Creagh D., Bradley D. (Eds.) Physical Techniques in the Study of Art, Archaeology and Cultural Heritage. Volume 2
Подождите немного. Документ загружается.


4.1.3. Application 1: blue paint
Identifying blue paints is important to the paintings conservator when choosing the right
colour for retouching (Morgans, 1982; Berns and Imai, 2002; Day, 2003a; van der Weerd
et al., 2003; Clarke, 2004). Blue, in particular, is difficult to match, and choosing the incor-
rect pigment can result in severe metamerism. Pigment identification was tested using
indigo, Prussian blue, light and deep cobalt blue, cobalt turquoise, cerulean blue, ultrama-
rine, phthalocyanine blue, and phthalocyanine turquoise in different binding media.
∑ Indigo blue is dark blue and fade prone. Indigo is found as glucoside indican on leaves
of Isatis tinctoria (woad) and Indigofera tinctoria from India (McLaren, 1983). It has
been exported to Europe from the twelfth century (McLaren, 1983). Synthetic indigo
from o-nitrophenyl acetic acid has been available since 1878.
∑ Prussian blue is a synthetic pigment developed in 1704 by Dippel and Diesbach. Also
called iron blue, it consists of ferric ferrocyanide (Fe
4
[Fe(CN)
6
]
3
) (Barnes, 1939b).
∑ Cobalt blue deep and light were isolated in 1735, but not developed as a pigment until
1802 to replace the similarly coloured smalt (Bergen, 1986). This blue contains oxides
of cobalt and aluminium
∑ Cobalt turquoise is a variety of cobalt blue additionally containing chromium.
∑ Cerulean blue is paler and greener than cobalt blue, and is an artificial pigment that
consists of oxides of cobalt and tin (Johnston-Feller, 2001).
∑ Ultramarine (French) is an artificial substitute for the expensive natural ultramarine
derived from lapis lazuli (Tate, Paint and Painting, 1982). It was discovered in 1826, but
not commercialised until 1830 by Guimet and Koetting (Bergen, 1986).
∑ Phthalocyanine blue was prepared by Von Diesbach and Von der Weid in 1927
(Loebbert, 1992). Its exceptional stability, lightfastness, and resistance to acids, alkalis,
and heat led to copper phthalocyanine being commercialised as a pigment by ICI in
1935, with IG Farben and DuPont starting soon after. Winsor and Newton produces
phthalocyanine blue under the name Winsor blue (Tate, Paint and painting, 1982).
∑ Phthalocyanine turquoise is a mixture of phthalocyanine blue and phthalocyanine
green (C
32
H
2
Cl
15
CuN
8
).
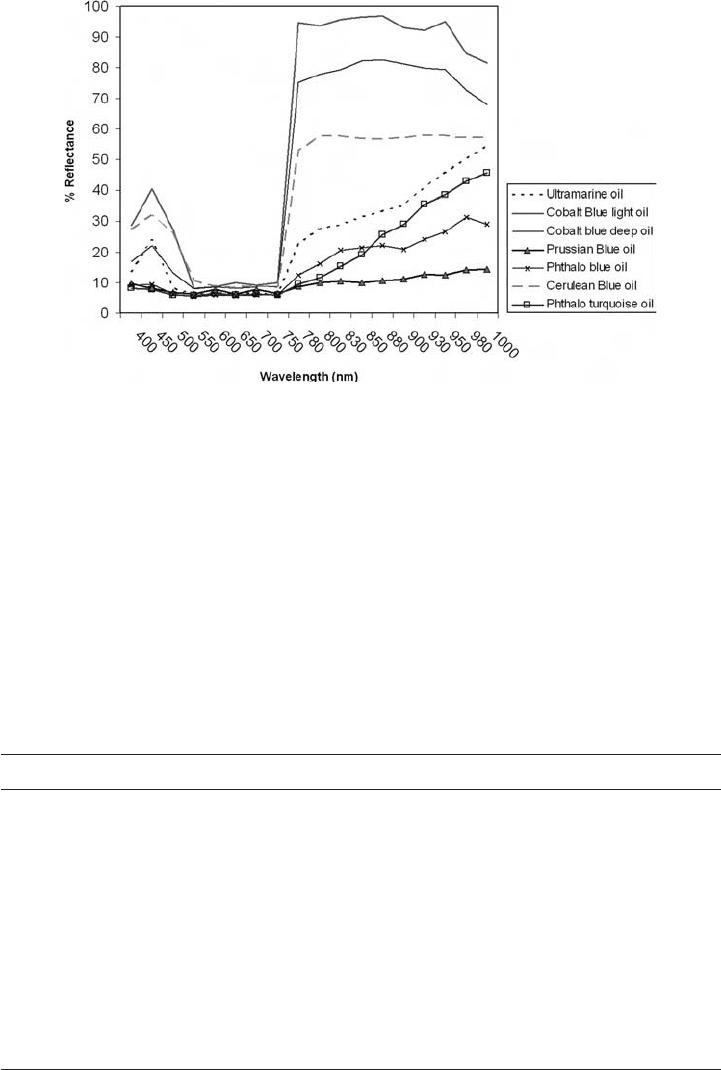
Reference spectra were collected directly from these common blue paints seen in Fig. 25.
