Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


rate of polyadenylation and the nuclear export of the mRNA (Mattaj,
1990). The sequence of the introns is not conserved and the bases GU and
AG in the 59 and 39 ends, respectively, are recognized as hallmarks of
almost all introns (Petitclerc et al., 1995). Although there are genes that are
efficiently expressed without the presence of introns, their inclusion in
expression vectors is generally recommended. They should be placed at
the 59 end of the transcription unit to avoid aberrant splicing as a result of
inactive or cryptic splicing sites present in the gene sequence (Huang and
Gorman, 1990).
Most mRNAs from eukaryotic cells contain in their 39 end an extension
of approximately 200 adenosine molecules, known as the polyA tail.
Similar to capping and splicing, the polyadenylation of the primary
transcript is a co-transcriptional process. It occurs at the 39 end of the
RNA and consists of a site-specific cleavage followed by polymerization
(adenylation). The polyadenylation is essential for the formation, stability,
and translatability of the mRNAs. The polyadenylation signal is composed
of a highly conserved sequence (AATAAA) located about 10–30 nucleo-
tides downstream from the stop codon, and positioned immediately up-
stream of a T- or TG-rich region (T/G box; Munroe and Jacobson, 1990).
In most cases, the initial step in mRNA degradation is the shortening of
the polyA tail. This gradual aging of the mRNA takes place in the
cytoplasm and is referred to as the ‘molecular clock9 of the RNA. The
polyadenylation signals more commonly employed in expression vectors
are those derived from the bovine growth hormone, mouse -globin, SV-
40 early transcription unit, thymidine kinase (TK), herpes simplex virus
(HSV), and hepatitis B antigen (Kaufman, 1990).
The sequence signaling the culmination of the transcription reaction is
localized between the polyadenylation signal and the 39 end of the mature
RNA. Although the transcription termination region of several eukaryotic
genes has been identified – consensus sequence: ATCAAA(A/T)TAGGA
AGA – the precise site of the termination is, in most of the cases,
unknown. The termination signal is required to prevent the transcriptional
machinery extending its activity to sequences located downstream of the
gene that is being transcribed, a phenomenon known as ‘transcriptional
interference.9 It has also been proposed that the introduction of termina-
tion sequences abolishes or diminishes the transcription of the comple-
mentary DNA chain, which would give rise to the formation of antisense
RNA, with the consequent suppression of the expression of the sense
RNA (Izant and Weintraub, 1985). The expression vectors for animal cells
harbor the well-characterized transcription terminator sequences from
prokaryotes.
3.3.2 Translational control elements
In the cytoplasm the codons of mRNA are translated into amino acids via
the concerted action of the ribosomes, transfer RNA (tRNA), and a large
protein complex. In addition to the mature RNA sequence (capped and
polyadenylated) other structural elements located in the 59 and 39 untrans-
lated regions (UTRs) of the mRNA may influence its translation.
42 Animal Cell Technology

59 Untranslated region
The initiation of the translation constitutes the rate limiting step in the
synthesis of proteins. The start codon (AUG) embedded in the consensus
sequence GCC(
A/G)CCAUGG, widely known as Kozak 9s sequence,
gives the appropriate context for an optimal beginning of the translation
(Kozak, 2005). Specific purines in the Kozak9s sequence (underlined)
provide an enhancer effect on translation. It has also been noted that the
existence of additional start codons in the 59 UTR impairs severely the
translation (Grens and Scheffler, 1990). The formation of secondary
structures (i.e. hairpin loops) in this region may inhibit the binding and/or
the advance of the translational complex on the RNA strand due to a steric
hindrance. Regions rich in G and C are prone to form structures of this
type, which are thermodynamically very stable (Grens and Scheffler,
1990).
39 Untranslated region
In mammalian cells, the degradation of the mRNA is triggered after a
significant shortening of the polyA tail and is catalyzed by 39-59 exoribo-
nuclease activity of a protein complex termed the exosome. Mechanisms
involving exonuclease activity that are independent of the polyA ‘‘aging’’
have also been described (Ina´cio and Liebhaber, 2003). In the 39 UTR of
the mRNA there are sequences able to alter the stability of the transcript.
