Dunn Colin E. Biogeochemistry in Mineral Exploration
Подождите немного. Документ загружается.

Recently, the concept of a ‘functional plant nutrient’ has been introduced (Sub-
barao et al., 2003). In their paper they note that the concept of ‘essentiality’ was
defined by Arnon and Stout (1939) as
those elements necessary to complete the life cycle of a plant. A few other elements such
as Na have an ubiquitous presence in soils and waters and are widely taken up and
utilized by plants, but are not considered as plant nutrients because they do not meet the
strict definition of ‘essentiality’. Sodium has a very specific function in the concentration
of carbon dioxide in a limited number of C4 plants and thus is essential to these plants,
but this in itself is insufficient to generalize that Na is essential for higher plants. The
unique set of roles that Na can play in plant metabolism suggests that the basic concept
of what comprises a plant nutrient should be re-examined. We contend that the class of
plant mineral nutrients should be comprised not only of those elements necessary for
completing the life cycle, but also those elements which promote maximal biomass yield
and/or which reduce the requirement (critical level) of an essential element. We suggest
that nutrients functioning in this latter manner should be termed ‘functional nutrients.’
Thus plant mineral nutrients would be comprised of two major groups, ‘essential nu-
trients’ and ‘functional nutrients.’ y other elements such as Si and Se may also confirm
to the proposed category of ‘functional nutrients.’
All 90 naturally occurring elements are found in most plant tissues, but with
many at extremely low concentrations. In addition to C, H and O, only 16 elements
are truly essential for growth of all plants (Table 2-I, first column). An additional
16 elements shown in the remaining columns of Table 2-I are essential to some
plants and/or beneficial to the metabolism of others. The remaining 55 elements are
mostly taken up passively in small quantities (traces or ultra-traces) as plants absorb
the nutrient elements that they need for growth and reproduction. At this time
they are not known to play an essential role in plants, but at ultra-trace levels
more elements may prove to be essential. This large group of trace elements can be
tolerated by most plants, with some species tolerating higher concentrations than
others.
In extreme cases ‘hyperaccumulation’ of a non-essential elem ent, such as Tl, can
take place although elements that can hyperaccumulate are mostly those that are
essential to many plants (e.g., Ni, Cu, Zn, Co, Mn). Hyperaccumulation of metals
(e.g., 1% Ni in Alyssum bertolonii) was first recognized by Minguzzi and Vergnano in
1948, but it was not until 1977 that the term was introduced by Robert Brooks and
his co-workers (Brooks et al., 1977). Brooks (1998) summarized the numbers of
plants that are known to be hyperaccumulators of eight elements (Table 2-II ). Since
that publication appeared there have been additional plants recognized (e.g., a sec-
ond genus that can hyperaccumulate Tl).
Some elements that are considered non-essential can, in some situations, substi-
tute for an essential element and perform a surrogate ‘essential’ role. Molybdenum
22
Plant Function, Chemistry and Mineralogy

and W are examples. A primary function of Mo is in nitrate reductase, an important
enzyme that converts nitrate to ammonium. Tungsten can substitute for Mo in the
synthesis of nitrate reductase with reduced effectiveness, but still maintaining some
function. Similarly, Br can substitute for Cl in some plants.
It is advantageous to have some knowledge of these plant requirements when it
comes to interpreting biogeochemical data with respect to mineral exploration. For
example, concentrations of Zn in plants normally appear quite high when compared
to other elements or to Zn concentrations in soils. Part of this enrichment is because
Zn is an essential element and, therefore, will be present at moderate concentrations
even when there is no Zn mineralization in the substrate. As a result, the geochem-
ically similar element Cd is likely to be a better ‘pathfinder’ element for Zn min-
eralization than Zn itself. However, if there is substantial sphalerite mineralization
near surface it is highly likely that it will be reflected as enhanced concentrations of
Zn in tissues of trees that are rooted within the geochemical envelope of Zn enrich-
ment emanating from the mineralization.
