Casing/Tubing design manual october 2005 Chevron
Подождите немного. Документ загружается.

Casing/Tubing Design Manual 13-27
October 2005
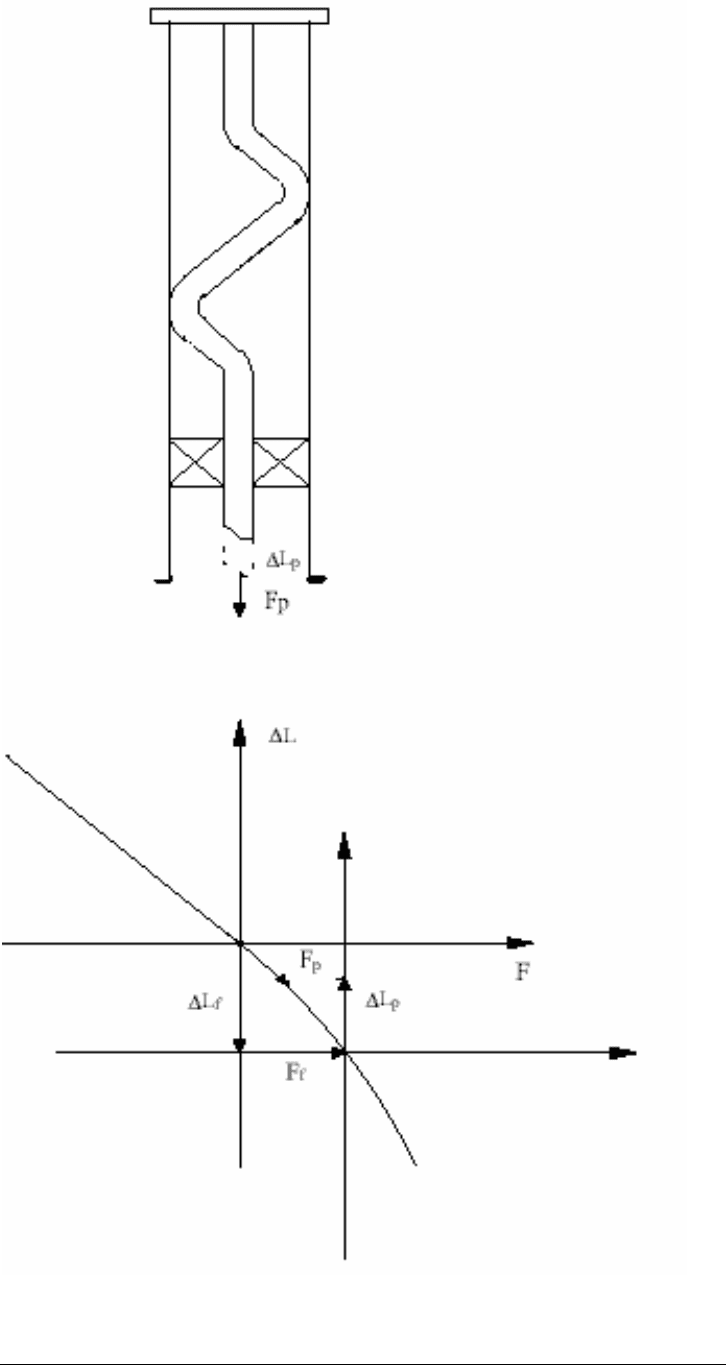
Figure 13-15. Graphical Representation of Movement
13-28 Casing/Tubing Design Manual
October 2005
13.15.2 Floating Tubing
Another method which may be used in some types of completions is that the
tubing is fully or partially limited in down-hole movement. In this method after the
packer is set, some of the weight of the string is set down on the packer, putting
the tubing into compression or slackened off (refer to Figure 13-16).
Figure 13-16. Limited Downward Movement
The shortening of the string caused by, ∆Pso, makes it possible to limit the length
variations of the string, for example, during an injection operation. Therefore,
∆Ltot, i.e., the total length variation calculated as the sum of the above described
effects, is decreased by ∆Lso. The ∆Lso value is determined using the following
formula:
EIw
rF
EA
LF
L
So
S
So
So
8
22
−−=Λ
(13-20)
where:
Fso = slack-off force released on the packer.

