Elder K. Human preimplantation embryo selection
Подождите немного. Документ загружается.


HUMAN PREIMPLANTATION EMBRYO SELECTION
to this a process of zona hardening in vitro has
been described, which might impede the blastocyst’s
attempt to hatch. Failed implantation may be due at
least in part to entrapment of the embryo as a conse-
quence of impaired zona-hatching.
23
Indeed, imple-
mentation of the assisted hatching (AH) technique
was demonstrated to be effective in patients where
chemical or physical changes in the zona pellucida
had occurred as a consequence of advanced reproduc-
tive age or elevated basal follicle stimulating hormone
(FSH) concentrations.
24,25
From these considerations
it might be speculated that the structure and function
of the zona is associated with specific changes within
the oocyte, and this may therefore also affect the
oocyte’s potential for successful embryo development.
The morphology of the zona pellucida can also
be influenced by extrinsic factors. The periovulatory
hormonal environment may have an impact on the
thickness of the zona pellucida. Studies by Bertrand
et al
26
demonstrated that the thickness of the zona
pellucida is also influenced by hormonal stimula-
tion in assisted reproductive technology (ART) cycles.
Therefore, ovulation induction might be an impor-
tant modulator of zona function in the process of
fertilization in vitro.
26
There is also increasing evi-
dence that suggests a further influence of ovulation
induction on the hatching procedure. This will be
discussed below.
MORPHOLOGICAL CHANGES IN THE ZONA
AS PREDICTORS OF ART OUTCOME
Criteria that can be assessed by routine microscopy
prior to fertilization or embryo transfer would be
useful in selecting embryos for transfer during ART
procedures; however, very few reliable, predictive and
non-invasive markers for oocyte quality have thus
far been identified. Nearly two decades ago it was
described that the zona pellucida of a proportion of
cleaving embryos had thinned areas in their zonae
pellucidae.
23
This could be expressed as a percentage
variation of zona pellucida thickness and was con-
sidered an important factor associated with implan-
tation of embryos.When the ‘best’ embryo had a zona
pellucida that varied more than 25%, 24 of 60 (40%)
resulted in pregnancy; pregnancies were not induced
(0/21) when the ‘best’ embryo had less than 10%
variation. The thinned areas appeared to be part of
a dynamic process that was already noticeable in
zygotes, but can increase during preimplantation
development, particularly in embryos with a good
prognosis.
27
Early studies showed that zona thinning
of the expanding blastocyst is not a unique process,
as it is preceded, at least in the human, by a gradual
thinning process correlated with increased viability
starting as early as the zygote and cleavage stages.
Several other studies have since reported on the
relationship between the thickness and morphology
of the human zona pellucida and embryo quality,
embryo development and pregnancy rates. Three
major approaches have been used to assess morpho-
logical criteria in the human zona, including direct
imaging with camera or video using an inverted
microscope with Hoffman modulation optics fol-
lowed by processing the film for manual measure-
ments.
23
This method has advantages in terms of
reliability but is slow and not helpful in real-time
prediction of viability. A second approach offered
by other investigators
27
uses a digitized imaging sys-
tem to store images, which can be measured with the
aid of a computer. Many different criteria have been
measured, but the two that have been most commonly
used are zona pellucida thickness (ZPT) and zona
pellucida thickness variation (ZPTV). In the majority
of studies, the zona was measured at three points as
suggested by Cohen and co-workers,
23
with the fol-
lowing calculations applied. Mean ZPT value and
ZPTV value computed as:
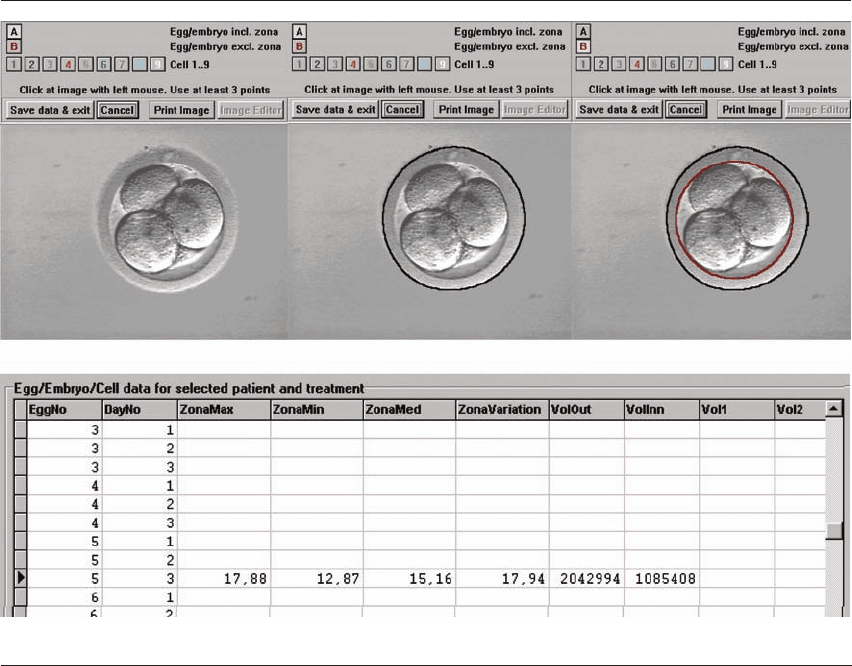
An example of this type of measurement is shown
in Figure 2.1, with multiple data sets used to increase
the accuracy of the measurements.
