Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


these sensations, which are translated as ‘aroma.’
According to international praxis, flavors are divided
into three categories – natural, nature identical, and
artificial. (See Sensory Evaluation: Taste.)
0010 Natural flavors are mainly based on essential oils
and extracts where natural aromatic substances dom-
inate. In addition to the aromatic components, they
also contain constituent parts from the fruit, which
change during storage and can worsen the taste of the
drink. For this reason, these natural aromatic sub-
stances are sometimes strengthened with certain
identical aromatic substances that contain aromatic
components only and give a better stability to the
taste. (See Essential Oils: Properties and Uses;
Isolation and Production.)
0011 Nature-identical flavors are based on ingredients as
they occur in the natural fruit but which are produced
by chemical methods. They have a good solubility, a
reasonable price and a long shelf-life, which makes
them very suitable for soft drinks production.
0012 Artificial flavors contain chemicals that do not
occur in nature but have a familiar taste such as
vanillin, which has the taste of vanilla. This is an
ever-decreasing category and in time will be replaced
by nature-identical flavors.
Water
0013 Water is the main raw material in soft drinks. In
weight terms, a soft drink is 90% water. Water of
high quality is therefore important. It must be pure
and from a source approved by the regulatory author-
ities for drinking purposes. This means that it must be
free from biological and chemical pollutants, such as
bacteria and poisonous substances. It should also
have the cleanest taste and be least interactive and
microbiologically stable. The quality of water has an
impact on the quality and taste of the soft drink.
Sweeteners
0014 Sweeteners can be divided into two main categories:
nutritive or caloric sweeteners and nonnutritive or
low calorie sweeteners. We will discuss only the
sweeteners important for the soft-drink industry.
Sugar
0015 Sugar is a generic name for a class of sweet carbohy-
drates, including fructose, glucose, maltose, sucrose,
and lactose, that are readily soluble in water, color-
less, odorless, and usually crystallizable.
0016 For soft drinks, the most commonly used is sucrose,
which can be supplied either as a liquid or in
granulated form. Liquid sugar is dissolved granulated
sugar in water, normally at 67
Brix.
0017Sugar is an important ingredient for soft drinks, not
only because of its properties as a sweetener, but also
because it is highly nutritious and is an important
carrier of the fruit aromas. The different fruit aromas
require different quantities of sugar, depending on
which fruits are in the drink. This means that the
composition of the sugar solution is critical if the
drink is not to be too ‘thin’ or taste too sugary. Two
other types of sugar are found in fruit – fructose (fruit
sugar) and glucose (grape sugar). Fructose is 50%
sweeter than ordinary sugar, but contains the same
number of calories per gram. A smaller quantity of
fructose gives the same sweetness. It is therefore
sometimes used to reduce the number of calories.
Honey is another less commonly used sweetener
that also contains glucose and fructose. (See Carbo-
hydrates: Classification and Properties; Glucose: Pro-
perties and Analysis; Honey; Lactose; Sucrose:
Properties and Determination; Dietary Importance.)
Nonnutritive Sweeteners
0018In the past, low-calorie drinks were only marketed as
diabetic products. Now, they have become increas-
ingly popular for both weight-watching and eco-
nomic reasons. Low-calorie drinks are an important
part of the total soft-drink market. When using a
nonnutritive sweetener, it is important to have up-
to-date information about the food regulations
concerning this subject, as they differ greatly from
country to country and are subject to many changes.
0019A sweetener has to comply with the following
standards, assuming it is allowed by the food laws
concerned:
.
0020It should not be hazardous to health.
.
0021It should give no off-taste.
Saccharin
0022Saccharin is a synthetic, white, crystalline powder, of
formula C
6
H
16
CONHSO
2
, which, in its pure state, is
550 times sweeter than sugar. In its commercial form,
saccharin is estimated to have a sweetening power
375 stronger than that of sugar.
0023The only synthetic, nonnutritive sweetener pres-
ently allowed in most countries in soft drinks is sac-
charin. It has been in use continuously since 1900.
The use of saccharin is mostly restricted to special
dietary foods and beverages appropriately labeled as
such.
0024For several years, saccharin has been under investi-
gation as a potential cause of cancer. Warning labels
were required on saccharin-containing foods. The
most recent studies indicate that it is a weak carcino-
gen in laboratory animals but does not, in moderate
use, present an increased cancer risk in humans. The
SOFT DRINKS/Chemical Composition 5347

recommended acceptable daily intake (ADI) is 1 g
per person per day. The tolerance level for soft drinks
is 0.4 mg ml
1
. This is an interim tolerance level,
and safety studies are continuing. (See Cancer:
Epidemiology; Carcinogens: Carcinogenic Substances
in Food: Mechanisms; Carcinogenicity Tests;
Saccharin.)
Cyclamate
0025 Cyclamate is an odorless, white crystalline powder.
The name usually denotes either calcium cyclamate or
sodium cyclamate, both of which are salts of cyclo-
hexylsulfamic acid. They have a very sweet taste, with
about 30 times the sweetening power of sucrose.
0026 After saccharin, cyclamate was the most commonly
used sweetener until it was banned in the USA in
1970, after which many countries followed suit.
Much of the scientific community doubted that
cyclamate was hazardous but for new FDA approval
more very costly research is needed and people would
rather concentrate on other sweeteners. (See Cycla-
mates.)