Casing/Tubing Design Manual 13-29
October 2005
With this type of anchoring it is, therefore, possible to limit the movements of the
tubing with respect to the packer and consequently the length of the packer seal-
assembly. If an anchored type constraint is considered, then the tubing-packer
force with respect to the anchored tubing can be reduced, e.g., in an injection
operation.
In practice, applying slack-off is the same as moving the packer upwards by
∆Lso, compressing the string and thus causing part of the length variation, which
would occur in any case at a later stage because of the effects described above.
The same considerations can be made if ∆Ltot < 0 during the operation while,
conversely, any elongation of the string would be prevented causing a force on
the packer which would be equal to that of the slack-off amount.
13.15.3 Landing Conditions
A particular problem arises in tubing tied to packer completions when using
hydraulic set packers. As pressure is applied to the tubing to set the packer, it
changes the length of the tubing during the setting process. In turn, this places
stress in the tubing after the packer is set and the pressure is bled off. This stress
needs to be taken into account to determine the total stress applied to the tubing.
Hydraulic packers are set by plugging the tubing below the packer either by
dropping a setting ball onto a shear-out ball seat or by installing a plug with
wireline.
The formulae for determine tubing length change are:
S
a
EA
LF
L
Λ
−=Λ
1
Hooke's Law
L
R
P
E
v
L
im
⎟
⎠
⎞
⎜
⎝
⎛
−
Λ
−=Λ
1
2
2
3
Ballooning
Where:
ii
PAF
Λ
−
=
Λ
α
iim
PP
Λ
=
Λ
13.16 Materials and Corrosion
A production well design should attempt to contain produced corrosive fluids
within tubing. They should not be produced through the casing/tubing annulus.
However, it is accepted that tubing leaks and pressured annuli are a fact of life.
As such, production casing strings are considered to be subject to corrosive
environments when designing casing for a well where hydrogen sulfide (H
2
S) or
carbon dioxide (CO
2
) laden reservoir fluids can be expected.
During the drilling phase, if there is any likelihood of a sour corrosive influx
occurring, consideration should be given to setting a sour service casing string

13-30 Casing/Tubing Design Manual
October 2005
before drilling into the reservoir. The BOP stack and wellhead components must
also be suitable for sour service.
13.16.1 Corrosion Considerations for
Development Wells
Casing corrosion considerations for development wells can be confined to the
production casing only.
Internal Corrosion
The well should be designed to contain any corrosive fluids (produced or
injected) within the tubing string by using premium connections. Any part of the
production casing that is likely to be exposed to the corrosive environment,
during routine completion/work over operations or in the event of a tubing or
wellhead leak, should be designed to withstand such an environment.
External Corrosion
Where the likelihood of external corrosion because of electrochemical activity is
high and the consequences of such corrosion are serious, the production casing
should be cathodically protected (either cathodically or by selecting a casing
grade suitable for the expected corrosion environment).
13.16.2 Contributing Factors to Corrosion
Most corrosion problems occur in oilfield production operations because of the
presence of water. Whether present in large amounts or in extremely small
quantities, it is necessary to the corrosion process. In the presence of water,
corrosion is an electrolytic process where electrical current flows during the
corrosion process. To have a flow of current, there must be a generating or
voltage source in a completed electrical circuit.
The existence, if any, of the following conditions alone or in any combination may
be a contributing factor to the initiation and perpetuation of corrosion:
• Oxygen (O2). Oxygen dissolved in water drastically increases its
corrosive potential. It can cause severe corrosion at very low
concentrations of less than 1.0 ppm. The solubility of oxygen in water is
a function of pressure, temperature, and chloride content. Oxygen is less
soluble in salt water than in fresh water. Oxygen usually causes pitting in
steels.
• Hydrogen Sulfide (H
2
S). Hydrogen sulfide is very soluble in water and,
when dissolved, behaves as a weak acid and usually causes pitting.
Attack due to the presence of dissolved hydrogen sulfide is referred to as
“sour” corrosion. The combination of H
2
S and CO
2
is more aggressive
than H
2
S alone and is frequently found in oilfield environments. Other
serious problems, which may result from H
2
S corrosion, are hydrogen
blistering and sulfide stress cracking. It should be pointed out that H
2
S
also can be generated by introduced micro-organisms.