28–30
Zona pellucida thickness variation is associated
not only with better outcome in terms of pregnancy,
ZPT
mean
(ZPT
1
ZPT
2
ZPT
3
)/3
ZPTV (ZPT
max
ZPT
mean
)/
⫽⫹⫹
⫽⫺ ZZPT
mean
100%.⫻
HPE_Chapter02.qxp 7/13/2007 5:24 PM Page 16
THE ZONA PELLUCIDA AND MARKERS OF OOCYTE AND EMBRYO VIABILITY
but also with better overall embryo morphology.
28
One might therefore speculate as to which variable
might be the better predictor. We investigated this
question in a smaller series, and found no significant
impact of the ZPTV in embryos that already had
optimal morphology measured as a function of devel-
opment rate and fragmentation, among other para-
meters. The ZPTV did predict viability of embryos
when other morphology criteria were inferior.
In reports where pregnancy rate was the meas-
ured outcome,
27–32
ZPT was not associated with an
improved pregnancy rate for all groups of patients,
but was found to be generally correlated with preg-
nancy in all of the studies, which included biometric
analysis of the zona pellucida at the time of embryo
transfer. The data suggest that assessment of ZPTV
should be included in criteria used to select
embryos of optimal quality prior to transfer.
A third exciting and real-time technique for eval-
uating zona pellucida morphology can be achieved
with the use of a Polscope.
33
With this system,
the retardance magnitude and the thickness of the
inner, middle and outer layers of the ZP are meas-
ured before embryo transfer. The authors found
that the magnitude of light retardance by the inner
layer of the zona pellucida appears to represent a
unique non-invasive marker for the developmental
potential of the oocyte. We were able to confirm this
observation in a small series of 20 oocytes prepared
for ICSI (Lindenberg, unpublished data).
Figure 2.1 The outer and inner zona is delineated and the zona pellucida thickness has been calculated.
HPE_Chapter02.qxp 7/13/2007 5:24 PM Page 17

HUMAN PREIMPLANTATION EMBRYO SELECTION
CONCLUSION
The human zona pellucida is not static, but is a highly
dynamic structure that serves several known and
unknown functions during oogenesis and embry-
onic development. Specifically, the zona seems to
have a vital impact on co-ordinating the influence of
the somatic cell compartment in the ovary on oocyte
maturation. Fertilization and remodelling of the zona
after cortical granule release are also classical features
of the zona pellucida’s role. With the use of conven-
tional microscopy to measure ZPTV, it has been
found that remodelling of the zona after fertilization
seems to influence both the immediate in vitro, and
later in vivo development of the human embryo.
ZPTV can be seen as early as the zygote stage and
increases in a subset of embryos over time. It is our
firm conviction that assessment of ZPTV should be
an integral part of the selection criteria for embryo
transfer. We therefore conclude that ZPTV meas-
urement is a well documented but clearly under-
appreciated tool that can be used to select embryos for
transfer on days 2–3 after fertilization. Furthermore,
this criterion appears to be even more important
when only embryos of suboptimal morphology are
available for transfer.
The ZPTV could also be used to select those
embryos that might benefit from assisted hatching.
26
These observations are a valuable addition to criteria
that may be of benefit in a policy of selected single
embryo transfer.
REFERENCES
1. Baranska W, Konwinski M, Kujawa M. Fine structure of the zona pellu-
cida of unfertilized egg cell and embryos. J Exp Zool 1975; 192: 193–202.
2. Herrler A, Beier HM. Early embryonic coats: morphology, function,
practical applications. Cell Tissue Organs 2000; 166(2): 233–46.
3. Carino C, Prasad S, Skinner S et al. Localization of species conserved
zona pellucida antigens in mammalian ovaries. Reprod Biomed
Online 2002; 4: 116–26.