For instance, motifs that have AU-rich elements (ARE) were found in
highly unstable mRNA. AREs were later shown to bind proteins with the
capacity to regulate either positively or negatively the decay rate of the
mRNA, that is, recruiting or blocking the exosome (Gray and Wickens,
1998).
Termination codon and codon usage
The completion of the translational process requires the binding of the
‘‘release factor’’ to the stop codon, which induces the disassociation of the
translational complex and thus, the end of polypeptide synthesis.
Although the presence of the stop codon is sufficient to interrupt the
translation, exhaustive studies have demonstrated that the contiguous base
can influence significantly the efficiency of this process. For example, it
has been reported that purines (A or G) occupying this position constitute
a much more effective termination signal than pyrimidines (C or U;
McCaughan et al., 1995). Two models were proposed to explain the effect
of the fourth base on the performance of the termination process: one
suggests that this base is recognized as a part of the stop codon and the
other claims that this base constitutes an independent signal for the
recruitment of the ‘‘release factor’’ (McCaughan et al., 1995).
It is worthwhile to mention that a considerable degree of preference for
certain codons has been observed for genes displaying high expression
levels, which correlated with a higher abundance of the complementary
tRNA in the cell (Fedorov et al., 2002). The optimization of codon usage
Cloning and expression of heterologous proteins in animal cells 43
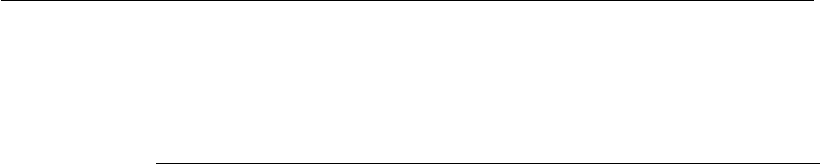
can be achieved by site-directed mutagenesis to increase the expression
level of some genes (Makrides, 1999).
3.4 Systems for heterologous expression in animal cells
All expression systems consist of a vector and a host. The vector is defined
as a molecule of DNA or RNA, which is genetically manipulated to carry
a molecule of foreign DNA or RNA, with the aim of producing proteins
(gene expression) or DNA (amplification, replication) once it is introduced
into a host cell. In the nucleus of the transfected cell the expression vector
can exist: (i) as an independent replication unit, namely episome, or (ii)
integrated to the host genome through a random or non-homologous
recombination process. In order to exist as an episome the vector must
have a signal (episomal replication origin) allowing for its autonomous
multiplication, non-linked to the replication of the host genome. Episomal
vectors render high transient expression levels of recombinant protein
(discussed below). However, the cytotoxicity generated by the high
concentration of exogenous DNA, the tendency to integrate into the
cellular genome and to undergo rearrangements (mainly in long-term
cultures) have been pointed out as the major obstacles inherent to its use
(Sanders, 1990). As explained below, the clonal variation due to the
‘‘positioning effect,’’ commonly observed for integrative vectors, does not
occur with episomal vectors.
The integration of the vector into the host’s genome takes place in a
random manner and the sequences/structures from either the vector or the
site of integration can affect the expression of the target gene. The fact that
nearly 95% of the genome from animal species consists of non-coding
sequences and that the coding regions are frequently silenced (heterochro-
matin) explains the low efficiency in obtaining highly productive clones
from vectors that integrate into the chromosomes. Possible rearrange-
ments of the vector9s DNA giving rise to mutations, insertions or deletions
that can alter the sequence or expression of the heterologous gene, may
take place when using this type of vector.
The expression systems available for the production of recombinant
proteins in animal cells can be classified as mammalian cell/viral or plasmid
vector, or insect cell/baculovirus.
