Charlton M., Humberston J.W. Positron Physics
Подождите немного. Документ загружается.

5.7 Inner shell ionization 259
100 eV impact energy. A small peak was observed close to 42 eV in the
ejected electron energy spectrum when both the electron and positron
were emitted at small angles to the forward (incident beam) direction.
These authors attributed this to the ECC process (see the discussion
in section 5.2) and therefore provided the first clear evidence for this
mechanism in positron impact ionization.
5.7 Inner shell ionization
In this section we describe experiments whose aim has been to determine
explicitly inner shell ionization cross sections (or ratios of electron and
positron cross sections). Section 5.5 contained an account of the influence
of inner shell processes on multiple ionization of the heavier noble gases.
The first experiments to study inner shell processes, and indeed the
first investigations of positron impact ionization, were those of Hansen,
Weigmann and Flammersfeld (1964), Hansen and Flammersfeld (1966)
and Seif el Nasr, Ber´enyi and Bibok (1974). These workers used β-
spectrometers to velocity-select positrons and electrons emitted from
radioactive sources, and they measured (by detection of the appropriate
X-rays) the ratio of the K-shell ionization cross sections, σ
K
i
, for the
two projectiles scattering from various targets at high energies, typically
hundreds of keV. Within the statistical accuracy of the experiment no
deviation from unity was found for the ratio σ
K
i
(e
−
)/σ
K
i
(e
+
). Ito et al.
(1980), also using β-spectroscopy, measured K- and L-shell cross section
ratios for a silver target for a range of positron and electron kinetic
energies above 100 keV. Although they found the ratio to be unity for
the L-shell processes, σ
K
i
(e
−
)/σ
K
i
(e
+
) was observed to be greater than
unity below approximately 150 keV, around six times the K-shell binding
energy. A similar trend was observed at relativistic energies by Schneibel
et al. (1976).
Turning to more recent experiments, a vertical section of the target
region used by Schneider, Tobehn and Hippler (1991, 1992) and Schneider
et al. (1993) is shown in Figure 5.22. The positron beam was provided
by the pulsed source at the Giessen electron linear accelerator (see e.g.
Ebel et al., 1990, and references therein). An electron beam could also be
produced, using a heated filament in close proximity to the target. The
kinetic energy of the projectiles was mainly determined by applying an
electrical potential to the target, which was tilted at 45
◦
to the beam axis.
The targets consisted of various thin silver and gold foils and gold–silver
multilayer arrangements (Schneider et al., 1993). The purpose of the
latter was to use the silver L-shell X-ray as a standard for comparison
of the positron and electron cross sections, since little difference between
them is expected in the energy range under investigation. X-rays emitted
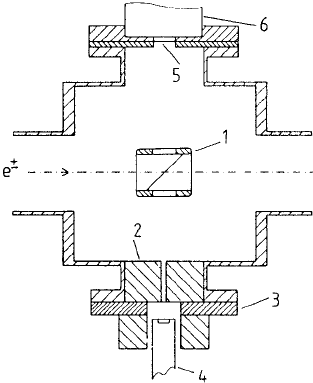
260 5 Excitation and ionization
Fig. 5.22. Experimental set-up for studies of positron impact inner shell ioniza-
tion (Ebel et al., 1989). Key: (1) target support; (2) lead collimator; (3) flange
with thin window for the transmission of X-rays to (4) a Si(Li) detector; (5)
aluminium window 0.1 mm thick; (6) NaI(Tl) gamma-ra
y detector.
into a small solid angle passed through a thin mylar window before being
registered by a Si(Li) detector. A NaI(Tl) detector was located directly
opposite the Si(Li) counter as a veto to suppress the Compton-scattered
annihilation γ-rays which may be registered by the latter.