As seen with the yellow pigments, the nine blue pigments separate easily, based on both
different value and shape reflectance peaks in the blue region (400–450 nm), and different
levels of reflection in the infrared (Fig. 25). Most pigments reflect light in the blue region
of the spectrum and absorb well into the red region. With the exception of phthalocyanine
blue, phthalocyanine turquoise, and Prussian blue, all blues show an upwards slope at 780 nm.
Cobalt blue deep and cobalt blue light may only be separated by percentage reflectance
peaks at 450 nm. Some of the deep blue/black pigments, Indigo in particular, extinguishes
the incoming beam in the visual region to a large extent, but the intensity of the long-
wavelength reflection of the spectrum (700–750 nm) is high. The deep colour of Prussian blue
is explained by its low reflectance curve, which reflects little light between 400–1000 nm.
This reflection spectrum is almost featureless. Some of the spectra were quite close in
shape and were therefore difficult to separate by spectrum (e.g. cobalt blue light and cobalt
blue deep). These results are confirmed by van der Weerd et al. (2003) and Berns and
(C
o
OS
no
)
⋅n
2
(CoO Al O ).
23
⋅n
232 M. Kubik
Imai (2002), who also found blues easy to separate. Berns and Imai incorrectly identified
manganese blue as phthalocyanine blue, which may have been due to similar incorrect
labelling of pigments.
Blue pigments also provided the opportunity to measure CIE L*a*b* colours using
Thermo Grams AI©. This program converted reflectance spectra using 2∞ observer angle
and standard illuminant CIE A, and calculated CIE L*a*b* values between 400 and
830 nm (Table 2). This demonstrates how easily CIE L*a*b* values are determined, useful
for recording colour change in an objective manner.
Hyperspectral Imaging: A New Technique for the Non-Invasive Study of Artworks 233
Fig. 25. Reflectance spectra of representative blue paints.
Table 2. CIE L*a*b* values derived from reflectance spectra of blue paints
Paint L* a* b*
Ultramarine blue oil 24.005 5.356 -43.318
Ultramarine blue tempera 29.673 3.576 -72.191
Ultramarine gamblin 25.130 7.061 -47.966
Cobalt blue deep tempera 29.530 -0.123 -54.090
Cobalt blue gamblin 31.121 1.450 -60.498
Cobalt blue light tempera 45.237 -6.571 -61.429
Prussian blue tempera 12.402 3.004 -14.658
Prussian blue gamblin 19.770 0.072 -11.424
Indigo oil 25.690 2.008 0.395
Manganese blue gamblin 40.015 -27.707 -54.071
Phthalo blue oil 23.443 -1.582 -12.553

4.1.4. Application 2: gouache painting in blue
To test the identification capacity for blue paints, the system was applied to a watercolour
and gouache painting made by the author (Fig. 26). This also validated methodology before
field testing. A number of blue Artist Spectrum (Australia) gouache paints and Windsor and
Newton (UK) watercolours were combined into a single portrait of a lady. Schmincke refer-
ence plates were used to attempt matching and identification of the “unknowns”. Figure 27
presents the location of these gouaches and watercolours that were tested.
All blues present in the reference set were identified (Figs. 28–32). Where overlap was pres-
ent between reference “known” paints and the “unknowns” of the painting, successful match-
ing was achieved, e.g. phthalocyanine blue (Fig. 31) and ultramarine (Fig. 28). Locations
for these identified paints mapped well across the image. While this is normally seen as
red highlights against a black and white image, reproductions in this chapter do not permit
colour and instead locations are mapped as bright white areas. Cerulean blue (Fig. 29) had
234 M. Kubik
Fig. 26. Blue painting with Schmincke reference plates.