4. Spargo SC, Hope RM. Evolution and nomenclature of the zona pellu-
cida gene family. Biol Reprod 2003; 68: 358–62.
5. Litscher ES, Wassarman PM. Egg extracellular coat proteins: from fish
to mammals. Histol Histopathol 2007; 22: 337–47.
6. Dean J. Reassessing the molecular biology of sperm-egg recognition
with mouse genetics. Bioessays 2004; 26: 29–38.
7. Sinowatz F, Topfer-Petersen E, Kolle S, Palma G. Functional morphol-
ogy of the zona pellucida. Anat Histol Embryol 2001; 30: 257–63.
8. Gook D, Martic M, Borg J, Edgar DJ. Identification of zona pellucida
proteins during folliculogenesis. Hum Reprod 2004; 19 (Suppl 1): 140.
9. Familiari G, Nottola SA, Macchiarelli G, Micara G, Aragona C, Motta
PM. Human zona pellucida during in vitro fertilization; an ultrastruc-
tural study. Mol Reprod Dev 1992; 32: 51–61.
10. Oehninger S. Biomedical and functional characterization of the human
zona pellucida. Reprod Biomed Online 2003; 7: 641–8.
11. Wasserman PM. Zona pellucida glycoproteins. Ann Rev Biochem
1988; 57: 415–42.
12. Bogner K, Hinsch KD, Nayudu P et al. Localization and synthesis of
zona pellucida proteins in the mammoset mokey ovary. Mol Hum
Reprod 2004; 10: 481–8.
13. Albertini DF, Riedler V. Patterns of intercellular connectivity in the
mammalian cumulus–oocyte complex. Microsc Res Tech J 1994; 27:
125–33.
14. Eppig JJ. Intercommunication between mammalian oocyte and
companion somatic cells. BioEssays 1991; 13: 569–74.
15. Rankin T, Talbot P, Lee E, Dean J. Abnormal zona pellucida in mice
lacking ZP1 result in early embryonic loss. Development 1999; 126:
3847–55.
16. Rankin T, Soyal S, Dean J. The mouse zona pellucida. Mol Cell
Endocrinol 2001; 163: 21–5.
17. Hoodbhoy T, Dean J. Insights into the molecular basis of sperm-egg
recognition in mammals. Reproduction 2004; 127: 417–22.
18. Soupart P, Strong PA. Ultrastructural observations on polyspermic
penetration of zona pellucida-free human oocytes inseminated in vitro.
Fertil Steril 1975; 26: 523–37.
19. Gardner AJ, Evans JP. Mammalian membrane block to polyspermy:
new insights into how mammalian eggs prevent fertilisation by multiple
sperm. Reprod Fertil Dev 2006; 18: 53–61.
20. Nichols J, Gardner RL. Effect of damage to the zona pellucida on devel-
opment of preimplantation embryos in the mouse. Hum Reprod 1989;
4: 180–7.
21. Willadsen SM. A method for culture of micromanipulated sheep
embryos and its use to produce monozygotic twins. Nature 1979;
277(5694): 298–300.
22. Cohen J, Malter H, Elsner C et al. Immuno-suppression supports
implantation of zona pellucida dissected human embryos. Fertil Steril
1990; 53: 662–5.
23. Cohen J, Inge KL, Suzman M,Wiker SR,Wright G. Videocinematography
of fresh and cryopreserved embryos: a retrospective analysis of embry-
onic morphology and implantation. Fertil Steril 1989; 51: 820–7.
24. Cohen J, Alikani M, Reing AM et al. Selective assisted hatching of
human embryos. Ann Acad Med Singapore 1992; 21: 565–70.
25. Cohen J. Assisted hatching of human embryos. J Assist Reprod Genet
1993; 17: 179–90.
26. Bertrand E, Van den Bergh M, Englert Y. Clinical parameters influ-
encing human zona pellucida thickness. Fertil Steril 1996; 66(3):
408–11.
27. Wright G, Wiker S, Elsner C et al. Observations on the morphology of
pronuclei and nucleoli in human zygotes and implications for cryo-
preservation. Hum Reprod 1990; 5: 109–15.
28. Gabrielsen A, Bhatnager PR, Petersen K, Lindenberg S. Influence of
zona pellucida thickness of human embryos on clinical pregnancy
outcome following in vitro fertilization treatment. J Assist Reprod
Genet 2000; 17: 323–8.
29. Host E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in
human cumulus cells in relation to zona pellucida thickness variation,
maturation stage, and cleavage of the corresponding oocyte after
intracytoplasmic sperm injection. Fertil Steril 2002 Mar; 77(3):
511–15.