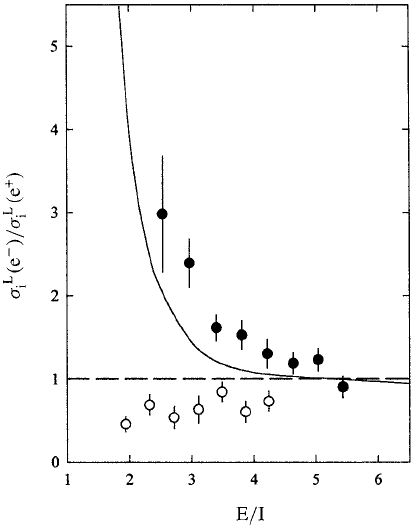
Figure 5.23 shows a summary of what has been obtained for the K-shell
ionization of various elements expressed as the ratio σ
K
i
(e
−
)/σ
K
i
(e
+
) versus
E/I, the reduced impact energy, where I is the K-shell binding energy.
There is good accord between the various experiments in the energy
range of overlap, with σ
K
i
(e
−
)/σ
K
i
(e
+
) varying smoothly throughout the
range E/I = 2–16. Above E/I ∼ 5 the ratio is unity, but below this
value the electron cross section rises above that for positrons, the ratio
reaching a value around eight at the lowest impact energy. This is most
clearly seen in the data of Ebel et al. (1989) for silver. The steep rise
in the ratio at the lower impact energies is reproduced by the results
of a plane wave Born approximation with a correction included to take
account of modification of the projectile wave function by the Coulomb
field of the target nucleus. It is notable that when this correction is
omitted the calculation completely fails in the low energy region. As
summarized by Knudsen and Reading (1992), the difference between the
K-shell ionization probabilities for the two projectiles is mainly governed
by the different deflections experienced by the particles in the Coulomb
field of the nucleus. The effect of this is expected to lessen with rising

5.7 Inner shell ionization 261
Fig. 5.23. Ratio of K-shell ionization cross sections for electrons and positrons
scattering
from silver and copper at various impact energies: ——, Born ap-
proximation calculation including a Coulomb correction (see text); – – –, Born
approximation without the Coulomb correction. The data are: •, Ag (Ebel et al.,
1989);
, Cu (Ebel et al., 1989); , Cu (Schultz and Campbell, 1985); , Ag (Ito
et al., 1980).
projectile energy (in accord with approach of the cross section ratio to
unity) and, as observed, with increasing principal quantum number of the
electronic shell.
Calculations by Gryzi´nski and Kowalski (1993) for inner shell ionization
by positrons also confirmed the general trend. Theirs was essentially
a classical formulation based upon the binary-encounter approximation
and a so-called atomic free-fall model, the latter representing the internal
structure of the atom. The model allowed for the change in kinetic energy
experienced by the positrons and electrons during their interactions with
the screened field of the nucleus.
Lennard et al. (1988) studied L-shell ionization ratios for gold, where it
was hoped that the Coulomb effect would be so reduced as to permit the
observation of certain basic differences between positron–electron scatter-
ing (Bhabha, 1936) and electron–electron scattering (Møller, 1932), for
large energy transfers. Later Schneider, Tobehn and Hippler (1991, 1992),
using the apparatus described above, measured the same quantities. As
262 5 Excitation and ionization
Fig. 5.24. Ratio of L-shell ionization cross sections for electrons and positrons
at various impact energies: ——, Born approximation calculation including the
Coulomb correction (see text). The data are: •, Schneider, Tobehn and Hippler
(1991) for a gold target; ◦, Lennard et al. (1988) for a silver target.
shown in Figure 5.24, a major discrepancy exists between the two sets
of measurements, which increases as the threshold is approached, with
σ
L
i
(e
−
)/σ
L
i
(e
+
) dipping below unity in the data of Lennard et al. (1988)
whilst exhibiting the opposite trend in the work of Schneider, Tobehn
and Hippler (1991). The solid line in this figure, which is in qualitative
accord with the Schneider data, was obtained using the plane wave Born
approximation, including exchange and allowance for Coulomb effects.
The Møller–Bhabha effects mentioned above were not included in this
calculation.
Although the general trend of the theories tends to support the work
of the Giessen–Bielefeld group, Gryzi´nski and Kowalski (1993) pointed
out that the differences between the results from the two experiments
may be explained by different target arrangements. They noted that the
orientation of the latter with respect to the beam (Ebel et al., 1989, target
at 45
◦
; Lennard et al., 1988, target perpendicular) may play a role if there
is some anisotropic orientation of the electron orbitals in the foil targets.