TABLE 2-I
Essential elements
The biogeochemistry of life – essential elements
Essential to all
animals and plants
Essential to several
classes of animals
and plants
Essential to a wide
variety of species in
one class
Essential to only a
few species
Recent work
indicates
essentiality, but of
unknown function
Hydrogen(H) Silicon (Si) Boron (B) Lithium (Li) Rubidium (Rb)
Carbon (C) Vanadium (V) Fluorine (F) Aluminium(Al) Tin (Sn)
Nitrogen (N) Cobalt (Co) Chromium (Cr) Nickel (Ni)
Oxygen (O) Molybdenum(Mo) Bromine (Br) Strontium (Sr)
Sodium (Na) Iodine (I) Barium (Ba)
Magnesium(Mg)
Phosphorus(P)
Sulfur(S)
Chlorine (Cl)
Potassium (K)
Calcium (Ca)
Manganese (Mn)
Iron (Fe)
Copper (Cu)
Zinc (Zn)
Selenium (Se)
Note: Elements in bold type are generally considered to be trace elements in plants.
Source: The United States Geological Survey website http://geology.er.usgs.gov/eastern/
environment/environ.html#1 (8th October 2006).
23Biogeochemistry in Mineral Exploration

ELEMENT UPTAKE AND FUNCTION
The uptake of elements by plants is dependent upon ionic size and charge, and the
microscopic structure of root surfaces.
some elements can go in and out at will,
some elements are physically excluded,
some elements are actively pulled through the root walls (i.e., osmosis), and
some elements are actively excluded.
An element can ‘plug’ a root wall aperture, and subsequently exclude other el-
ements from passing through the wall or it may change the charge of the wall. This is
the ‘barrier’ mechanism described in the Russian literature (e.g., Kovalevsky, 1987).
Furthermore, the size of the apertures depends upon the temperatur e and availability
of water, and can change during the course of the year, giving rise to seasonal
variability in plant composition.
The role of various elements in plant metabolism is summarized in Table 2-III.As
noted above, many elements play a role in plant metabolism yet others may have no
function. Gold, for example, is not known to be of any use to plants, yet in plants
growing over mineralization it can be absorbed and concentrated a thousand-fold
over its usual background levels.
Additional information on the forms and principal functions of trace elements
essential for plants is provided by, amongst others, Kabata-Pendias and Pendias
(1992), Kabata-Pendias (2001), and by Chapin and Eviner (2005).
Photosynthesis is one of the most metal-sensitive processes of plant metabolism,
hence when a plant absorbs unusually large amounts of some metals (e.g., Cu)
photosynthesis can be affected, such that a plant may show stress, yellowing of leaf
TABLE 2-II
Plant hyperaccumulators of eight elements and the families in which they are most often found
(after Brooks, 1998)
Element No. of species Family
Cadmium 1 Brassicaceae
Cobalt 26 Lamiaceae, Scrophulariaceae
Copper 24 Cyperaceae, Lamiaceae, Poaceae, Scrophulariaceae
Manganese 11 Apocynaceae, Cunoniaceae, Protaceae
Nickel 290 Brassicaceae, Cunoniaceae, Euphorbiaceae,
Flacourtiaceae, Violaceae
Selenium 19 Fabaceae
Thallium 1 Brassicaceae
Zinc 16 Brassicaceae, Violaceae
24 Plant Function, Chemistry and Mineralogy

tips (chlorosis), or subtle changes in spectral reflectance that hyperspectral imagery
may be able to differentiate.
Uptake of an excess of trace elements can result in the induction of deficiencies of
other essential elements. These interactions trigger secondary respon ses, involving
enzymes, which either protect membranes against further damage or may partially
bypass metal-sensitive reactions. As a result the physiological state of the cell may be
altered and the plant may become more metal-tolerant.