HPE_Chapter02.qxp 7/13/2007 5:24 PM Page 18
THE ZONA PELLUCIDA AND MARKERS OF OOCYTE AND EMBRYO VIABILITY
30. Gabrielsen A, Lindenberg S, Petersen K. The impact of the zona pellu-
cida thickness variation of human embryos on pregnancy outcome in
relation to suboptimal embryo development. A prospective random-
ized controlled study. Hum Reprod 2001; 16(10): 2166–70.
31. Garside WT, Loret D, Mola JR et al. Sequential analysis of zona thick-
ness during in vitro culture of human zygotes: correlation with
embryo quality, age, and implantation. Mol Reprod Dev 1997; 47:
99–104.
32. Palmstierna M, Murkes D, Csemiczky G, Andersson O, Wramsby H.
Zona pellucida thickness variation and occurrence of visible mononu-
cleated blastomeres in preembryos are associated with a high preg-
nancy rate in IVF treatment.J Assist Reprod Genet 1998; 15: 70–5.
33. Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High
magnitude of light retardation by the zona pellucida is associated with
conception cycles. Hum Reprod 2005; 20: 1596–606.
HPE_Chapter02.qxp 7/13/2007 5:24 PM Page 19
HPE_Chapter02.qxp 7/13/2007 5:24 PM Page 20

INTRODUCTION
The process of fertilization is a complex sequence of
events the signs of which are perceptible as early as
several seconds after sperm–oocyte fusion.
1
However,
the demonstration of very early signs of fertilization
requires recourse to special techniques, most of
which are destructive for the fertilized oocyte. Hence,
since the first successful clinical application of human
in vitro fertilization (IVF),
2
two signs of fertilization
that are detectable by non-invasive inspection of
inseminated oocytes have been used: the extrusion
of the second polar body and the development of
pronuclei.
The extrusion of the second polar body is a much
earlier sign of fertilization than is the development
of pronuclei. However, the high incidence of first
polar body division or fragmentation compromises
the accuracy of second polar body detection in many
cases. Consequently, the development of pronuclei
after IVF has become the gold standard of fertiliza-
tion assessment. The possibility of distinguishing
between normal fertilization (2 pronuclei), partheno-
genetic oocyte activation (1 pronucleus), and poly-
sper
mic oocyte penetration (⬎2 pronuclei) is an
addi
tional advantage of pronucleus-based evalua-
tion systems, because parthenogenetically activated
and polyspermically penetrated oocytes both show
the same pattern of second polar body extrusion as
does a normally fertilized oocyte. There are a few
exceptions to these common observations: firstly,
eggs can be activated by a sperm cell without
decondensation. This is apparently quite common,
and may be overlooked as abnormal fertilization,
activation or failed fertilization.
3
Secondly, a single
pronucleus can be the result of the close association
of male and female genomes in human zygotes.
4
Thirdly, some eggs may activate without extruding
the second polar body, rendering the zygote digynic.
The idea that analysis of pronuclei can provide
something more than simply evidence of fertiliza-
tion arose from studies performed in the late 1980s
which showed that pronuclear development reflects
the activity of developmentally important oocyte
cytoplasmic factors,
5
and is also related to an early
period of RNA synthesis in the zygote.
6,7
At the same
time, the relationship between characteristics of
pronuclear development, nucleolar distribution/
movement and embryo viability/implantation poten-
tial, was established by Wright et al;
8
this was con-
firmed and expanded upon by our team and others
some years later.
9–11
Here we outline the biological basis that under-
lies pronuclear development, as well as the patho-
logical conditions that lead to abnormal pronuclear
development. This information should help to under-
stand the relationship between pronuclear mor-
phology and IVF outcomes. It can also serve as a
background for further studies aimed at refining the
existing pronuclear scoring systems and defining
new clinical applications and relationships.
THE PHYSIOLOGY OF THE HUMAN ZYGOTE
The beginning of the zygote’s existence is marked
by sperm–oocyte fusion. At this point in time, the
fertilizing spermatozoon triggers a cascade of cell sig-
nalling events in the oocyte, collectively termed oocyte
activation. A series of repetitive increases in free intra-
cellular calcium concentration (calcium oscillations)
has become the most well studied aspect of human
oocyte activation.