Ultramarine
Cobalt Blue
Prussian Blue
Cerulean Blue
Zinc White
Turquoise
Marine Blue
Fig. 27. Location of Gouache and watercolour “unknowns”.
Hyperspectral Imaging: A New Technique for the Non-Invasive Study of Artworks 235
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
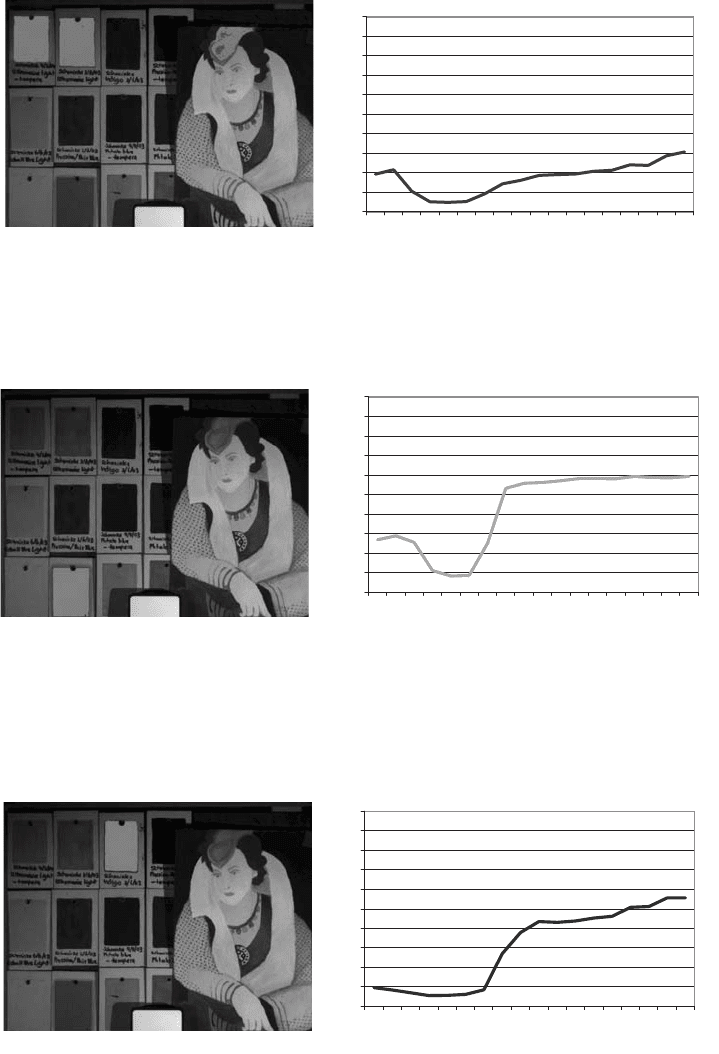
Fig. 28. Ultramarine locations and spectrum.
Fig. 29. Cerulean blue locations and spectrum.
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
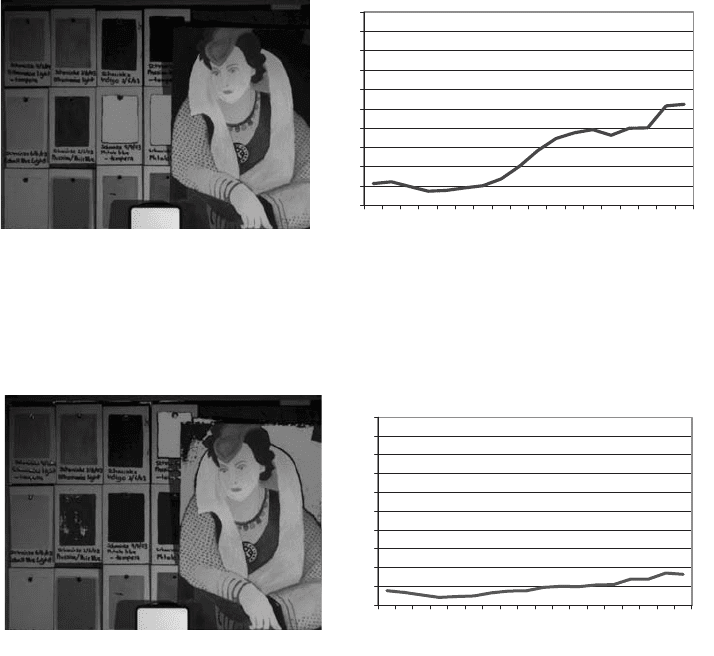
Fig. 30. Indigo blue locations and spectrum.