5.7 Inner shell ionization 263
This could lead to anisotropies in the emission of the X-rays. Further
experimental work is needed to clarify this issue.
Schneider et al. (1993) developed their group’s technique further in
order to assign an absolute scale to the ionization cross sections by electron
and positron impact for the K-shell of silver and the L
3
-shell of gold.
Magnitudes were of the order of 10
−23
cm
2
and 10
−22
cm
2
respectively,
though strongly energy dependent as the relevant threshold is approached.
6
Positron annihilation
6.1 Introduction and theoretical considerations
Before the advent of low energy beams, the only means of investigating
positron interactions with atoms and molecules was to study their annihi-
lation. Information could thereby be obtained directly on the annihilation
cross section but only indirectly for other processes such as elastic scatter-
ing. In this chapter we consider the annihilation of so-called free positrons
in gases. The fate of positrons which have formed positronium prior to
annihilation is treated in Chapter 7.
The basic physical principles governing positron annihilation were de-
scribed in subsection 1.2.1, where the non-relativistic limit of the Dirac
cross section for the annihilation of a free positron–electron pair into two
gamma-rays was given as σ
2γ
=4πr
2
0
c/v, equation (1.3). If the electron
density in the vicinity of the positron is n
e
, then the annihilation rate is
λ
2γ
=4πr
2
0
cn
e
. However, as shown in subsection 1.2.2, annihilation into
two gamma-rays requires the positron–electron pair to be in a singlet spin
state, but only one quarter of the electrons in an unpolarized ensemble
would form such a state with the positron. The remaining electrons would
form a triplet spin state with the positron, for which annihilation is into
three gamma-rays at a much lower rate (less than 1% of the two-gamma
rate). Thus, the total free-positron annihilation rate is
λ
f
πr
2
0
cn
e
. (6.1)
If the electrons are bound in atoms or molecules, each having Z elec-
trons, and the number density of atoms or molecules is n, the electron
density is n
e
= nZ. Therefore, if there were no distortions of the positron–
atom system, the free annihilation rate would be given by
λ
f
= πr
2
0
cnZ. (6.2)
264
6.1 Introduction and theoretical considerations 265
In practice, the positron does influence the charge distribution in the
atom or molecule, in such a way as to enhance the electron density in its
vicinity. Allowance for this can be made by replacing Z by an effective
number of electrons, Z
eff
. Thus, the annihilation rate may be expressed
as
λ
f
= πr
2
0
cnZ
eff
=0.201ρZ
eff
(µs
−1
), (6.3)
where ρ is the gas density in amagat (1 amagat ≡ 2.69 × 10
25
m
−3
), and
the annihilation cross section is therefore
σ
2γ
= λ
f
/(vn)=πr
2
0
cZ
eff
/v. (6.4)
Distortion of the target atoms or molecules is particularly pronounced
at very low positron speeds, and Z
eff
may then be considerably larger
than Z; however, as the speed increases and the electrons have less time
to react to the perturbing field of the positron, Z
eff
initially decreases.
The value of Z
eff
is a measure of the probability that the positron is
at essentially the same position as any of the electrons in the target, and
it can be calculated from the wave function representing elastic positron
scattering by the target. If the wave function is Ψ(r
1
, r
2
,...,r
Z+1
), where
r
1
is the positron coordinate and r
2
,...,r
Z+1
are the coordinates of the
Z electrons in the target system, then
Z
eff
=
Z+1
i=2
|Ψ(r
1
, r
2
,...,r
Z+1
)|
2
δ(r
1
− r
i
) dr
1
···dr
Z+1
= Z
|Ψ(r
1
, r
2
,...,r
Z+1
)|
2
δ(r
1
− r
2
) dr
1
···dr
Z+1
, (6.5)
since the wave function is antisymmetric in all the electron coordinates.
In this calculation the positron wave function must be normalized in such
a way that its asymptotic form has unit amplitude, i.e.
Ψ(r
1
, r
2
,...,r
Z+1
) ∼
r
1
→∞
exp(ik · r
1
)Φ(r
2
,...,r
Z+1
), (6.6)
where Φ(r
2
,...,r
Z+1
) is the wave function of the target atom.