Casing/Tubing Design Manual 13-31
October 2005
• Carbon Dioxide (CO
2
) When carbon dioxide dissolves in water, it forms
carbonic acid, decreases the pH of the water, and increase its
corrosivity. It is not as corrosive as oxygen, but usually also results in
pitting. The important factors governing the solubility of carbon dioxide
are pressure, temperature, and composition of the water. Pressure
increases the solubility to lower the pH; temperature decreases the
solubility to raise the pH. Corrosion primarily caused by dissolved carbon
dioxide is commonly called “sweet” corrosion.
• Using the partial pressure of carbon dioxide as a yardstick to predict
corrosion, the following relationships have been found:
o Partial pressure >30 psi usually indicates high corrosion risk.
o Partial pressure 3 to 30 psi may indicate high corrosion risk.
o Partial pressure <3 psi generally is considered non corrosive.
• Temperature. As with most chemical reactions, corrosion rates generally
increase with increasing temperature.
• Pressure. Pressure affects the rates of chemical reactions and corrosion
reactions are no exception. In oilfield systems, the primary importance of
pressure is its effect on dissolved gases. More gas goes into solution as
the pressure is increased and this may increase the corrosivity of the
solution.
• Velocity of fluids within the environment. Stagnant or low velocity fluids
usually give low corrosion rates, but pitting is more likely. Corrosion rates
usually increase with velocity as the corrosion scale is removed from the
casing exposing fresh metal for further corrosion. High velocities and/or
the presence of suspended solids or gas bubbles can lead to erosion,
corrosion, impingement, or cavitation.
13.16.3 Forms of Corrosion
The following forms of corrosion are addressed in this manual:
• Corrosion caused by H
2
S (Sulphide Stress Cracking)
• Corrosion caused by CO
2
and Cl
• Corrosion caused by combinations of H
2
S, CO
2,
and Cl
Corrosion in injection wells and the effects of pH and souring are not included.
The procedure adopted to evaluate the corrosivity of the produced fluid and the
methodology used to calculate the partial pressures of H
2
S and CO
2
will be
illustrated in the following sub-sections.
13.16.3.1 Sulfide Stress Cracking (SSC)
The SSC phenomenon usually occurs at temperatures below 80°C and with the
presence of stress in the material. The H
2
S comes into contact with H
2
O, which is
an essential element in this form of corrosion, by freeing the H+ ion. Higher
temperatures e.g., above 80°C, inhibit the SSC phenomenon; therefore,