3.4.1 Viral vectors
The first attempts to express foreign genes in mammalian cells were
carried out using viruses as vectors (Goff and Berg, 1976; Hamer et al.,
1977). According to the nature of the viral nucleic acid, these vectors are
classified as DNA or RNA viruses, or chimeras. If the genetic material of
the virus is surrounded by a protein envelope (capsid), it enters the cell
by a mechanism called infection, which generally occurs with high
efficiency. If the nucleic acid of the vector lacks a capsid (‘‘naked’’), its
incorporation to the cell is carried out by means of transfection techni-
ques (discussed in Section 3.7). In a permissive cell, a viral vector
develops a lytic cycle, in which the replication of the viral DNA and its
44 Animal Cell Technology

later encapsulation generates a large number of virions (progeny) that
will finally cause cell lysis and death, thus precluding the establishment
of a stable cell line. Such limitations can be overcome by using: (i)
nonpermissive cells, wherein the virus replicates as an independent entity
integrated into the host’s genome and induces only a morphological
transformation of the cells; (ii) defective viruses, whose genome has been
modified in such a way that it cannot complete the lytic cycle and
depends for its propagation on either a helper virus or a specific cell line
that provides the absent elements/functions (i.e. COS cells/SV-40 virus,
Section 3.6.1); or (iii) genetically modified permissive cells that allow the
episomal replication of the virus, increasing the productive yield while
preventing the lytic cycle, although the high concentrations of extrachro-
mosomal DNA can be cytotoxic (Sanders, 1990). The main advantage
offered by the viral systems is that tedious screening for high producing
clones is not required as is the case when using integrative plasmid
vectors. Some disadvantages displayed by viral vectors are: (i) the
physical limitations of the viral packaging restrict the size of the foreign
gene; (ii) recombinant viruses are generally defective and must be
propagated with a helper virus or a specific cell line; (iii) stable recombi-
nant cell lines cannot be established with a virus that presents a lytic
cycle; (iv) the specificity of the virus to infect certain cell types restrains
the selection of the expression host.
Vectors derived from DNA viruses
SV-40 and polyoma viruses belong to the group of the papovavirus
(Family: Polyoma virus), both presenting a small ‘‘naked’’ genome (5
kb) that is able to replicate in primate and murine cells, respectively. SV-40
is considered the pioneer vector in terms of heterologous expression in
mammalian cells (Goff and Berg, 1976; Hamer et al., 1977), and is
preferred over polyoma viruses for production of recombinant proteins
due to its higher replication rate and infection efficiency. The target gene
is inserted in the early or late replication regions of the viral genome,
producing a recombinant defective virus (Muller et al., 1983; Zhu et al.,
1984). The advantages of employing SV-40 as an expression vector can be
summarized in the higher expression levels and short time required to
evaluate the production of recombinant protein. The drawbacks of this
expression system are: (i) the DNA sequence to be inserted has a maxi-
mum size of 2.5 kb; (ii) the host selection is limited because the viral
infection is cell-specific; and (iii) successful recombinant expression is
variable and unpredictable (Levinson, 1990).
Adenoviruses possess a genome of about 35 kb, which allows insertion
of a larger heterologous sequence without affecting viral replication. This
expression system offers high expression levels since viral infection inhi-
bits synthesis of cellular proteins and, in the case of type-2 adenoviruses,
they allow the use of a wide range of host cells (i.e. human, primate,
rodent). Owing to the reduced number of restriction sites available in the
adenoviral genome, insertion of foreign sequences is performed by homo-
logous recombination instead of molecular cloning. In this respect, the
viral DNA is co-transfected with a plasmid containing the gene of interest
Cloning and expression of heterologous proteins in animal cells 45

flanked by the regions of the viral genome where recombination will take
place. The frequency of recombination is variable and the generation of
stocks of recombinant virus requires purification of the viral progeny.
Sequences up to 8 kb can be inserted into and expressed by adenoviruses.
Heterologous promoters (from SV-40 or cytomegalovirus) have been
successfully used to express genes with this system (Sandig et al., 1997).
The cell line HEK-293 has been generated to complement a region absent
in recombinant adenoviruses, thus obviating the need for a helper virus to
complete the viral cycle. The adenoviruses are reliable vectors for the
production of recombinant proteins. The generation of novel adenoviral
vectors is described in detail by Bourbeau et al. (2003).
The adeno-associated viruses (AAVs) belong to the Parvovirus family,
contain a small genome (5 kb), and are not pathogenic. AAVs have a
biphasic life cycle in the host cell. They can either integrate to the host
genome or persist in an episomal form, both mechanisms leading to a
latent infection in the absence of a helper virus. In the presence of a
helper virus (i.e. adenovirus or herpesvirus), AAVs undergo a productive
infection. In addition to the lack of pathogenicity, these vectors present
other advantages such as high transfection efficiency, reduced cytotoxi-
city at elevated multiplicity of infection, and a wide range of hosts. The
main limitation of these vectors lies in the size of the gene that can be
expressed (, 5 kb). Although the rate of integration of AAVs to the host
genome is low, undesirable chromosomal mutations can be expected
when employing the helper virus approach (Xiao, 2003). The recent
discovery of the minimal set of adenoviral genes required for efficient
generation of progeny AAV particles (Matsushita et al., 1998) allows the
production of recombinant AAVs without the need of adenoviral co-
infection.