Within a plant, elements may be variously mobile. The mobility determines where
deficiency or toxic symptoms will be expressed and how elements will accumulate
with increasing age. Table 2-IV lists the uptake mechanisms and relative mobility in
TABLE 2-III
The roles of essential and beneficial elements in vascular plants (‘S’ ¼ structural;
‘E’ ¼ enzymatic). Modified after compilation by Scagel (in Dunn et al., 1993a)
Element Class Use
Al E Colloidal properties of cells
B S, E Carbohydrate metabolism, flavinoid synthesis
Ca S Cell walls, N-metabolism
Cl E Enzymes, osmotic functions, stomatal movement
Co E Coenzyme
Cu S, E Enzymes, coenzymes, phosphorylation
F E Respiration
Fe S, E Chloroplasts, respiratory enzymes, phosphorylation
I E Protein function
K E Enzyme activity, osmotic regulation, stomatal movement
Li E Salt metabolism
Mg S, E Central element in chlorophyll molecule, enzymes,
phosphorylation
Mn E Chlorophyll synthesis, enzyme activator
Mo E Nitrogen metabolism, enzymes
N S Amino acids, proteins, cell membranes, enzymes, chlorophyll
synthesis, protein synthesis
Ni E Translocation of N
P S, E Nucleoproteins, phospholipids, high-energy phosphate bonds,
energy transfer, phosphorylation
Rb E Partial analogue for K
S S Amino acids, proteins, coenzymes, phosphorylation
Si S Cell walls
Sr S Partial analogue for Ca
Ti E Nitrogen fixation
V E Nitrogen metabolism
Zn E Enzymes, hormone synthesis
25Biogeochemistry in Mineral Exploration

the phloem (downward movement) of essential and trace elements in plants. These
results (Bukova c and Wittw er, 1957) are complicated by the fact that the mobility of
elements classified as ‘intermediate’ varies with species, the stage of growth and the
concentration in the plant (Loneragan, 1975). Consequently, this classification
should be viewed as only a general indication of the relative ability of element
movements. Elements that are mobile will express deficiency symptoms in older
plant parts and toxic symptoms in the youngest parts. Low-mobility elements will
TABLE 2-IV
Classification of some chemical elements found in plants according to their uptake, transport
and mobility in the phloem (after Bukovac and Wittwer, 1957)
Element Quantities Uptake transport Phloem mobility
Ag Trace Passive
Al Micro Active
B Micro Active Low mobility
Ba Trace Passive Low mobility
Ca Macro Active Low mobility
Cl Micro Passive Mobile
Co Micro Passive
Cr Trace Passive
Cu Micro Active Intermediate
Fe Micro Active Intermediate
I Trace Passive
K Macro Active Mobile
Li Trace Passive Low mobility
Mg Macro Active Mobile
Mn Micro Active Intermediate
Mo Micro Active Intermediate
N Macro Active Mobile
Na Trace Passive Mobile
Ni Micro Active
P Macro Active Mobile
Rb Trace Passive Mobile
REE Trace Passive
S Macro Active Mobile
Se Trace Passive
Si Micro Active
Sr Trace Passive Low mobility
Ti Trace Passive
U Trace Passive
V Micro Passive
Zn Micro Active Intermediate
26 Plant Function, Chemistry and Mineralogy
express deficiency symptoms in the youngest plant parts and toxic symptoms in the
oldest plant parts.
ROOT FORM AND CONTROLS ON ELEMENT UPTAKE
Roots are complex structures that are highly variable in their morphology and in
their lateral extent and depth of penetration. Revi ews of roots and chemical inter-
actions in their rhizosphere are given by Richards (1986), Jones (1998) and Arienzo
(2005). The combined length of roots, rootlets and their mycorrhizal fungi (con-
sidered to be essential for tree survival) can be immense. Roots have been reported at
a depth of 53 m (Phillips, 1963) and there are anecdotal reports of roots penetrating
to greater depths from their presence in the roofs of mine galleries. It has been
estimated that a single rye plant, just over 1 m tall can have a combined length of
these roots, rootlets and mycorrhizae totalling over 600 km, and through millions of
microscopic apertures a root system constantly extracts from the soil and ground-
water those nutrients that the plant requires along with other elements that the plant
is able to tolerate. The additional roles that bacteria play are discussed in Chapter 12.