236 M. Kubik
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
0
10
20
30
40
50
60
70
80
90
100
Wavelength (nm)
% Reflectance
Fig. 31. Phthalocyanine blue locations and spectrum.
Fig. 32. Prussian blue locations and spectrum.
been used in a diluted manner across the face and hands; however, the diluted spectrum of
these areas was not strong enough for identification. Limits were further presented where
reference spectra were not available for particular paints present, e.g. turquoise blue
(Fig. 33), marine blue, and cobalt blue. This highlights the need for either an extensive
reference set to be included in the analysed image, or a searchable library database of
reflectance spectra to be accessible for comparison.
4.1.5. Application 3: small war memorial paintings
With the assistance of David Keany, senior paintings conservator of the Australian War
Memorial, two studies in oil measuring 7 cm ¥ 12 cm were provided on loan for further
investigation (Fig. 34). These were deemed suitable due to their small size, and the appar-
ently large variety of blues employed.
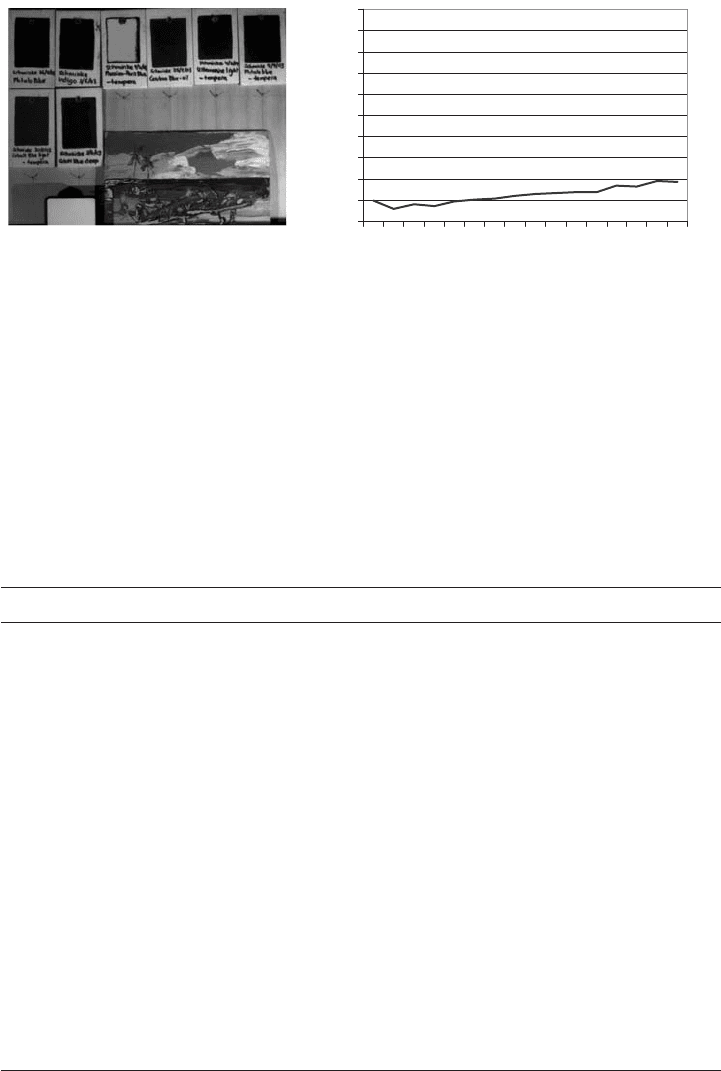
The locations of pure Prussian blue could easily be identified by comparison of a refer-
ence plate in the same image (Figs. 35 and 36). Mixtures using lead white to obtain lighter

0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
Fig. 33. Turquoise blue locations and spectrum.
Fig. 34. Small war memorial painting (Wings) with selected blue reference plates.