12
Relatively less is known about
downstream elements of the oocyte-activating sig-
nal transduction cascade, which can be expected to
affect regulatory elements that control the oocyte’s
cell cycle checkpoints and the function of cytoskele-
tal elements; these promote the exit of the oocyte
3. Morphology and kinetics of human pronuclei
Jan Tesarik, Raquel Mendoza-Tesarik, Ermanno Greco, and Carmen Mendoza
HPE_Chapter03.qxp 7/13/2007 5:22 PM Page 21

HUMAN PREIMPLANTATION EMBRYO SELECTION
from metaphase II (MII) arrest and its entry into
the regular mitotic cell cycle.
13,14
Studies performed in different mammalian species
have shown that early embryonic development
occurs in the absence of active gene expression
during a period which is species dependent. In the
human, a major activation of nuclear RNA synthesis
occurs at the 4-cell stage,
15,16
and the first biochemi-
cal
17
and morphological
18
signs of embryonic gene
expression are detectable between the 4- and the 8-
cell stage. The messages required to guide develop-
mental processes that take place prior to embryonic
gene activation are derived from a pool of mRNAs
that have been synthesized during oocyte growth and
are stored in the oocyte cytoplasm as polyadenylated
mRNA. It is tempting to speculate that the drawn-out
and spatially differentiated series of periodic calcium-
driven signalling events observed throughout human
zygote development
19
may determine the timing of
deadenylation and expression of specific maternal
mRNAs stored in different regions of the oocyte and
zygote. In fact, spontaneous
12
and drug-induced
20
disturbances of calcium oscillations in the human
zygote were shown to be associated with abnormal-
ities of pronuclear morphology and developmental
arrest.
From the developmental point of view, the main
function of the zygote is to ensure a timely start for
the embryonic cell cycle after the long period of arrest
that both the sperm- and the oocyte-derived genomes
have experienced in their respective gametes. This
function includes the correct timing of DNA syn-
thesis during S-phase and the subsequent fusion
of paternal and maternal genomes to merge into a
unique genome for the future embryo.
THE DEVELOPMENT AND FUNCTION OF
PRONUCLEI
EXPERIMENTAL APPROACHES
Most of the basic knowledge about the structure
and function of human pronuclei is derived from
invasive studies in which either normal zygotes were
sacrificed, or polypronuclear eggs were formed for
research purposes by inseminating zona-free human
oocytes. These studies used electron microscopy and
autoradiography to analyze changes in pronuclear
ultrastructure and nucleic acid synthesis.
5–7,21
More recently, a number of non-invasive studies
were carried out, based on observations of pro-
nuclei in living human zygotes. These studies, nicely
reviewed by Scott,
22
could not use the same structural
detail and experimental rigor as the earlier invasive
studies. However, they have the merit of having
established the existence of relationships between
the appearance of pronuclei and further embryonic
development, including data about implantation
and post-implantation events. Both types of studies
are thus complementary.
THE ESTABLISHMENT OF PRONUCLEI
Soon after sperm–oocyte fusion, the nuclear enve-
lope of the fertilizing spermatozoon disintegrates,
and both sperm- and oocyte-derived chromatin is
thus directly accessible to oocyte cytoplasmic fac-
tors.
1
However, sperm chromatin differs from the
oocyte telophase chromosomes by its higher degree
of condensation, due to its association with sperm-
specific nuclear protamines. Sperm nuclear prota-
mines are removed and replaced with oocyte-derived
histones before the male pronucleus is formed.
1
This step is obviously dependent on the availability
of histones in the oocyte. Relative insufficiency of
oocyte histones may thus slow down the transforma-
tion of the sperm nucleus into the male pronucleus,
and consequently cause asynchrony of pronuclear
development.
The establishment of pronuclei is not completed
until a new nuclear envelope is formed around the
sperm- and oocyte-derived chromatin. This process
has been studied in human polyspermically pene-
trated zona-free oocytes,
21
and was shown to
involve alignment and stepwise fusion of vesicles
and tubules of endoplasmic reticulum around the
chromatin (Figure 3.1). Once nuclear envelope for-
mation is completed, the pronuclei can be easily
visualized in living zygotes with the use of phase con-
trast, Nomarski differential interference contrast or
Hoffman modulation contrast optics.
HPE_Chapter03.qxp 7/13/2007 5:22 PM Page 22
MORPHOLOGY AND KINETICS OF HUMAN PRONUCLEI
DEVELOPMENTAL CHANGES IN
PRONUCLEAR STRUCTURES
Newly formed pronuclei contain finely dispersed
chromatin with disseminated small electron-dense
bodies.
21
The latter progressively aggregate and fuse
with each other, and are closely associated with large
chromatin clusters (Figure 3.2). This process leads
to the formation of still larger aggregates, consisting
of material derived from the electron dense intra-
nuclear bodies and chromatin, merged in compact
structures that develop into functionally active nucle-
oli three cell cycles later.