Thus, the root system of a single plant can integrate the geochemical signature of
many cubic metres of soil, groundwater and sometimes the precipitated oxides that
coat the surfaces of joints and faults in bedrock, and mineral surfaces. Locally, the
extraordinary physical power of roots can be observed in road-cuts or cliff faces
revealing the way the root system of a tree strives to firmly anchor itself, sometimes
by wedging apart and propagating rock fractures while seeking out sufficient water
and nourishment for its very existence. Furthermore, on a hot sunny day 100–150 l of
water (with its dissolved elements) may be transported through the roots and stem to
the leaves of a large tree (Kramer and Kozlowski, 1979). Given these sobering sta-
tistics it is evident that a tree can be considered as an efficient integrator of the
chemistry of a large volume of soil, groundwater and sometimes bedrock, and
thereby reflects a comprehensive geo chemical signature of the substrate.
Practically all of the movement of elements from roots to foliage occurs in the
xylem sap, carried by mass flow in the transpiration stream with subsequent flow
down the phloem to complete the fluid cycle. Not all elements move in the xylem at
the same rate, with some slowed by adsorption on cell walls during xylem flow. Thus,
some elements get to the growing parts of the plant more quickly than others, thereby
accounting for seasonal fluctuations in concentrations of some elem ents. In the au-
tumn, soon before the leaves fall, some elements return to twigs where they are stored
over the winter months before they are required for the next year’s growth cycle.
There are a number of environmental factors that can modify the capabilities of
roots to absorb e lements. The pH immediately around the roots is generally a mi-
croenvironment that can be considerably more acidic than the surrounding rooting
environment of the soil, and values as low as pH 1 have been reported. The non-
woody roots and root tips are surrounded by a mucilage sh eath that is acidic and
27
Biogeochemistry in Mineral Exploration
may modify element fluxes. Many trace elements are relatively stable at slightly acidic
pH due to the stability of the ligand chelates with which they are associated
(Pb>Cu>Ni>Co>Zn>Cd>Mn). Seasonal variation in pH influences the avail-
ability of several elements (e.g., Cu, Zn, Pb, Cd, Cr, Ni) thereby further accounting
for some of the seasonal variations in metal uptake and emphasizing the importance
of conducting a survey using live tissues in as short a time frame as possible (few
weeks). The relative mobility of elements is shown in Table 2-V.
The salt content of a groundwater solution determines what elements are taken up
or not in competing for active sites on the roots. In particular Na is readily taken up
by many plants, but blocks the subsequent uptake of K. Notes should be kept of sites
from near alkaline and seaside locations because the uptake of some elements may be
modified by such conditions. Along major roadways in northern climates, the salt
content of plants may be elevated because of the application of salt to road surfaces
in winter months to dissolve ice.
Redox potential determines the availability of many elements to plants. For in-
stance, arsenic becomes readily available under low redox conditions. Elements that
are particularly affected are those that require active uptake. Plant root respiration
cannot occur at low redox potentials and elements cannot be actively transported.
Redox potential also determines the form (i.e., chemical species) of an element in the
soil solution.
Decaying organic matter tends to bind trace elements and remove them from soil
solution, thereby preventing their uptake by plants. Consequently, consideration
should be given to the environmental conditions of a survey area, because by com-
bining samples from trees growing on organic-rich and inorganic soils, there may be
differences in distribution patterns of some elements that are largely attributable to
local ground conditions.
Several factors can influence root developm ent and subsequent metal uptake.
Slope and aspect can modify thermal conditions increasing the length of the
growing season and regulating transpiration rates.
Moisture levels that can regulate the length of the growing season and degree of
soil aeration.
History – stand age and tree age. Young trees have a wider lateral to depth ratio of
roots than older trees.
Soil depth and texture.
Pathology – insects and fungi.