Aspartame
0027 Aspartame is a sweetener made from the natural
amino acids l-aspartic acid and l-phenylalanine.
l-Aspartic acid is tasteless, and l-phenylalanine is
slightly bitter, so the taste of aspartame could never
have been predicted. Aspartame was discovered by
accident in the laboratories of the Searle Company in
1965 and was first approved for use in food products
in Canada 15 years later. In 1983, approval was
granted by the FDA, followed by many authorities
in other countries. The only negative aspect of aspar-
tame is that it can be hazardous for people who suffer
from phenylketonuria, an inherited disorder that can
lead to brain damage in those exposed to phenylalan-
ine. This disease is rare, however. Since aspartame is
so sweet (about 200 times sweeter than saccharin),
only a small quantity is needed, and it is therefore put
into the same category as energy-free sweeteners. The
calorie content can be reduced by 95% compared
with corresponding drinks sweetened with saccha-
rates. During digestion, aspartame is treated as pro-
tein, and so diabetics are able to eat and drink food
sweetened with aspartame. (See Amino Acids: Proper-
ties and Occurrence; Aspartame; Protein: Chemistry;
Food Sources; Determination and Characterization;
Requirements; Functional Properties; Interactions
and Reactions Involved in Food Processing; Quality;
Digestion and Absorption of Protein and Nitrogen
Balance; Synthesis and Turnover; Deficiency; Heat
Treatment for Food Proteins; Sources of Food-grade
Protein.)
Acesulfame-K
0028Acesulfame-K was developed by Hoechst in 1973,
was approved by the British Food Regulations in
1983, and has since been adopted in countries like
Germany, Belgium, and Denmark. Acesulfame-K is
not metabolized at all and therefore has no calorific
value. It is absorbed quickly in the intestine, and
excretion is similarly fast. (See Acesulfame/Acesul-
phame.)
Stevia (
Stevia rebaudiana Bertoni
)
0029Stevia contains natural compounds, especially stevio-
side and rebaudioside A, that are estimated to be
150–400 times sweeter than saccharose. Used for
centuries in parts of South America, stevia has been
discovered in recent years by much of the calorie-
conscious modern world. It is now widely and legally
consumed by millions of people, from South Korea,
Israel, and the People’s Republic of China, but no
country has done more to demonstrate stevia’s dietary
and economic potential than Japan, where the herb
and its extract have been used since 1970s.
0030The Japanese, having subjected stevia extract to
extensive safety testing and found it without health
risk, now incorporate it in numerous food products,
including soft drinks.
Sucralose
0031Sucralose was approved by the FDA in 1998 for use in
a wide variety of food products including soft drinks.
Sucralose is a low-calorie, high-intensity sweetener
that is about 600 times sweeter than sugar. It is sold
under the brand name of ‘Splenda.’ Sucralose and
sucrose (sugar) have been shown to have similar
taste and flavor profiles.
0032A number of other fascinating low-calorie sweet-
eners are currently undergoing safety evaluations for
future use. These include alitame, a compound simi-
lar to aspartame that is remarkably 2000 times
sweeter than sucrose, and various naturally occurring
plant derivatives, such as stevia and thaumatin.
Acids
0033Acids are used as pH buffers in beverages and to
impart an astringent taste that offsets the sweetness
of the sugar and enhances or complements the associ-
ated flavor. Thus, the characteristic flavor of a bever-
age is developed in part through proper acidulation.
Acids also help in preventing microbiological spoilage.
0034All acids used in beverages must be ‘edible grade’
or ‘food grade.’ Those commonly used are citric-,
phosphoric-, and malic acid. Each has the property
of being weak and nonharmful at the concentrations
5348 SOFT DRINKS/Chemical Composition

used. Inherent chemical differences in the various
acids impart different taste characteristics, and
in substituting one acid for another, these differ-
ences have to be kept in mind. Table 2 shows
the acidification strength of some acids relative to
citric acid.
0035 The kind of acid and the concentration employed
are very important for adjusting the flavor of the
beverage.
Citric Acid
0036 Traces of citric acid are found in numerous plants and
animals, because it is a nearly universal intermediate
product of metabolism. Large amounts of the acid are
found in the juice of citric fruits. Fermentation of
sugar by Aspergillus niger is the chief commercial
source of the acid. Since it is a natural ingredient of
citrus fruits, it adapts itself well to beverages with
such a flavor.
0037 Citric acid may be used in the anhydrous form or
containing one molecule of water. It occurs as color-
less, translucent crystals or as a white, granular-to-
fine crystalline powder. It is odorless with a strong
acid taste, and the hydrous form is efflorescent in dry
air. (See Citrus Fruits: Types on the Market; Compos-
tion and Characterization; Oranges; Processed and
Derived Products of Oranges; Lemons; Grapefruits;
Limes.)
Phosphoric Acid
0038 Phosphoric acid is a very popular acidulant because
of its strength and very low cost; in fact, it is the
cheapest acid used in the industry. It is used mainly
in cola-type beverages, in which it improves not only
the acid taste but also the typical cola bite.
Malic Acid
0039 Malic acid is found in fruits such as apples, apricots,
and cherries. The food industry finds the tartness of
this additive a valuable flavor booster for candies,
jams, ice creams, fruit, and soft drinks. (See Ice
Cream: Methods of Manufacture; Properties and An-
alysis; Dietary Importance; Microbiology; Jams and
Preserves: Methods of Manufacture; Chemistry of
Manufacture; Sweets and Candies: Sugar Confec-
tionery.)
Carbon Dioxide
0040Carbon dioxide is present in the air in very small
quantities (approx. 0.03%) and is about 1.5 times as
dense as air. It is a colorless, odorless, and tasteless
gas and has a good solubility in water. When confined
within a suitable pressure vessel, CO
2
can exist as a
solid, liquid, or gas.