16,23
Thus, these structures
were given the name of nucleolar precursor bodies
(NPBs).
23
In the experimental system based on polysper-
mically penetrated human zona-free oocytes, the
process of NPB formation began as early as 4 hours
after in vitro insemination, and the first fully devel-
oped NPBs appeared 12 hours after in vitro insemi-
nation.
21
This transformation coincides with the
development of typical nuclear pores all around the
pronuclei, and with the appearance of characteristic
vesicles between the two membranes of the
nuclear envelope.
21
The function of these vesicles,
also observed in blastomeres of cleaving human
embryos,
24
is unknown.
DEVELOPMENTAL CHANGES IN
PRONUCLEAR FUNCTION
One of the main functions of pronuclei is to ensure
adequate conditions for the first phase of DNA
synthesis after fertilization so that the sperm- and
oocyte-derived chromatin is eventually mixed. An
experiment in which
3
H-thymidine was incorpo-
rated into the pronuclei of polyspermically pene-
trated human zona-free oocytes, evaluation and
analysis by autoradiography, showed the first signs of
DNA synthesis no earlier than 12 hours after in vitro
insemination, and only in those pronuclei that had
completed ultrastructural differentiation of NPBs.
6
Interestingly, human paternal pronuclei can only com-
plete their NPB differentiation when they have previ-
ously carried out a limited RNA synthesis, detected in
polyspermically penetrated human zona-free oocytes
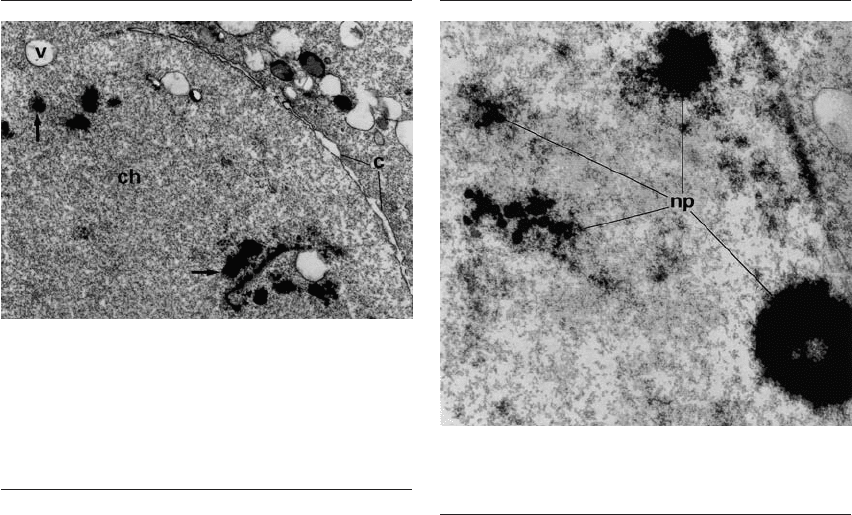
Figure 3.1 Electron micrograph of a fully decondensed sperm
nucleus in the course of nuclear envelope assembly, occurring
in an early phase of male pronucleus development, 4 hours
after in vitro insemination. Chromatin (ch) is surrounded
by a discontinuous series of vesicles (v) and cisternae (c) of
endoplasmic reticulum. Arrows indicate intranuclear dense
bodies. ⫻20 000. Reprinted from Tesarik et al.
18
Figure 3.2 Electron micrograph showing a part of a developing
male pronucleus with nucleolar precursors (np) at different
phases of their assembly. ⫻44 000. Reprinted from Tesarik et al.
18
HPE_Chapter03.qxp 7/13/2007 5:22 PM Page 23
HUMAN PREIMPLANTATION EMBRYO SELECTION
as early as 4 hours after in vitro insemination.
5
Consequently, delayed NPB development is likely to
signal a failure or delay of the early pronuclear RNA
synthesis, a condition which will in turn cause a
delay in the onset of DNA synthesis.
The nature of the transcripts resulting from pronu-
clear RNA synthesis is largely unknown. However, it
has been shown that paternal Y-linked transcripts are
expressed in paternal pronuclei of human zygotes.
25
Early RNA synthetic activity has also been observed
in mouse pronuclei, similar to that observed in
humans.
26
Interestingly, chromatin-mediated repres-
sion of promoter activity that is almost total is
imposed on maternal pronuclei, but not on paternal
pronuclei.
27,28
Consequently, the ongoing rate of tran-
scription by endogenous genes in the mouse zygote is
four to five times greater in the paternal pronucleus
than in the maternal pronucleus.