Climatic events – drought, winter desiccation and frost.
SUMMARY COMMENTS ON CHEMICAL REQUIREMENTS OF PLANTS
There is a formidable amount of literature on plant physiology, nutritional re-
quirements and plant chemistry in general. Clearly, controlled pot and greenhouse
tests have done much to determine the conditions under which metals can accumulate
28
Plant Function, Chemistry and Mineralogy

in plants and the levels that can occur prior to the onset of toxicity. However, ex-
periments in the natural forest are far less common, and of necessity they need to be
extremely long term – tens if not hundreds of years.
It appears that in natural forest, over a number of years plants establish equi-
librium with their environment. Many factors can interact to govern element uptake,
TABLE 2-V
Relative mobility of different trace elements in relation to pH. Immobilizing factors: Fe/
Mn ¼ Fe/Mn oxides; OM ¼ organic matter; C ¼ clay; Red. ¼ reducing conditions; Ca ¼ cal-
cium carbonate (modified after compilation by Scagel, in Dunn et al., 1993a)
Element Acidic (opH
5.5)
Weakly acidic to
neutral (pH 5.5–7.0)
Alkaline
(pH>7.0)
Immobilizing
factors
Ag High Medium–low Very low Fe/Mn, OM, Red.
As Medium Medium Medium Fe/Mn, C
Au Low mobility Low mobility Low mobility
Ba Low Low Low Red., Ca, C
Be Low Low Low Fe/Mn; OM; C
Bi Low Low Low Fe/Mn, Red.
Cd Medium Medium Medium Red., OM
Ce Poorly soluble Poorly soluble Poorly soluble
Co High Medium–low Very low
Cu High Medium–low Very low Fe/Mn, OM
F High High High
Hg Medium Low Low Fe/Mn
Li Low Low Low Fe/Mn, C
Mo High High High Fe/Mn, Ca, Red.
Nb Poorly soluble Poorly soluble Poorly soluble
Ni High Medium–low Very low
Pb Low Low Low Ca, Red.
Pt Poorly soluble Poorly soluble Poorly soluble
Ra High High High Fe/Mn, OM
Rb Very high Very high Very high
Sb Low Low Low Fe/Mn, Red.
Se High High Very high Fe/Mn, Red.
Sn Poorly soluble Poorly soluble Poorly soluble
Ta Poorly soluble Poorly soluble Poorly soluble
Te Very. low Very low Very low
Th Very low Very low Very low C
U Low–medium High Very high Fe/Mn, OM, Red.
V High High High Red.
W Poorly soluble Poorly soluble Poorly soluble Fe/Mn
Zn High High–medium Low–Very low Fe/Mn, OM, Ca
29Biogeochemistry in Mineral Exploration
but there is sufficient stability in plant chemistry for the biogeochemical method to be
a robust approach to mineral exploration provided consistency in collection, prep-
aration and analysis of tissues is maintained. An awareness of the factors that can
modify element uptake needs to be constantly maintained, and there should be a
general appreciation of the complexities of the natural environment. Consequently,
brief notes on field conditions should be made and, if possible, broadly quantified in
order to assist in data interpretation. Quantification of field parameters need only be
a simple broad categorization. For example, aspect can be recorded as ‘south facing’
and degree of slope can be simply recorded as steep, moderate, gentle or flat. Thus a
field notation of 1S would indicate a gentle south-facing slope, whereas 3N would
denote a steep north-facing slope. By recording parameters in this manner they can
be related to chemical factors (by simple observation or statistically) during inter-
pretation of the subsequent elemental data.