0041When drinking a carbonated beverage, the devel-
opment of CO
2
in the mouth, because of the warmth
of the throat, causes a light anesthetic effect on the
taste buds, which diminishes and sometimes even
stops the sensation of thirst. Carbon dioxide makes
the drink more refreshing through its stimulation of
the mouth’s mucous membranes. This adds to the
sensation that the soft drink is colder than it actually
is and also brings out the aroma.
0042Carbon dioxide also checks microbiological
growth, which means that the product lasts longer.
Many bacteria die after a certain time in carbonated
drinks. This applies equally to carbonated soft drinks,
mineral water, and beer. There are good grounds
for advising people, for hygienic reasons, to drink
carbonated drinks, particularly in hot climates. (See
Beers: History and Types.)
Preservatives
0043The low pH value of soft drinks is good for the life
of the product from a microbiological point of
view since bacterial growth is checked. Carbon
dioxide also has a positive effect. Sometimes, how-
ever, this is not enough, and preservatives have to
be used.
0044Preservation denotes any method that extends
the shelf-life of a product. Methods such as pick-
ling, freezing, drying, sterilizing, smoking, or the
addition of a chemical agent with a bactericidal ac-
tivity all have undesirable effects on the odor, taste,
or digestibility of the foodstuffs. (See Drying:
Theory of Air-drying; Freezing: Principles; Pickling;
Smoked Foods: Principles; Production; Sterilization
of Foods.)
0045The history of the soft-drink industry has demon-
strated that beverages are good nutrient media for
certain acidophilic microorganisms, particularly
yeasts. It has been demonstrated by various investi-
gations that over 90% of all cases of microbiological
spoilage of soft drinks are caused by yeast. Spoilage of
soft drinks by yeasts is evidenced by visible sediment,
gas formation, off-odor, off-taste, and changes in
beverage color and clarity.
tbl0002 Table 2 Acidification strength (%) relative to citric acid
Type Strength (%)
Citric acid 100
Tartaric acid 93
Malic acid 100
Ascorbic acid 43
Lactic acid 60
Phosphoric acid 125
SOFT DRINKS/Chemical Composition 5349

0046 To prevent soft drinks spoiling, it is necessary to
use good manufacturing practices to control the
growth of microorganisms. High hygienic standards
should be applied during production, together with
the creation of unfavorable environments for micro-
organisms, such as acidifying, cooling and pasteuriz-
ing, and, if necessary, chemical preservation. Only
small quantities of preservatives are allowed in soft
drinks. The preservatives allowed are subject to the
food and drugs laws of the different countries in-
volved. However, chemical preservation alone is not
sufficient. Therefore, these types of products must
always be pasteurized.
0047 The most common preservatives are sodium benzo-
ate (sodium or potassium sorbate can be used as a
replacement) and sulfur dioxide, particularly if it is
required to prevent the color darkening.
Benzoic Acid
0048 Benzoic acid or benzene-carbonic-acid is a monobasic
aromatic acid, moderately strong, white crystalline
powder, very soluble in alcohol, ether, and benzene,
but poorly soluble in water (0.3 g of benzoic acid in
100 g of water at 20
C).
0049 Benzoic acid has the advantage that it does not
affect the odor or taste of the soft drink, if used in
small quantities. The preserving quality of benzoic
acid is based on its activity to delay the multiplication
of several groups of microorganisms, which, however,
are not killed by this product. The low solubility
of benzoic acid in water complicates its applica-
tion in products containing large amounts of water.
Therefore, the water-soluble salt sodium benzoate is
used.
0050 This product, which is the salt of benzoic acid,
has no preserving activity by itself. Therefore, after
addition of sodium benzoate, the acidity of the soft
drink is increased (pH < 3.5), with the result that
free undissociated benzoic acid is formed, which has
a preserving property. In an alkaline environment,
benzoic acid is split into ions and thus loses its pre-
serving activity.
0051 Sodium benzoate is the sodium salt of benzoic acid
used as a white crystalline or amorphous (without
crystal structure) powder, very soluble in water (66 g
of sodium benzoate in 100 g of water at 20
C) but
poorly soluble in alcohol.
Sorbic Acid
0052 Sorbic acid, potassium sorbate, and calcium sorbate
are novel, highly efficient, safe, and nonpoisonous
food preservatives. They are the substitute for the
benzoic acid as a traditional preservative. Sorbic
acid, potassium sorbate, and calcium sorbate
approved worldwide are often now successfully used
as standard products in many branches of the food
industry. As they are acidic preservatives, it is better
to use them at pH 5–6.
0053Sorbic acid, potassium sorbate, and calcium sor-
bate are unsaturated fatty acids and salts of unsatur-
ated fatty acids, which participate in the normal fat
metabolism in human body and are oxidized into
carbon dioxide and finally water. They do not accu-
mulate in the human body. (See Fatty Acids:
Properties; Trans-fatty Acids: Health Effects.)
Sulfur Dioxide
0054The preservative effect of sulfur dioxide, like benzoic
acid, is greatly increased by a corresponding decrease
in pH. Undissociated H
2
SO
3
is responsible for most
of the preservative action. In contrast to sodium
benzoate, sulfur dioxide kills microorganisms instead
of delaying their growth.
0055Sulfur dioxide also combines regularly with many
compounds present in juices and soft drinks, and the
combined SO
2
exhibits little or no preservative effect.
This must be taken into account when deciding on the
levels required to preserve soft drinks, but it must be
remembered that the legal limit is often based on the
total free and combined SO
2
in the product. Sulfur
dioxide can be added as the salt of sodium or potas-
sium metabisulfite or as a solution of sulfurous acid.