26
The existence of
similar differences between transcriptional activity
in male and female pronuclei in the human zygote
still remains to be determined, although the male
pronucleus in general contains more nucleoli than
the female pronucleus.
29
PRONUCLEAR MOVEMENT
The male and female pronuclei are initially separate
spatially, and they then move in the zygote cytoplasm
to approach each other. This process culminates in a
close apposition of both pronuclei.
1
It appears that
apposition of the two pronuclei is achieved near the
spindle if a spermatozoon has penetrated close by, and
in the center of the zygote if the spermatozoon has
entered the oocyte through a region opposite the
spindle.
30,31
It was hypothesized that the movement of the
pronuclei is integrated within an overall cytoplasmic
movement (rotation) in the oocyte and zygote.
32,33
Direct evidence for zygote cytoplasmic rotation comes
from time-lapse video recordings of human sperm-
injected oocytes showing that the sperm centro-
some can organize contraction waves, resulting in
clockwise rotations of cortical granulated cyto-
plasm. This was noted in 95% of mature human MII
oocytes, beginning 2–3 hours before second polar
body extrusion following ICSI, and ending as the
second polar body was extruded.
34
Indirect evidence
for oocyte cytoplasmic rotation near the time of fer-
tilization comes from the observation that second
polar body extrusion often occurs distant from the
first polar body after fertilization by ICSI.
33
The correct function of the sperm-derived cen-
trosome, acting as a microtubule-organizing center
(MTOC), is a necessary prerequisite for pronuclear
apposition.
33
If a sperm-derived centrosome (aster)
has an inherent defect that prevents it from nucleat-
ing the formation of microtubules, the apposition
of pronuclei and syngamy in the human zygote
fail.
34
This mechanism also functions between the
pronuclei of the same gamete (sperm) origin in
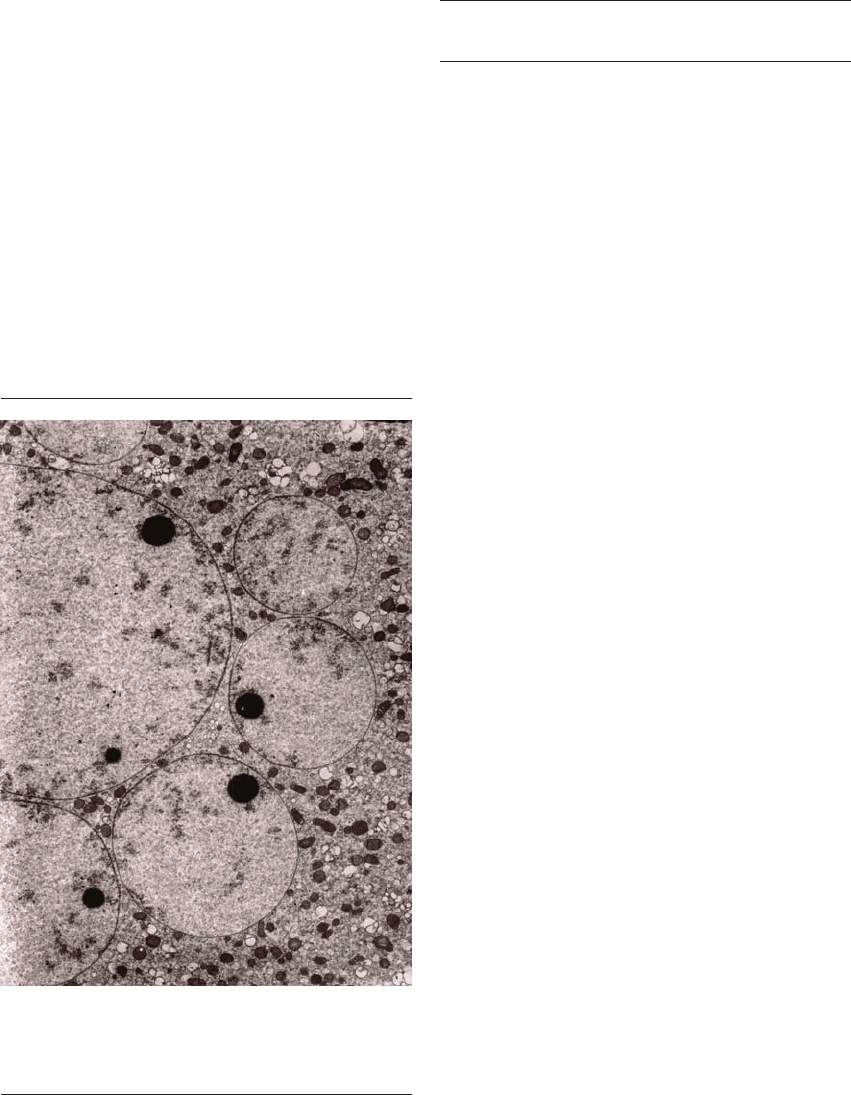
polyspermically penetrated human zona-free oocytes
(Figure 3.3). A similar mechanism may be in place
in parthenogenetic human embryos, where mosaicism
is commonly observed.