13-32 Casing/Tubing Design Manual
October 2005
knowledge of temperature gradients is very useful in the choice of the tubular
materials because differing materials can be chosen for various depths.
Evaluation of the SSC problem depends on the type of well being investigated. In
gas wells, gas saturation with water will produce condensate water and,
therefore, create the conditions for SSC. In oil wells, two separate cases need to
be considered, vertical and deviated wells. The cases are:
1. In vertical oil wells, generally corrosion occurs only when the water cut
becomes higher than 15%, which is the “threshold” or the “critical level,” and
it is necessary to analyze the water cut profile throughout the producing life
of the well.
2. In highly deviated wells (i.e., deviations >80 degrees), the risk of corrosion by
H
2
S is higher because the water, even if in very small quantities, deposits on
the surface of the tubulars. The problem can be likened to the gas well case
where the critical threshold for the water cut drops to 1% (WC <1%).
The following formulae are used to calculate the value of pH
2
S (partial pressure
of H
2
S) in both the cases of gas (or condensate gas) wells or oil wells.
The potential for SSC occurring is evaluated by studying the water cut values
combined with the type of well and deviation profile. If the conditions specified
above are verified, then the pH
2
S can be calculated.
13.16.3.1.1 Gas or Condensate Gas Well
H
2
S partial pressure is calculated by:
pH
2
S = SBHP x Y(H
2
S)/100 (13-21)
where:
SBHP = Static bottomhole pressure [atm]
Y(H
2
S) = Mole fraction of H
2
S
pH
2
S = Partial H
2
S pressure [atm]
SSC is triggered at pH
2
S >0.0035 atm and SBHP >4.5 atm.
13.16.3.1.2 Oil-Bearing Well
The problem of SSC exists when there is wetting water; i.e.:
• Water cut >15% for vertical wells
• Water cut >1% for horizontal or highly deviated wells (>80
o
)
• If the GOR >800 Nm 3 /m 3
The pH
2
S calculation is different for undersaturated and oversaturated oil.
13.16.3.2 Undersaturated Oil
Oil where the gas remains dissolved because the wellhead and bottom-hole
pressures are higher than the bubblepoint pressure (Pb) at reservoir temperature
is termed “undersaturated.”

Casing/Tubing Design Manual 13-33
October 2005
In this case, the pH
2
S is calculated in two ways:
• Basic method
• Material balance method
If the quantity of H
2
S in gas at the bubblepoint pressure [mole fraction = Y(H
2
S)],
is unknown or the values obtained are not reliable, the pH
2
S is calculated using
both methods and the higher of the two results is taken as the a reliable value.
Otherwise, the basic method is used.
Basic Method
This method is used, without comparison with the other method, when the H
2
S
value in the separated gas at bubblepoint conditions is known and is reliable or if
Y(H
2
S), molar fraction in the separated gas at bubblepoint pressure (Pb) is higher
than 2%.
The pH
2
S is calculated by:
pH
2
S = Pb x Y(H
2
S)/100 (2@) (13-22)
where:
Pb = Bubblepoint pressure at reservoir temperature [atm]
Y(H
2
S) = Mole fraction in the separated gas at bubblepoint (from PVT data if
extrapolated)
pH
2
S = Partial H
2
S pressure [atm]
Material Balance Method
This method is used when data from production testing is available and/or when
the quantity of H
2
S is very small (<2,000 ppm) and the water cut value from is
lower than 5%. (This method cannot be used when the water cut values are
higher.) The value of H
2
S in ppm to be used in the calculation must also be from
stable flowing conditions.
NOTE: H
2
S sampled in short production tests is generally lower than the actual
value under stabilized conditions.
The following algorithm is used to calculate the pH
2
S:
pH
2
S is calculated at the separator (pH
2
Ssep)
pH
2
Ssep= (Psep x H
2
Ssep)/106 (13-23)
where:
Psep = Absolute mean pressure at which the separator works (from tests) in atm
H
2
Ssep= Mean H
2
SH2S value in the separator gas (generally measured in ppm)
The mean molecular weight of the produced oil, PM:

13-34 Casing/Tubing Design Manual
October 2005
()
⎟
⎠
⎞
⎜
⎝
⎛
−
+
=
6.23
29
6.23
1000
1000
GOR
PM
d
GOR
giac
PM
γ
γ
(13-24)
where:
PM = mean molecular weight of the reservoir
⎥
⎥
⎥
⎥
⎦
⎤
⎢
⎢
⎢
⎢
⎣
⎡
⎟
⎠
⎞
⎜
⎝
⎛
=
∑
=
100
1
n
i
ii
MC
PM
Ci = Mole % of the ith component of the reservoir oil
Mi = Molecular weight of the ith component of the reservoir oil
d = Density of the gas at separator conditions referred to air =1
The quantity of H
2
S in moles/liter dissolved in the separator oil is calculated:
[H
2
S]oil = (pH2Ssep/H1 x (
γ
x 1000)/ PM ) (13-25)
where:
H1 = Henry constant of the produced oil at separator temperature (atm/Mole
fraction). See the procedure for calculating the Henry constant.
PM = Mean molecular weight of the produced oil
γ = Specific weight g/l of the produced oil
The quantity of H
2
S in the gas in equilibrium is calculated (per liter of oil):
[ H
2
S]gas = (GOR/23.6 x H
2
Ssep/10 6 ) (13-26)
where:
GOR = Gas oil ratio Nm 3 /m 3 (from production tests)
23.6 = Conversion factor
The pH
2
S is calculated at reservoir conditions:
pH
2
S = (([H2S]oil + [H
2
S]gas)/K ) x H2 (13-27)
where:
K =(g x 1,000/ PM + GOR/23.6) total number of moles of the liquid phase in the
reservoir
H
2
= Henry constant for the reservoir temperature and reservoir oil (see 13.16.3.3
Procedure for Calculating Henry Constant).
In general, H
2
S corrosion can occur at either the wellhead or bottom hole without
distinction. There is SSC potential if pH
2
S >0.0035 atm and STHP >18.63 atm.

Casing/Tubing Design Manual 13-35
October 2005
13.16.3.3 Procedure for Calculating the Henry Constant
The value of the Henry constant is a function of the temperature measured at the
separator. The mapping method can be applied for temperatures at the separator
of between 20°C and 200°C given the diagram in Figure 13-17, which represents
the functions H(t) for the three types of oils:
1. Heptane PM =100
2. N-propyl benzene PM = 120
3. Methylnaphthalene PM =142
13.16.3.3.1 Remarks on the H1 Calculation
Having calculated the molecular weight of the produced oil PM using the formula
in equation 5d the reference curve is chosen (given by points) to calculate the
Henry constant on the basis of the following value thresholds:
• If PM > 142, the H(t) curve of methylnaphthalene is used.
• If PM > 120, the H(t) curve of propyl benzene is used.
• If PM > 100, the H(t) curve of heptane is used.
• If 100 <PM <120, the mean value is calculated using the H(t) curve of
propyl benzene and the H(t) curve of methylnaphthalene.
• If 120 <PM <142 the mean value is calculated using the H(t) curve of
heptane and the H(t) curve of propyl benzene.
• Given FTHT, wellhead flowing temperature, the H1 value is interpolated
linearly on the chosen curve(s). For this purpose, the temperature values
immediately before and after the temperature studied are taken into
consideration.
13.16.3.3.2 Comments on the H2 Calculation
Having calculated the molecular weight of the reservoir oil PMres using
temperature measured at the separator, H2 is measured in a similar way as H1.

13-36 Casing/Tubing Design Manual
October 2005
Figure 13-17. H(t) Reference Curves
13.16.3.4 Oversaturated Oil
Oil is considered oversaturated when the gas in the fluid separates because the
pressure of the system is lower than the bubblepoint pressure. Two situations
can arise:
Case A
• FTHP <Pb
• FBHP >Pb
Case B
• FTHP <Pb
• FBHP <Pb
13.16.3.4.1 Calculation of Partial Pressure in Case A
Calculation is of the partial pressure in the reservoir: In this case, pH
2
S is
calculated in the way described for undersaturated oil. The calculation is of the
partial pressure at the wellhead, i.e., when FTHP <Pb: The data result from the
production conditions and only the basic method is used.
Basic Method
pH2S = STHP x Y(H
2
S)/100 (13-28)
where:
STHP = Static tubing head pressure [atm]