This compound gives off SO
2
gas and therefore must
be stored in airtight containers. A disadvantage of the
use of SO
2
as a preservative is its relatively bad taste
at a dosage above 10 mg l
1
.(See Sodium: Properties
and Determination.)
Coloring Matter
0056Food that looks beautiful when served, e.g., nicely
served and has a beautiful color, affects the consumer’s
experience of taste positively. Color is an important
signal of identification that complements the label and
is a dominant factor in consumer acceptance of a
beverage. An attractive, natural-looking color tempts
one to taste and consume a beverage.
0057Before the mid-1970s, many more colorings were
used than is the case today. The soft-drinks industry,
itself, has reduced the number of coloring matters
used. However, the pure fruit-juice content of soft
drinks makes it difficult to create the right colors.
For this reason, identical coloring agents of the sort
found in the fruits are used extensively. The most
important is b-carotene, which is the predominant
coloring agent in carrots and oranges.
0058Brown drinks are colored with caramel. Different
drinks require caramel with different qualities. This
5350 SOFT DRINKS/Chemical Composition

can be achieved by having different nitrogen com-
pounds present during the heating phase of caramel
production.
0059 Colors can be either natural or artificial. Natural
colors include: anthocyanins (from berries and
grapes), caramel, xanthophyl, and carotenoids. Arti-
ficial colors include: tartrazine NS, yellow FCS, and
Amaranth AS. (See Caramel: Properties and Analysis;
Carotenoids: Occurrence, Properties, and Determin-
ation; Colorants (Colourants): Properties and Deter-
mination of Natural Pigments.)
0060 Legal requirements for colors used in soft drinks
differ from country to country, as do the required
declarations for each country. The overall move
toward more health-oriented products has increased
requests for natural colors. When using natural
colors, ascorbic acid has to be added to improve the
stability (better resistance to light). A positive side-
effect of using ascorbic acid is a reduced incidence of
can corrosion. The disadvantages of natural colors
are the high cost, the complication of the manufactur-
ing process, and the poor stability. (See Ascorbic
Acid: Properties and Determination.)
Antioxidants
0061 Antioxidants prevent reactions, which destroy aro-
matic substances. The most common antioxidant is
ascorbic acid, i.e., vitamin C. Sulfur dioxide, named
above as a preservative, is also used as an antioxidant.
(See Antioxidants: Natural Antioxidants; Synthetic
Antioxidants.)
Other Additives
0062Emulsifying agents, stabilizing agents, and thickening
agents are all used to ensure that the contents of
the drinks remain evenly distributed. The fat peel oil
from oranges, for instance, would otherwise form
lumps and create a ring around the neck of the bottle.
Since the oil is the carrier of the aroma and sometimes
the coloring agents, the drink would soon become
uneven in taste and look unattractive. Examples of
stablizing agents and thickening agents are pectins,
which are obtained from citrus fruits or apples,
and alginates and carragheen, which are obtained
from algae. (See Emulsifiers: Organic Emulsifiers;
Phosphates as Meat Emulsion Stabilizers; Uses in
Processed Foods; Pectin: Properties and Determin-
ation; Food Use; Stabilizers: Types and Function;
Applications.)
0063Soft drinks generally contain no significant
amounts of protein, fat, fiber, or vitamins. The
small amounts of minerals (calcium, iron, magne-
sium) and trace elements (copper, manganese, zinc,
fluoride) that may be naturally present will vary
depending on the local water supply. However, some
soft drinks now contain added vitamins (C, niacin,
B
6
,B
12
, biotin, pantothenic acid, and folic acid) and/
or increased levels of potassium (from added juice).
tbl0003 Table 3 Calorie, nutrient, and ingredient content of major types of soft drinks
Flavor types Calories Carbohydrates
(gml
1
)
Total sugars
(gml
1
)
Sodium
(mgml
1
)
Potassium
(mgml
1
)
Phosphorus
(mgml
1
)
Caffeine
(mgml
1
)
Aspartame
(mgml
1
)
Regular
Cola 0.4–0.5 0.10–0.12 0.10–0.12 0–0.08 0–0.05 0.11–0.21 0.08–0.13 0
Caffeine-free cola 0.4–0.5 0.10–0.12 0.10–0.12 0–0.08 0–0.05 0.11–0.21 0 0
Cherry cola 0.4–0.5 0.10–0.12 0.10–0.12 0–0.04 0–0.03 0.13–0.15 0.03–0.13 0
Lemon–lime (clear) 0.4–0.5 0.10–0.12 0.10–0.12 0–0.15 0–0.01 0–0.003 0 0
Orange 0.5–0.6 0.11–0.14 0.11–0.14 0.04–0.12 0–0.05 0–0.17 0 0
Other citrus 0.3–0.5 0.08–0.14 0.08–0.14 0.03–0.14 0–0.33 0–0.003 0–0.18 0
Root beer 0.4–0.5 0.10–0.14 0.10–0.14 0.01–0.17 0–0.05 0–0.05 0 0
Ginger ale 0.3–0.4 0.08–0.11 0.08–0.11 0–0.08 0–0.01 0–trace 0 0
Tonic water 0.3–0.4 0.08–0.10 0.08–0.10 0–0.03 0–0.01 0–trace 0 0
Other regular 0.4–0.6 0.10–0.15 0.10–0.15 0–0.12 0–0.06 0–0.26 0–0.12 0
Juice added 0.4–0.6 0.10–0.14 0.10–0.14 0–0.06 0.08–0.33 0–0.21 0 0
Diet
Diet cola <0.03 0–0.003 0 0–0.17 0–5.0 0.07–0.16 0–0.16 0–0.53
Caffeine-free diet cola <0.03 0–0.003 0 0–0.2 0–10.0 0.07–0.16 0 0–0.53
Diet cherry cola <0.03 0–<0.001 0–trace 0–0.02 1.5–5.0 0.07–0.11 0–0.13 0.50–0.52
Diet lemon–lime <0.03 0–0.003 0 0–0.26 0–6.9 0–trace 0 0–0.53
Diet root beer <0.06 0–0.013 0 0.11–0.28 0–3.0 0–0.05 0 0–0.58
Other diets <0.2 0–0.05 0–0.05 0–0.27 0.3–10.1 0–trace 0–00.19 0–0.57
Club soda, seltzer, and
sparkling water
0 0 0 0–0.27 0–0.5 0–0.003 0 0
Diet juice added <0.1 0.003–0.017 0.003–0.017 0–0.06 0–0.3 0–0.17 0 0.38–0.53
SOFT DRINKS/Chemical Composition 5351

Table 3 shows the calorie, nutrient, and ingredient
content of major types of soft drinks.