Epstein–Barr virus (EBV) is a member of the herpesvirus family that
became useful as an expression vector for mammalian cells. A peculiarity
of this virus is that it can develop either a latent infection or a lytic cycle in
the host. The first leads to transformation/immortalization of the host cell,
and the second to cell lysis. The identification of the elements necessary
for EBV replication extends the range of permissive cells from human B
lymphocytes to fibroblastic and epithelial cells of human, primate, and
canine origin (Sugden et al., 1985; Lutfalla et al., 1989). The use of this
virus as an expression vector offers stable transfection and high levels of
gene expression, simplicity for the selection of highly producing clones,
easy recovery of the episomal DNA, and the possibility to produce
authentic human glycoproteins if human cells are used as the host
(Teshigawara and Katsura, 1992). Some disadvantages of the EBV-based
expression systems are the need for a selection marker to assure the
persistence of the DNA in an extrachromosomal form, a certain degree of
variability in the expression levels depending on the cell type, and the
probability of genomic integration with a consequent reduction in the
expression rate of the heterologous gene (Levinson, 1990).
Another member of this family is HSV (herpes simplex virus). HSV has
a genome size of 125 kb, although half of the genes are not essential for
growth, allowing large molecular inserts. HSVs infect a wide range of
hosts and show a high infectivity. The relative simplicity of preparing large
46 Animal Cell Technology

stocks of recombinant virus is another advantage presented by HSV
(Burton et al., 2003).
Papillomaviruses contain a genome of 8 kb, usually have a single host
and replicate episomally. Bovine papillomavirus (BPV) is a representative
of this family, which induces phenotypic changes in the host facilitating
the identification and selection of transformants. Although BPV-based
vectors replicate episomally, the selection pressure achieved by the addi-
tion of selection markers (i.e. neomycin or cadmium; see Section 3.8) has
been reported to induce a stable integration of multiple copies of the viral
DNA into the host genome (Niwa et al., 1991). The susceptibility of the
virus to undergo rearrangements with the concomitant risk of altering the
sequence of interest is perhaps its main drawback (Levinson, 1990). The
optimization of the expression system based on papillomavirus has been
thwarted by the lack of progress in understanding the biology of the viral
replication and transcription.
Vaccinia virus is a representative of the Poxviruses. Members of this
family are unique among vertebrate viruses because they possess their own
transcription machinery encoded in a genome of 100–300 kb. The fact that
the host metabolism is dispensable allows vaccinia to have a high rate of
replication and protein biosynthesis, making it an excellent choice for the
expression of heterologous genes (Moss, 1996). Mammalian and avian cells
are suitable hosts for vaccinia. The viral genome can accept DNA frag-
ments up to 25 kb, which are incorporated by homologous recombina-
tion, where in general, the gene encoding for viral thymidine kinase (TK)
is replaced and selection of recombinant virus is carried out in TK-
deficient cells (Mackett et al., 1982). It is worthwhile to note that the
transcription mechanism of vaccinia is unable to perform splicing; there-
fore, this vector allows only the expression of complementary DNA
(cDNA) sequences (Moss, 1996). Poxvirus-based systems are commonly
used in eukaryotic cells due to the stability of the viral genome, the wide
range of hosts, the ease of production and manipulation of the virus, and
the high expression levels (Carroll and Kovacs, 2003).