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
Fig. 35. Wings Prussian blue locations and reflectance spectrum.
shades of blue are further investigated. The blue pigment composition was confirmed
using Raman spectroscopy (Lee et al., 2005). Results of Raman analysis using the Kremer
database are presented in Table 3, while sample locations are given in Fig. 37. It appears
the artist has used a very simple palette: the lead white is almost ubiquitous, while all blue
areas consisted of various mixtures of Prussian blue and lead white.
238 M. Kubik
0
10
20
30
40
50
60
70
80
90
100
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
Table 3. Location and identification of AWM plane pigments using Raman spectroscopy
Sample Description Identification
P1 Blue sea Prussian blue
P2 White cloud Lead white, small amount of Prussian blue
P3 Green earth Prussian blue + lead white + possibly kibeni
orange or priderite yellow
P4 Light brown ground Lead white, other inclusions seen but no spectra
generated.
P5 Medium blue Prussian blue
P6 Medium blue sky Prussian blue, lead white
P7 Green foreground Prussian blue + lead white + possibly kibeni
orange or priderite yellow
P8 Purple pants Prussian blue
P9 Pink man on wing Lead white
P10 Red hair on LH man Possibly kibeni orange or priderite yellow
P11 Red hair on LH man Possibly kibeni orange or priderite yellow
P12 Dark blue wing Prussian blue, small carbon peaks indicating
carbon black
P13 Light brown fabric coating Lead white
P14 Light blue Lead white, low amount of Prussian blue
Fig. 36. Plane Prussian blue locations and reflectance spectrum.
These dark blue regions have also been identified as Prussian blue using imaging spec-
troscopy. This shows that identification by imaging spectroscopy has been partially
successful in identifying the blue paint used, although lighter mixtures using lead white
have made this less than straightforward.
4.1.6. Application 4: Sidney Nolan
In order to test the portability and effectiveness of the instrument setup, the imaging
system was taken to the Australian War Memorial (Fig. 45). Here, it could be tried with
ambient lighting conditions and actual paintings with unknown pigment composition. It
also allowed for field testing, seeing how portable the instrumentation was and how easy
it was to carry and set up in a museum location with uncontrolled lighting. This was an
important consideration for the system’s design. Application proved to be very straightfor-
ward, with the entire system being able to be dismantled, carried by hand, and fitted in the
back of a car. The War Memorial venue consisted of an active conservation studio, with
diffuse natural light, fluorescent light, and incandescent lights, simultaneously. As in the
lab, the camera was positioned at 150 cm from the painting, which was sufficient to
capture the entire image. Projectors could be placed at slightly longer distances as the
allowed space was wider, in this case 120 cm compared to 90 cm. This affected maximum
exposure readings for the Spectralon reference, so that calibration had to be performed
before images were captured.
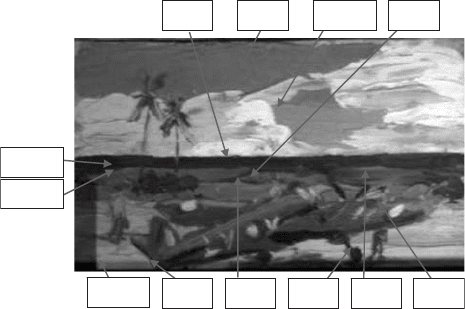
The Angry Penguins group was of particular interest, with little materials research
performed to date on some of Australia’s best known artists, e.g. Sydney Nolan (1917–1992).
This has led to the formulation of incorrect hypotheses about his materials, and incorrect
labelling in catalogues (Kubik, 2007). Nolan was unusually experimental in his approach
to paints, in part due to influence from overseas artists, but also caused by rationing and cost
of materials during the early stage of his career. Returning to interviews (Nolan, 1962; 1980;
1987) and letters written by Nolan (Nolan, 1943b; Nolan, 1943c; Nolan, 1943d; Nolan,
1943e; Nolan, 1947; Nolan, 1948) reveals what may be expected in materials analysis.
Hyperspectral Imaging: A New Technique for the Non-Invasive Study of Artworks 239
P6 P14 P2 P1
P11
P10
P9P4P3P7
P13
P8
Fig. 37. AWM plane locations for Raman sampling.