0064 Sodium and potassium values do not include the
levels contributed by water, which will vary
depending on geographic location and season. Most
soft drinks are very low in sodium, and some are
sodium-free. (See Potassium: Properties and Deter-
mination.)
See also: Ascorbic Acid: Properties and Determination;
Caramel: Properties and Analysis; Carbohydrates:
Classification and Properties; Citrus Fruits: Types on the
Market; Colorants (Colourants): Properties and
Determination of Natural Pigments; Glucose: Properties
and Analysis; Lactose; Potassium: Properties and
Determination; Preservatives: Classifications and
Properties; Saccharin; Sensory Evaluation: Aroma;
Taste; Sodium: Properties and Determination; Sucrose:
Properties and Determination; Dietary Importance
Further Reading
Colonna WJ, McGillivray T, Samaraweera U and Torgeson
T (1996) Proceedings Conference of The Sugar Process-
ing Research Institute.
Henkel J (1999) Sugar Substitutes: Americans Opt for
Sweeteners and Lite. USA: FDA.
Production
C Matthews, NET International, Gloucester, UK
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 Soft drinks have been consumed, in one form or
another, for thousands of years from the first time it
was discovered that the addition of acid materials
such as lemon juice and/or the incorporation of only
5% of alcohol from fermentation of grape and other
juices resulted in the preservation of dietary water.
Although milk, wine, and beer have all been around
for well over a thousand years, and tea and coffee for
many centuries, carbonated soft drinks are approxi-
mately 200 years old, dating from the commercial
production of seltzers and sodas in the late 1700s.
Revitalization of the squashes, syrups, or concen-
trated still soft drinks market has been facilitated
in the last few decades by the introduction of aseptic
packaging techniques, enabling continuous filling of
juices and ready-to-drink squash-type products into
foil laminates, pouches, and cartons. Some would
argue, with justification, that this is a completely
new sector of soft drinks made possible by advances
in production and packaging technology.
0002The production of soft drinks falls into three main
areas of activity:
1.
0003Supply, handling and treatment of ingredients
(including the water supply).
2.
0004Blending of these ingredients and the processing of
the blends/combinations so achieved.
3.
0005Packing or packaging of these blended ingredients
into the finished product for distribution and sale
(this may involve a further processing step, as in
the case of aseptic filling or dilution, and carbon-
ation, as with carbonated soft drinks).
Supply, Handling, and Treatment of
Ingredients
0006Most ingredients will be supplied in a user-friendly
and stable form by the ingredient manufacturers or
suppliers, with detailed specifications and usage in-
structions. During the time these ingredients are held
at the site of the soft drinks manufacturer it will
simply be necessary for them to be stored according
to the storage instructions of the supplier and used
within shelf-life. Some of the storage conditions and
shelf-lives of usually encountered raw materials will
be found in Table 1.(See Storage Stability: Param-
eters Affecting Storage Stability.)
0007It is normally the responsibility of the quality con-
trol department to approve the quality of all incoming
materials and insure correct rotation for use prior to
expiry of shelf-life.
0008This leaves the main ingredient in soft drinks –
water – which requires special treatment to insure
sensory, microbiological, and physical acceptable
quality. The chemical quality of water may be char-
acterized as follows.
1.
0009Appearance free from sediment, color, and cloud.
2.
0010Taste free from taint or materials such as chlorine,
hypochlorite, or nitrates which are capable of
reacting with other components or materials (e.g.,
cans).
3.
0011Free from toxins of any description.
4.
0012Free of water hardness which can destabilize fruit
colloidal suspensions via the calcium and magne-
sium which cause water hardness. (See Quality
Assurance and Quality Control.)
This is achieved in a soft drinks production plant in a
number of ways and the following list comprises the
main parameters covered in the specification (and
5352 SOFT DRINKS/Production

hence treatment, where necessary) of water: appear-
ance; pH; total dissolved solids; total hardness;
alkalinity; nitrogenous compounds; chloride; organic
content; microorganisms; phosphate; silicates; trace
metals; chlorinated compounds.
Water Treatment
0013 Chemical coagulation is widely used in soft drink
production plants as a means of removing unwanted
impurities. The incoming water passes into a reaction
vessel where coagulating chemicals are added. The
reaction products and impurities are precipitated
and form a gelatinous sludge through which the
treated water flows to a clear zone at the top of the
vessel. Since small particles of the flocculant will
travel with the treated water, it is normal practice to
pass water treated in this way through a sand-bed
filter. A typical system of this type is illustrated in
Figure 1.(See Water Supplies: Water Treatment.)