Vectors derived from RNA viruses
In retroviruses, the genetic information is encoded in the form of RNA
(5–10 kb), which is retro-transcribed to DNA in the host cell by means of
a virus-specific reversed transcriptase enzyme. Since this intermediate
DNA cannot integrate into the genome of the infected cell, retroviruses
are employed as transference and expression vectors (Holmes-Son et al.,
2001). Several cell lines that enable the production of defective recombi-
nant retroviruses have been generated to circumvent the use of helper virus
(Cone and Mulligan, 1984). The use of retrovirus as an expression vector
presents the following advantages: (i) it allows the expression of genes in
different cell types and species; (ii) stable recombinant cell lines can be
obtained since the genetic material of the virus integrates reliably and
stably in the host genome, and the viral infection does not produce cell
death; (iii) it allows increased infectivity and higher viral titers; (iv) it
allows high efficiency for introduction of foreign genes in animal cells
when compared with the commonly used transfection methods. Two
Cloning and expression of heterologous proteins in animal cells 47

relevant points should be considered before employing retroviral vectors:
(i) although they can express genomic sequences containing introns, the
progeny will not harbor them, only copies of the cDNA; (ii) polyadenyla-
tion signals in the target sequence must be avoided because they induce a
premature polyadenylation and the consequent absence of full mRNAs
(Shimotohno and Temin, 1981). The acceptable yields obtained with retro-
viral vectors are explained either by their capacity to integrate into
transcriptionally active loci or, once inserted, they trigger the activation of
the loci (Kaufman, 1990). However, in terms of biotechnological applica-
tions, retroviruses can hardly compete with other viral vectors and present
the following limitations: the size of the foreign DNA to be expressed
(, 6–7 kb), the moderate and variable expression level (one or few copies
of the retrovirus integrate into the chromosomes), and the incapacity to
co-express two heterologous sequences from a single retrovirus due to
epigenic effects (Emerman and Temin, 1984). Parolı´n and Palu´ (2003) have
provided an excellent review on recent progress in the design and applica-
tions of retroviral vectors.
Within the family of Togavirus, two members of the genus Alfavirus,
namely Sindbis virus and Semliki Forest virus, stand out for their use in
biotechnology. They are very simple RNA viruses with a genome encod-
ing for replication signals, structural proteins and for an RNA-dependent
polymerase. The main expression product of the infected cells is the
heterologous gene, since the viral infection down-regulates the synthesis
of host proteins (Strauss and Strauss, 1994). The generation and manipula-
tion of these vectors is simple, and well-established methods exist for the
production of recombinant proteins in different cell lines on a large scale
(Blasey et al., 1997). Lundstrom (2003) has recently outlined the applica-
tions of alfavirus as expression systems.
Among the single-strand RNA viruses, coronaviruses present the
largest genome allowing the insertion of large heterologous sequences
(27 kb). The virus replicates in the cellular cytoplasm and does not
undergo an intermediate stage as DNA. Therefore, its integration into the
host genome is an unlikely phenomenon. A description of the different
strategies available nowadays for the generation and use of coronaviruses
as expression vectors has been published by Enjuanes et al. (2003).
3.4.2 Baculoviruses
Baculoviruses encompass a family of viruses that infect different species of
arthropod insects. The name derives from the fact that the DNA genome,
with a size between 80 and 200 kb, is packed in nucleocapsids that acquire
the shape of a walking cane or baculo. A schematic representation of the
life cycle of the virus is shown in Figure 3.1. The polyhedrin gene is not
essential for virus growth in cell cultures and, if deleted, extracellular viral
particles (ECVs) will be released from the cells. This constitutes the basis
for the use of baculovirus as an expression system: the polyhedrin gene is
replaced by the sequence of interest, whose transcription will, thus, be
under the control of the potent polyhedrin promoter. The recombinant
expression unit is transferred by homologous recombination to the locus
of the polyhedrin gene of a wild-type virus.
