). Geochemical
and isotopic data indicate that suprasubduction
zone magmatism formed the Dalgaringa Supersuite
at an Andean-type margin during this event; this may
represent the coming together of the combined
Pilbara Craton and Glenburgh Terrane with the
Yilgarn Craton.
The Capricorn Orogeny from 1830–1780 Ma
(Figure 3) has previously been regarded as marking
the collision between the Pilbara and Yilgarn
Cratons. Neodymium isotopes show that melting of
pre-existing, Early Palaeoproterozoic crust without
the introduction of mantle-derived material formed
Figure 1 North, South, and West Australian cratons, showing the main outcrops of Precambrian rocks in Australia.
AUSTRALIA/Proterozoic 209
voluminous granitic magmatism, which, together
with associated deformational and metamorphic
events, extended across the entire orogen. The Capri-
corn Orogeny may represent an intracratonic event
resulting from reactivation, possibly during amal-
gamation of the West Australian Craton with the
North Australian Craton. The 1840- to 1805-
My-old upper part of the Ashburton Basin and the
1805-My-old Blair Basin developed as a foreland
basin along the northern margin of the orogen at this
time. Uplift of the Gascoyne Complex and the Yilgarn
Craton, together with a presently unexposed Early
Palaeoproterozoic terrane, provided the sediment de-
posited in the upper Ashburton Basin and the Blair
Basin, the 1840-My-old upper part of the Yerrida
Basin and the 1840- to 1800-My-old Earaheedy Basin
(Figure 3).
In the Rudall Complex along the eastern margin
of the Pilbara Craton (Figure 3), the Talbot Terrane
contains quartzite, amphibolite, and serpentinite as
inclusions within complex orthogneisses that contain
components that crystallized, respectively, 2015,
1970, and 1800 Ma. A younger sequence (from
<1790 Ma) of clastic metasedimentary rocks was
possibly deposited in a foreland basin. In the adjacent
Connaughton Terrane, a sequence of deformed and
metamorphosed mafic volcanic rocks, and chemical
and clastic sedimentary rocks, may have been de-
posited in a rift prior to 1780 Ma. Deformation,
metamorphism, and further granite intrusion took
place in the Talbot and Connaughton terranes
during the Yapungku Orogeny (1790–1760 Ma).
West-verging thrusting and high-P metamorphism
may have accompanied collision of the West Austra-
lian Craton with the North Australian Craton, which
palaeomagnetic evidence indicates were unified by
1700 Ma.
The Mount Barren Group and equivalent sediment-
ary rocks were deposited on the south-eastern part of
the Yilgarn Craton 1700 Ma (Figure 4). Reactiva-
tion of the Capricorn Orogen took place between
1670 and 1620 Ma with the intrusion of granitic
rocks and the occurrence of medium- to high-grade
metamorphism, which was synchronous with local-
ized shear zones and hydrothermal alteration. In the
Albany–Fraser Orogen, the Biranup Complex con-
sists of granitic gneisses that include both Archaean
(2630–2575 Ma) (Figure 2) and Palaeoproterozoic
(1700–1600 Ma) protoliths (Figure 3). In the Pin-
jarra Orogen, a monzogranite in fault contact with
Figure 2 Assembly of the West Australian Craton, 2800–1900 Ma.
210 AUSTRALIA/Proterozoic