0014 The types of coagulants used are ferrous sulfate,
aluminum sulfate, and Lapofloc PAC (a solution of
polyaluminum chloride).
0015 Chlorination is the next stage in the treatment of
water. Free chlorine is usually used as the sterilizing
agent at a level of about 6–8mgl
1
of chlorine after
the sand filter to insure completion of the reaction.
Either chlorine gas or hypochlorite solution may
be used. Where ferrous sulfate is employed as the
coagulant, free chlorine oxidizes the ferrous salt
to ferric salt and is usually added to the coagulation
tank where the residence time may be included as
part of the contact time necessary for sterilization.
Where aluminum sulfate is used, the chlorine is
usually added after the sand filter and residence
time in the coagulant tank is not part of the contact
time.
0016 Organic matter, sulfites, nitrites, and ammonia (or
its compounds) absorb or react with chlorine and the
bactericidal action of chlorine will not be effected
until after these reactions have taken place. Chlorine
is best added to the coagulation tank or immediately
after, since contact with raw, untreated water
containing humic organic substances produces tri-
halomethanes (THMs) which would result in an un-
pleasant taint to the water if present in quantities in
excess of 100 mgl
1
. Via the chlorination process
all impurities will be oxidized, removing all taste
and odor, including those due to phenol and its
derivatives.
0017The next stage in the process is the dechlorination
of the water after sterilization by passing the water
through an activated carbon filter which is housed in a
vessel similar to that of the sand filter (Figure 1). The
quantity of carbon will be selected to achieve a con-
tact time of approximately 5 min with the water,
removing all of the chlorine and any remaining
organic molecules. Carbon filters are fitted with
steam injection at the base to allow sterilization
when required to reduce the gradually increasing
microbiological contamination from its use.
0018Quality control on a continuous basis of all aspects
of the process must be effected to insure optimum
performance of the process. In particular it must
always be able to indicate an imminent breakdown
of the carbon bed when it requires regeneration.
0019The above process is the classical procedure for
water treatment. There are, however, other methods
of either total treatment or treatment for specific
requirements of the water. They are as follows:
.
0020Ion exchange will perform softening; dealkaliza-
tion; nitrate removal; organic compound removal.
.
0021Reverse osmosis will effect removal of high levels
of dissolved solids, resulting in a water which may
be used directly or further treated for carbonated
soft drinks production.
.
0022Ultrafiltration removes colloidal substances and
may be substituted for the polishing filter in a
conventional process.
.
0023Alternative sterilization processes include ultra-
violet light, ozonization, and micropore filtration.
tbl0001 Table 1 Storage conditions of soft drink raw materials
Material Storage conditions Recommended shelf-life
Fruit
Frozen Below 18
C 2–3 years
Preserved Ambient 3–9 months
Aseptic c.4
C Up to 6 months