48 Animal Cell Technology
There are three different approaches to insert genes in a baculovirus
genome: (i) by homologous recombination; (ii) by site-specific transposi-
tion to a bacmid (a modified Escherichia coli that contains a copy of the
baculovirus genome) mediated by the transposon 7 (Tn7); or (iii) by
molecular cloning (Condreay and Kost, 2003). Transcriptional promoters
that operate in insect cells are not active in mammalian cells; therefore,
baculovirus-based expression can only be achieved by replacing the poly-
hedrin promoter with CMV or SV-40 promoter sequences (pCMV,
pSV-40). These modified vectors display an extensive diversity of hosts
(hematopoietic cells excepted) but an expression level that is cell line-
dependent. Variability of expression is, at least partly, associated with
transcriptional silencing of the expression cassette as a result of its
incorporation into the nucleosomes in the genomic DNA (Condreay et
al., 1999). Besides its application for the transient expression of recombi-
nant proteins, it is also possible to obtain stable cell lines by adding to the
vector a cassette with a biochemical marker (Merrihew et al., 2001). The
baculovirus system offers: (i) versatility and diversity of hosts; (ii) absence
Fusion
Solubilization of
protein matrix
(insect gut)
Ingestion
Secondary
infection
Secondary
infection
Primary infection (insect)
Replication
Occlusion
Endocytosis
OV
ECV
Figure 3.1
Life cycle of baculoviruses. The infection of insect cells with baculovirus produces
two types of progeny, the extracellular (ECV) and occluded (OV) viral particles,
which differ structurally and functionally. The nucleocapsid of ECVs is surrounded
byamembraneenvelopeandtheseparticlescausein vitro cell to cell infection but
are not infective to insects. In contrast, the nucleocapsid of OVs is coated by a
protein matrix, formed by polyhedrin or granulin, for the nuclear polyhedrosis or
granulosis virus, respectively. OVs are responsible for de novo infection of insects
but cannot infect c ells cultivated in vitro. The matrix protects the particles against
environmental agents (e.g. UV light, drying), but is degraded at the alkaline pH of
the insect gut. The removal of the protein coat enables the infection of the gut
cells by the virus, which migrates to the nucleus to accomplish the replication and
transcription of its genetic material. At this stage the two viral progenies can be
formed from the nucleocapsids: those accumulated in the nucleus and assembled
to the polyhedrin or granulin give origin to OVs, while those emerging from the
nucleus and cell membrane are ECVs.
Cloning and expression of heterologous proteins in animal cells 49

of cytotoxicity; (iii) transient or stable gene expression; (iv) integration as
a single copy to the genome; (v) easy manipulation and scaling-up of the
production; (vi) stability and conservation of the viral particles; (vii)
capacity to accept large inserts (40 kb); and (viii) high biosafety since
baculovirus is only able to replicate in invertebrates (King and Possee,
1992). The main limitations of this system are related to the use of insect
cells as a platform for the production of recombinant proteins. In this
respect, the expression is discontinuous (batch), because of cell lysis/death,
and the glycosylation pattern is simpler than that of mammalian cells (with
relatively unbranched sugar chains) and with a high content of mannose.
This is usually undesirable for some proteins with therapeutic application
(see Chapter 6 and Luckow, 1995).
3.4.3 Plasmid vectors
The discovery and functional characterization of several of the elements
that control the expression of genes in eukaryotic cells and viruses, in
addition to progress in the field of genetic engineering, allowed the
generation of plasmid expression vectors. These types of vectors consist of
naked DNA with a size between 2 and 20 kb. The genetic elements they
contain dictate whether they will exist as episomes or integrated into the
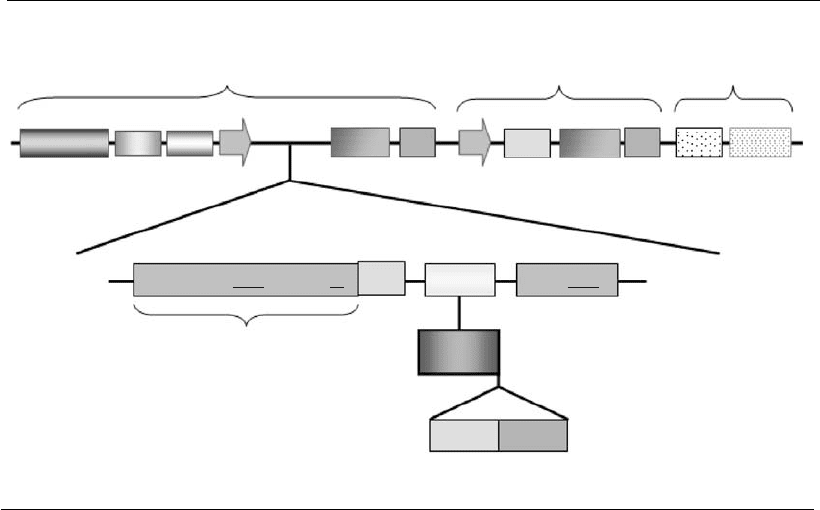
host genome. The basic structure of the plasmid employed for expression
in animal cells is made up of three cassettes: (i) an eukaryotic expression
unit; (ii) a biochemical marker; and (iii) a prokaryotic replication unit (see
Figure 3.2). The first cassette drives the expression of the exogenous gene.