Whereas the error in the calculated value of the elastic scattering phase
shift is usually of second order in the error in Ψ, the error in Z
eff
is of
first order; the values of Z
eff
therefore tend to be rather less accurate than
the corresponding phase shifts. Consequently, the value obtained for Z
eff
provides a sensitive test of the accuracy of a wave function, although
admittedly in a very restricted region of configuration space where the
positron is close to one of the electrons. Drachman and Sucher (1979)
developed an alternative method of calculating Z
eff
in which the delta
function δ(r
1
− r
i
) is replaced by a global operator but, because it is
266 6 Positron annihilation
more difficult to implement, the method has had very limited use (Ujc
and Stauffer, 1985).
In the first Born approximation the total wave function is taken to
be a plane wave for the positron multiplied by the undistorted target
wave function, and consequently Z
eff
= Z. This approximation is valid at
sufficiently high energies, and one might expect the calculated value of Z
eff
to tend to Z as the positron energy increases. However, the calculation
should only be carried out for positron energies below the positronium
formation threshold, E
Ps
. At higher energies, the explicit representation
of the open positronium channel in the total wave function, when inserted
into equation (6.5), yields an infinite value for Z
eff
, the interpretation of
which is as follows. Above E
Ps
, the cross section for positronium formation
is several orders of magnitude larger than the annihilation cross section.
Once formed, positronium certainly undergoes annihilation, and therefore
the positronium formation and positron annihilation cross sections can be
considered to be equivalent, implying a very large value of Z
eff
. At ener-
gies just below the positronium formation threshold, the positron tends
to form virtual positronium with one of the atomic electrons, resulting
in an enhanced electron density in its vicinity and, consequently, a very
rapid increase in the value of Z
eff
as the threshold is approached (Van
Reeth and Humberston, 1998).
Examples of the energy dependence of Z
eff
for atomic hydrogen and
helium are given in Figure 6.1. These results were obtained using the
very accurate elastic scattering wave functions described in detail in sub-
sections 3.2.1 and 3.2.2. The only molecule for which reasonably accurate
calculations of Z
eff
have been made is H
2
, where Armour, Baker and
Plummer (1990) used the elaborate variational wave functions obtained
from their studies of low energy scattering (see subsection 3.2.4). How-
ever, such is the sensitivity of the value of Z
eff
to the quality of the wave
function that even this calculation only yielded the value 10.2, compared
to the experimental result of 14.8 at room temperature. Nevertheless, this
is much closer to the measured value than any other theoretical result for
this molecule.
For each of these systems the value of Z
eff
exceeds the corresponding
value of Z by a significant factor, particularly in the case of atomic
hydrogen, for which it is almost nine times greater at very low energies,
whereas for helium the factor is only two. In a quite highly polarizable
atom, such as hydrogen, the outer electrons are readily attracted towards
the incident positron, enhancing the probability for annihilation. In an
early study of the correlation between the value of Z
eff
and the dipole
polarizability of the target, α, Osmon (1965) found that, for many simple
atoms and molecules, a reasonably good fit to the experimental data was
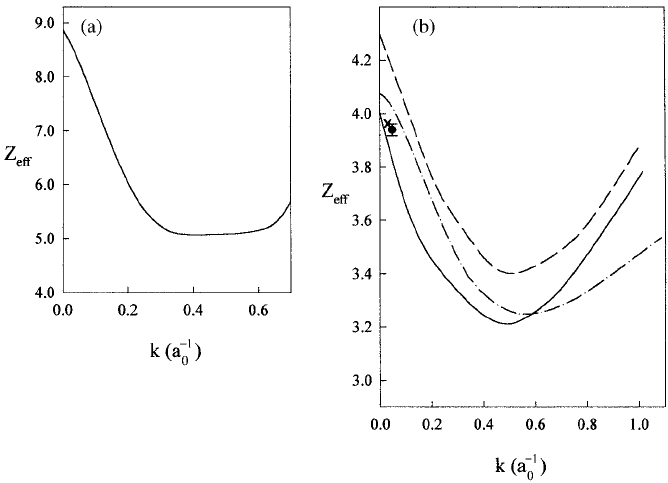
6.1 Introduction and theoretical considerations 267
Fig. 6.1. Variation of Z
eff
with positron momentum. (a) Atomic hydrogen,
Humberston and Wallace (1972). (b) Helium: ——, Campeanu and Humberston
(1977b) for helium model H5; – – –, Campeanu and Humberston (1977b) for
helium model H1; — · —, McEachran et al. (1977); ×, Roellig and Kelly (1965)
(Fraser, 1968); •, Coleman et al. (1975b).