Sugar syrup Dependent on concentration
Some may be stored at ambient 48 h
Glucose syrup (GS)/high-fructose GS 35
C 48 h–1 week
Citric acid solution Ambient 1 week
Volatile and natural flavoring materials 4
C 3–6 months
Nature-identical and artificial flavoring materials Dark UK; ambient 6–12 months
Water Ambient On demand
SOFT DRINKS/Production 5353
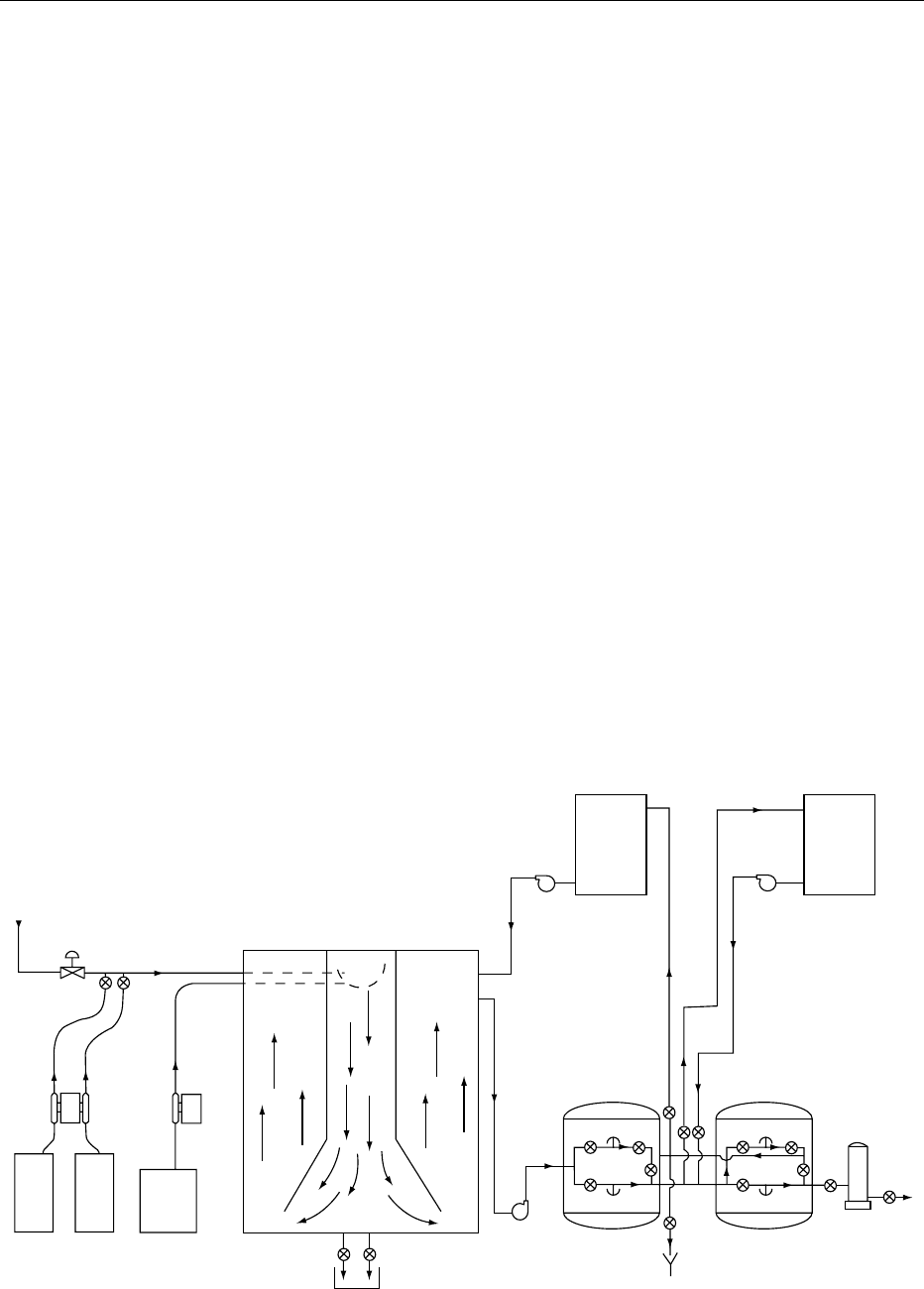
Blending and Processing of Key
Ingredients in Soft Drinks
0024 There are two basic methods of manufacture of soft
drinks; these are the single batch production and
continuous production. Batch manufacture is the
process where a tank with a capacity of up to
25 000 may be employed to manufacture the product
and/or intermediate syrup to feed the filling line over
a period of time. Continuous production utilizes two
tanks of only 50–100 l each, with a computer, con-
tinuously making one batch after another in a type of
tandem operation. The main advantages of the latter
system are associated with line problems and interup-
tions, particularly where a heat treatment is part of
the process, since only the 50 or 100 l of product
already manufactured will be affected, and may easily
be ‘ditched’ without significant economic conse-
quences. In the case of batch manufacture, however,
the 25 000 l of product or intermediate syrup will
either remain under nonoptimum conditions or, at
worst, continue to be heat-processed (with a return
to the batch tank) for the duration of the line down-
time.
0025 In each case the steps and safeguards it is necessary
to take in the production process are the same
and will be covered in the same way. The schematic
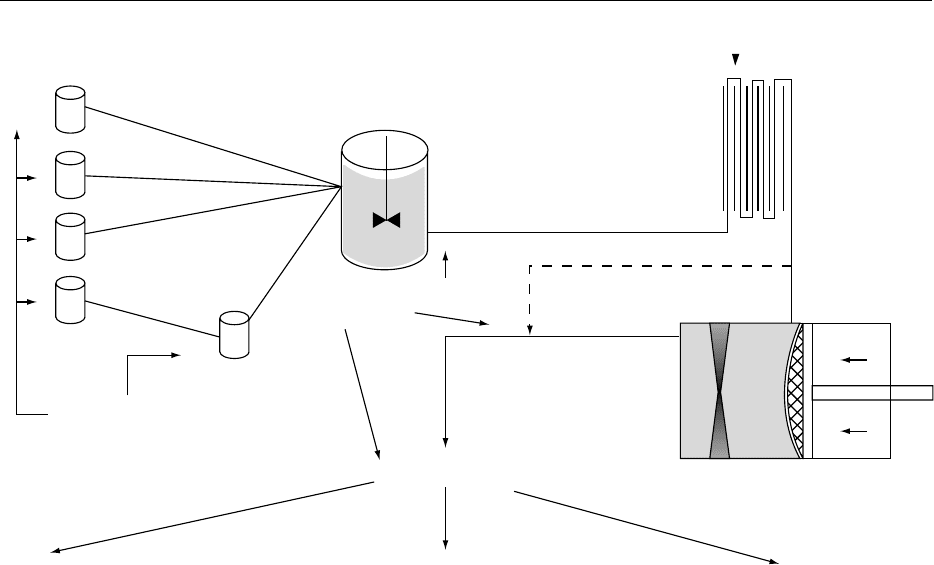
representation of the production of a soft drink is
shown in Figure 2. It can be seen that the processing
required for the production of a soft drink involves
blending the individual ingredients with heat treat-
ment and/or homogenization where necessary. In
reality all ingredients are not added separately to the
main blending vessel, since this would cause signifi-
cant logistical problems for items that are difficult to
handle, such as solid sugar (dissolution problems) or
thickeners or stabilizers (localized viscosity build-up,
lack of powder wetting). There are basically four
streams of ingredients:
1.
0026Colors, flavorings, etc.
2.
0027Sugars (i.e., all carbohydrates).
3.
0028Fruit materials.
4.
0029Citric acid, stabilizers, emulsifiers, etc. (this stream
utilizes a premix blending vessel with a signifi-
cantly increased blending power-to-volume ratio
for difficult-to-dissolve/disperse ingredients).
0030Many soft drink production sites are now convert-
ing to tanks located on load cells so that the vessel
may be tared and ingredients weighed directly into
them.
0031If liquid carbohydrates are being used, these may
be added directly from their optimum storage pos-
ition to the main blending vessel via an inline sieve or
Backwash water
storage tank
(optional)
Chlorinated water
storage tank
(optional)
Sand-bed
filter
Carbon-bed
filter
Polishing
filter
Sludge
blow-down
Raw-water
inlet
Chemical
feed
pumps
Chemical feed
storage tanks
fig0001 Figure 1 Typical water treatment process in the production of soft drinks.