In his letters, there are several requests by Nolan for a particular blue dye (Nolan, 1943f),
which has never been identified. By 1954, Nolan had switched to mixing his own pigments
into polyvinyl acetate binder (Llyn, 1967). Advantage was taken of the War Memorial’s
collection of Nolan paintings to shed light on these details. The following three paintings
containing blue paint were tested:
(1) Gallipoli Landscape, half-lit (1958): Synthetic polymer paint on coated card, ART
91243
(2) Cove at Hydra (1956): Synthetic polymer paint on coated card, ART 91261
(3) Gallipoli Landscape with gun (ca. 1963): Acrylic with yellow oil crayon on gloss art
board, ART 90217
All three contained unknown blue paint in either sky or sea, so that these could be tested
against known references and the library database created earlier. The blues also appeared
in different hues, so that several types of pigments were expected.
The first painting tested was Gallipoli Landscape, half lit. This showed dappled blue
paint, almost ultramarine in colour. Nolan’s technique at this stage involved wiping paints
using a squeegee and sponge (Moore, 1998). The painting was placed on an easel, and
projector lights placed at 1.0 m at 45∞ incident to the surface plane of the painting. As had
been established at ANU, the CCD camera was placed at 150 cm from the painting, which
was sufficient to capture the entire image, as well as six blue reference plates and the
Spectralon reflectance standard in the middle of these (Fig. 38). The positions of the blue
references remain consistent throughout the three Nolan paintings tested. The white
balance was calculated at 4000 maximum and the exposure times adjusted accordingly.
Images were captured sequentially between 400 and 1000 nm, and stacked into a data cube
240 M. Kubik
Cerulean
Prussian
Indigo
Ultramarine
Phthalocyanine
Cobalt
Fig. 38. Nolan’s Gallipoli Landscape, half lit with six reference plates of blue pigments
and the Spectralon standard.
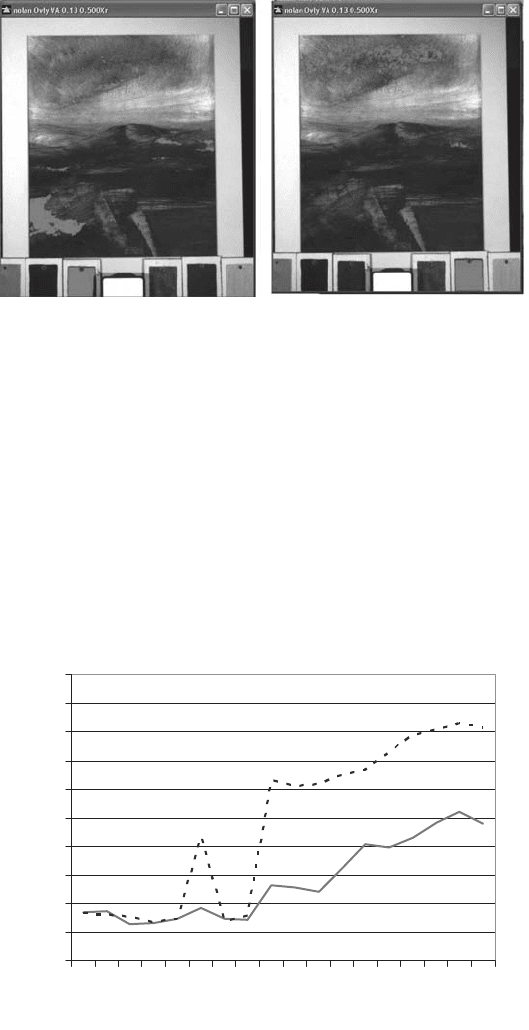
using the Hypercube software. From this, identification could either be achieved by select-
ing the six standards and finding similarities within the image, or reversely by selecting
key points in the sky and allowing the search to find similarities within the reference plates.
After collating the images in Hyperspec and searching both the distribution of unknown
blue areas and the references, both indigo and phthalocyanine blue were found to be pres-
ent as a mixture (Figs. 39 and 40). The two different colours appeared thoroughly mixed
on the canvas, and were only discernable using imaging spectroscopy. This was therefore
a positive outcome, as it indicates the possibility of identifying individual components
within a mixture.
Hyperspectral Imaging: A New Technique for the Non-Invasive Study of Artworks 241
0
10
20
30
40
50
60
70
80
90
100
400
450
500
550
600
650
700
750
780
800
830
850
880
900
930
950
980
1000
Wavelength (nm)
% Reflectance
Fig. 39. Gallipoli Landscape, half lit locations for indigo (left) and phthalocyanine blue
(right).
Fig. 40. Reflectance spectra of indigo (dotted line) and phthalocyanine blue (solid line).