35
POLARIZATION OF INTRAPRONUCLEAR
STRUCTURES AND INTERPRONUCLEAR
SYNCHRONY
Concomitantly with the apposition of the male and
female pronuclei, chromatin and NPBs in both
pronuclei polarize, and rotate to face the adjacent
pronucleus. Moreover, in both pronuclei, chromatin
that faces the other pronucleus becomes highly con-
densed during apposition.
31
This mechanism seems
to work only when pronuclei originate from different
gametes (male and female), because only an insignif-
icant, if any, intrapronuclear polarization can be
observed in multiple male pronuclei developing in
polyspermically penetrated human oocytes, even
though the pronuclei lie in close apposition to each
other (Figure 3.3).
Circumstantial evidence suggests that the pater-
nal pronucleus imposes, or collaborates in, the
formation of polar axes in mammalian eggs.
33
Chromatin located near the sperm tail becomes polar-
ized facing the oocyte interior as the differentiating
paternal pronucleus rotates, and this occurs even
while the sperm head lies distant from the maternal
pronucleus.
31
The failure of pronuclei to come into apposition
is usually associated with a failure of cleavage and
HPE_Chapter03.qxp 7/13/2007 5:22 PM Page 24
MORPHOLOGY AND KINETICS OF HUMAN PRONUCLEI
arrest at the zygote stage. Zygotes with pronuclei of
uneven size nearly all develop into mosaic embryos.
36
On the other hand, failure of NPB polarization can
occur even if pronuclei are in apposition, and this
usually involves only one of the two pronuclei. Such
interpronuclear asynchrony was shown to be associ-
ated with abnormalities of further embryonic devel-
opment.
10
Based on the finding that intrapronuclear
RNA synthesis is required for NPB development
and growth,
7
together with the observation that the
failure of NPBs to polarize is typically associated with
a reduced NPB size,
10
interpronuclear asynchrony
seems to be caused by abnormal gene expression in
the pronucleus that is lagging behind.
ANALYSIS OF PRONUCLEI IN LIVING
HUMAN ZYGOTES
VISUALIZATION OF PRONUCLEI
Using Hoffman modulation contrast optics, the most
widely used optical system in the current IVF and
ICSI practice, pronuclei can be easily observed in liv-
ing human zygotes only after completion of pro-
nuclear envelope formation (Figure 3.4). According
to electron microscopic studies,
21
this occurs about
10–12 hours after fertilization. When pronuclear
differentiation and DNA synthesis are completed,
the pronuclear envelopes disintegrate, the chromatin
of both pronuclei forms a single spindle, and the
zygote enters the first cleavage division. This can
occur as early as 18–20 hours after fertilization. The
optimal time window for pronuclear observation is
thus between 12 and 16 hours after in vitro insemi-
nation or ICSI.
Both immature and fully developed NPBs can be
visualized by Hoffman modulation contrast optics.
Since the fully developed NPBs are formed by fusion
between several NPBs that are immature with the
participation of chromatin,
6,21
with advancing pro-
nuclear development, fewer NPBs can be visualized,
and the size of each NPB is greater. This process
coincides with NPB migration towards the area of
interpronuclear contact. Hence, the number, size, and
position of NPBs are key elements of any pronuclear
scoring system used in living human zygotes.Changes
in the intrapronuclear distribution of chromatin,
which is also a part of pronuclear developmental
changes (see above), are inaccessible to observation
in the living state.
POSSIBILITIES AND LIMITS OF NON-INVASIVE
PRONUCLEAR EVALUATION
The development of pronuclear scoring systems was
initially largely motivated by the need to select the
best embryos for fresh transfer and to determine
which embryos were to be cryopreserved, as early as
the zygote stage.
8
The first clinical study to describe a
coherent pronuclear scoring system
9
was performed
Figure 3.3 Electron micrograph showing multiple
sperm-derived pronuclei in a polyspermically penetrated
human oocyte. The pronuclei are in close apposition with each
other. ⫻12 000.
HPE_Chapter03.qxp 7/13/2007 5:22 PM Page 25