MINERALOGY OF PLANTS
Skinner and Jahren (2005) gives a comprehensive overview of biomineralization
in plants and animals, eloquently describing those phases related primarily to sul-
phur, iron, silica, phosphorus and carbonates. It has long been established that
inorganic phases, such as silica phytoliths, develop on plant surfaces, giving grasses
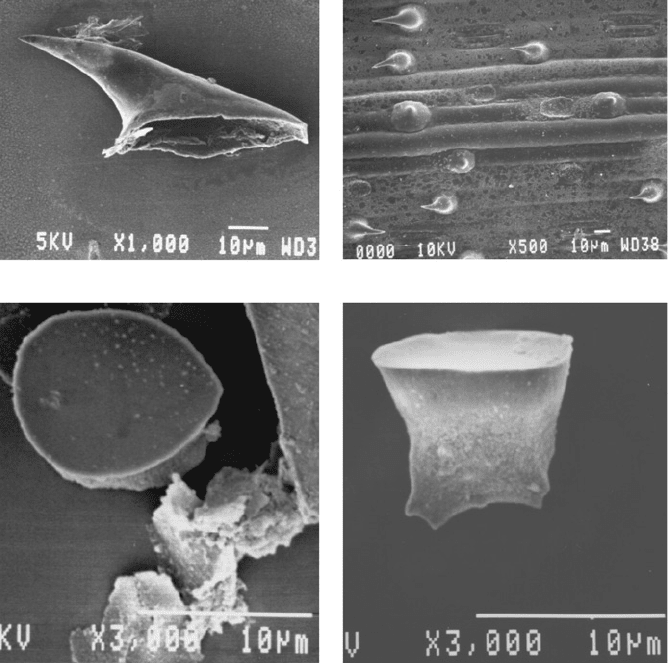
and horsetails (Equisetum) their roughness. Figure 2-1 shows examples of some forms
of phytolith associated with the cultivar ‘Einkorn wheat’, from the Gramineae
Family, and provides clear evidence of why cereal s and grasses can be harsh to the
touch.
The scanning electron microscope (SEM) is required for detailed observation of
plants for crystalline and other metal phases, because they are rarely more than
100 mm in size. Figure 2-2 shows the development of a manganese phosphate phase,
about 100 mm 10 mm, which developed within the inner trunk wood of the conif-
erous tree ‘mountain hemlock’ (Tsuga mertensiana) from Mt. Washington on Van-
couver Island. This phase appears to be non-crystalline, and is wedged between the
longitudinal fibres.
There are more than 100 families of tropical plants that contain silica and 215
families that contain calcium oxalates (Skinner and Jahren, 2005). The most fre-
quently occurring mineral phases observed in plant structures are crystals of calcium
oxalate (CaC
2
O
4
). They are commonly most concentrated in the bark (Fig. 2-3a, 3b),
although they occur, too, within twigs and foliage (Fig. 2-3c, 3d). Whereas Ca oxa-
late is a simple mixture of Ca, O and C, it is not absolutely pure. Pharmaceutical
analyses indicate that it typically has 0.5% Ba and 0.4% ‘Poorly soluble residues’.
These poorly soluble residues are likely to be primarily Si, Al, Fe, K, Mg and Na (all
of which can form independent oxalates), but in addition minor and trace elements
may be located within the crystal lattices of Ca oxalates in plants. Those reported
30
Plant Function, Chemistry and Mineralogy
include Sr, Rb, REE, S, F, Cl and Br. To date, no multi-element ICP-MS analysis of
Ca oxalate resid ues from plants appears to have been published, so it is quite feasible
that many additional trace and ultra-trace elements may be sequestered within the Ca
oxalate crystal structures.
On most modern SEMs the practical resolution is an object approximately 0.1 mm
in diameter. Examination of plant structures under the SEM quite commonly reveals
metal phases that occur as crystals or amorphous nucleations that are o2 mmin
diameter. Whereas some mineral phases seen on plant surfaces are not definitively
(a) (b)
(c) (d)
Fig. 2-1. Scanning electron micrographs (SEM) of silica phytoliths on einkorn wheat
(Triticum monococcum) – (a) hair cell; (b) lamina – abaxial section; (c) and (d) inflorescence,
top and side views.
Source: http://home.byu.net/tbb/tball/index2.html (Terry Ball, Brigham Young University),
Feb. 2004.
31Biogeochemistry in Mineral Exploration