The biochemical marker allows for selection of clones that have stably
integrated the plasmid DNA into their genome (see Section 3.8). In this
cassette, the expression of the selection gene is most preferably dictated by
a weak promoter to increase the chances of isolating high producer cell
lines (Wurm, 2004). All the genetic manipulations required during the
construction of the recombinant plasmids, as well as their amplification
(replication) in sufficient amounts for future transfections, are routinely
carried out in E. coli. Thus, unlike the viral vectors, plasmids also contain
sequences controlling their replication and selection in a prokaryotic host.
Some novel elements of plasmids, which have extended significantly the
range of applications in the biotechnology area, are discussed below.
Inducible and specific promoters. The expression of a heterologous
gene from a constitutive promoter is not recommended if the recombinant
product is cytotoxic or affects cell growth. In such a case, it will be
necessary to regulate the expression levels that can be achieved by means
of an inducible promoter. An ideal inducible system should meet the
following requirements. (i) Specificity: unresponsive to endogenous acti-
vators and absence of interference with the host physiology. (ii) Efficiency:
null to low expression levels in the non-induced state, and quick response
to activation or induction. (iii) Dose dependency: homogenous regulation
of the expression levels. Several sequences and ligands that regulate posi-
tively or negatively the transcriptional promoters of a large number of
animal cells have been identified and incorporated into expression vectors.
These elements can respond to different external stimuli such as heat
shock, hormones, heavy metals, cytokines, or hypoxia. However, these
50 Animal Cell Technology
stimuli present pleiotropic effects in the host cells, compromising their
specificity. To overcome this, regulatory elements of evolutionary distant
species are preferred, the most common being the lactose- (Lac), tetracy-
cline- (Tet) or erythromycin- (E; isolated from E. coli), streptogramin or
pristinamycin-operon/repressor (PIP; originating from Streptomyces coeli-
color or S. pristinaespiralis). Operators are DNA sequences that, bound to
specific molecules (polypeptides, metabolites, etc.), can diminish or in-
crease the affinity of the RNA polymerase for the neighboring promoter,
thus repressing or promoting the transcriptional activity of the adjacent
gene. In the case of the lactose operon, the inducer is isopropyl 1-thio -
D-galactopyranoside (IPTG), which binds to the lactose repressor protein,
alters its conformation, and results in the dissociation of the protein from
the operon, thus de-repressing the expression of the contiguous gene. One
of the disadvantages of this system is that IPTG is toxic at high concentra-
tions (50 mM), restricting its application on a large scale (Makrides, 1999).
Ward et al. (1995), using a recombinant vaccinia virus, designed a thermo-
regulable variant of the lac repressor that showed a temperature-dependent
Eukaryotic expression cassette
Eukaryotic
selection marker
Prokaryotic
cassette
polyA
polyA
SV-40 ori
SE
*
Enh
PTT
TT
P
Neo
Amp
ColE1
5 UTR⬘
3 UTR⬘
GCCGCC( )CCATGA/G G
ts
TE
MCS
ORF
*
PCS FUS
TAA( )A/G
**
Figure 3.2
Basic and optional component s of an expression vector for animal cells. The basic structure of a
plasmid vector can be divided into three cassettes: (1) a eukaryotic expression unit containing as
basic elements: promoter (p), enhancer (Enh), eukaryotic origin of replication (e.g. SV40 ori) if an
episomal replication is preferred, polyadenylation (polyA) and transcription termination signals (TT),
and a multiple cloning site (MCS), where the sequence of interest (ORF) is inserted. Other additional
elements that can be included are: SARs or LCRs (structural elements, SE), targeting signal (ts),
sequence encoding for a fusion protein or tag (FUS) and a protease cleavage site (PCS). The gene to
be expressed (ORF) should contain the Kozak sequence or translational enhancer (TE) and an
appropriate stop codon (TAA); (2) a biochemical marker (e.g. Neo – neomycin-phosphotransferase)
containing the basic elements that regulate its transcription; and (3) a prokaryotic replication unit,
composed of a replication origin (ColE1) and a selection marker (Amp, ampicillin) that allows the
genetic manipulation of the vect or in E. coli. Asterisks indicate optional elements.
Cloning and expression of heterologous proteins in animal cells 51