provided by the relationship Z
eff
∝α
1.25
. However, more recent data
are consistent with Z
eff
∝α (Wright et al., 1983). Some molecules,
particularly large organic molecules (e.g. Iwata et al., 1995) do not fit this
pattern and have values of Z
eff
several orders of magnitude greater than
the number of electrons in the molecule; for example, Z
eff
is around 18 000
for benzene, and greater than 10
6
for anthracene. One theory is that these
very large values arise because the positron forms a pseudo-bound-state
or resonance with the molecule, in which the positron is trapped in its
vicinity for much longer than the usual collision time. Molecules with
very large values of Z
eff
also have low threshold energies for positronium
formation, E
Ps
, the relationship being reasonably well represented by
lnZ
eff
≈A/E
Ps
+ B,
where A and B are constants (Murphy and Surko, 1991). This fact
prompted Laricchia and Wilkin (1997) to develop an alternative positron-
trapping mechanism based on the increasing significance of virtual positro-
nium formation as the threshold energy for real positronium formation

268 6 Positron annihilation
is lowered. Exploiting the time–energy uncertainty relationship, they
assumed that a positron with an incident energy an amount ∆E below
the positronium formation threshold will form virtual positronium and
be trapped in the vicinity of the target system for a time ∆t /∆E,
during which it might annihilate with one of the electrons. The value of
Z
eff
is then expressed as
Z
eff
=
σ
el
v
πr
2
0
c
γ[1−exp(−λ
d
t
c
)]+(1−γ){1−exp[−∆t(λ
sa
+λ
po
)]}
, (6.7)
where σ
el
is the elastic scattering cross section, λ
d
and λ
po
are the direct
and pick-off annihilation rates, λ
sa
is the spin-averaged annihilation rate of
positronium and γ = exp(−∆t/t
c
), where t
c
is the collision time. The pre-
dictions of this model are in qualitative agreement with the experimental
data and also with the theoretical results of Van Reeth and Humberston
(1998) mentioned above. Further discussion of positron annihilation on
large molecules has been given by Iwata et al. (2000).
The wave function of the ion that remains after annihilation is a super-
position of eigenstates of the Hamiltonian of the ion, the relative proba-
bilities of which may be determined from the wave function used in the
calculation of Z
eff
. The annihilation process takes place so rapidly, com-
pared with normal atomic processes, that it is reasonable to assume the
validity of the sudden approximation. Consequently, the wave function
of the residual ion when the positron has annihilated with electron 2 at
the position r
1
= r
2
is
F (r
2
; r
3
,...,r
Z+1
)=Ψ(r
1
= r
2
, r
2
, r
3
,...,r
Z+1
), (6.8)
where we have used the same nomenclature as in the positron–atom wave
function. The relative probability that the residual ion is in its n
ion
th
eigenstate, with wave function Φ
n
ion
(r
3
,...,r
Z+1
), is then obtained by
projecting the function F (r
2
; r
3
,...,r
Z+1
) onto this state, squaring the
result and integrating over the positron coordinate to give
P (n
ion
) ∝
|F (r
2
; r
3
,...,r
Z+1
)Φ
n
ion
(r
3
,...,r
Z+1
) dr
3
···dr
Z+1
|
2
dr
2
.
(6.9)
By exploiting closure in the summation over all the states of the ion,
it may be shown that the normalized probability of the ion being in the
state n
ion
is
P (n
ion
)=
n
ion
Z
eff
F (r
2
; r
3
,...,r
Z+1
)
×Φ
n
ion
(r
3
,...,r
Z+1
) dr
3
...dr
Z+1
|
2
dr
2
, (6.10)