5354 SOFT DRINKS/Production
filtration system to remove any small particles. If
solid sugar is used, a simple syrup must be prepared
of the sugar and water at approximately 50
C to aid
dissolution; this is then cooled before addition to the
main blending vessel. All other ingredients are added
to the carbohydrate syrup in the blending vessel in an
order which takes into account their chemical reactiv-
ity with other ingredients. For example, sodium
benzoate must always be added prior to the citric
acid, since benzoic acid is only sparingly soluble in
acid solution. Certain clouding agents and stabilizers
must not be added in close proximity to a flavoring
that contains a high level of an alcohol as solvent,
since they will interreact.
003 2 With a high level of carbohydrate or thickening
agent in the syrup in the main blending vessel, it is
very easy to entrain air with too much vigorous agi-
tation, and this would result in fobbing during filling,
particularly of carbonated products. As long as agita-
tion has not been excessive, then 2 h standing after
mixing, with gentle agitation to keep all ingredients
evenly dispersed, will be sufficient to allow entrained
air to escape. This gentle agitation should be con-
tinued for the duration of any pumping or direct
bottling so that particulate materials, such as fruit
comminute, are evenly dispersed throughout the
liquid.
Homogenization
0033This process is used for many fruit-containing
concentrates as part of the finished beverage manu-
facture enroute to the bottling line and for inter-
mediate syrup processing for some fruit-containing
carbonates and ready-to-drink still beverages. The
process is a means of reducing the particle size of
particulate matter (e.g., fruit comminute) by means
of energy input resulting in a more stable suspension
and hence better appearance. The conditions which
are normally used are 1000–3000 psi at ambient
temperature.
Carbonation
0034The simplest type of carbonator is a pressure vessel in
which the liquid and the carbon dioxide are allowed
to stay in contact with each other. There are three
basic ways to achieve this: (1) vessel partly filled with
liquid and pressurized with carbon dioxide; (2) liquid
falling through carbon dioxide in a pressurized con-
tainer; (3) carbon dioxide bubbling through liquid
in a pressurized vessel or pipeline. All carbonators
operate on one or more of these principles.
0035One of the essential requirements of a production
carbonation system is control over the degree of sat-
uration of the carbonated product, i.e., how close to
Colors, flavors, etc.
Ingredients
Main blending
with water
Sugars
Fruit
Citric acid
Heating
93 8C; 15s
Regeneration
Pasteurization
Cooling
Juice products
1000−3000 psi
Whole-fruit
products
Homogenization
Still
ready-to-drink
Filling line
Carbonates
Concentrates
(Quality control
check)
(Quality control
check)
Premix blending
fig00 02 Figure 2 Schematicrepresentationoftheproductionofsoftdrinks.SeeFigure3forindividualkeystages.
SOFT DRINKS/Production 5355

the maximum possible volume of carbon dioxide is
allowed to dissolve. An uncontrolled release of gas
from solution is known as fobbing. Partially saturated
solutions are more stable than saturated ones owing
to the additional pressure available over the equilib-
rium pressure. This additional pressure is known
as the overpressure and is used in the control of the
process. When the necessary degree of overpressure
may be maintained over a range of temperature and
carbonation, this is known as variable saturation. The
ready-to-drink, dilute product may be carbonated by
a reduction in temperature and injection of carbon
dioxide via one or more of the above techniques,
or the concentrated intermediate syrup may be
blended with a stream of carbonated water on line to
the filling apparatus, resulting in the correctly diluted,
carbonated, finished product in the pack.
Hygiene
0036 Throughout the production of all types of soft drinks
it is essential that plant hygiene is maintained at the
optimum level, whether chemical preservatives and/
or heat processing are used.
0037 Good manufacturing practice is a vital and integral
part of insuring minimal microbiological contamin-
ation of the product and will only be achieved if
attention is paid to certain areas of operation:
.
0038 Hygienic practices in receiving, handling, and pro-
cessing materials.
.
0039 Tight specifications for clean raw materials.
.
0040 Hygienic attitudes and understanding in all person-
nel.
.
0041 Hygienic design of plant and equipment.
.
0042 Hygienic operations of suppliers (especially of fruit
and carbohydrate materials).
Heat Processing
0043 An examination of the different types of processing
for concentrates, carbonates, and ready-to-drink soft
drinks is shown in Figure 3. This shows clearly that,
in addition to chemical preservation, blending,
carbonation, and basic hygiene and quality control
requirements, heat processing is a vital part of the
production of soft drinks.
0044 Although a manufacturer’s main protection against
microorganisms is the hygienic operation of the
manufacturing plant and that of suppliers, it may
still be necessary with certain microbiologically sensi-
tive formulations to enlist the help of both chemical
preservatives and heat processing. The use of heat
during the processing of soft drinks is covered in
outline here as it relates to the microbiological in-
tegrity of the product formulation. (See Preservation
of Food.)
0045Heat processing, or pasteurization, may be sub-
divided into four main categories for soft drinks
and/or material processing: (1) hot-fill; (2) in-pack
(or tunnel) pasteurization; (3) flash pasteurization:
(a) nonaseptic conditions or (b) aseptic conditions;
and (4) microfiltration. The selection of the required/
desired heat-processing conditions for any given soft
drink, intermediate syrup/blend, or raw material is
determined by a number of factors relating to the
following:
.
0046Presence of fruit materials and their origin, quality,
and microbiological status.
.
0047Presence of chemical preservatives – qualitative
and quantitative factors apply.
.
0048pH of the product.
.
0049Solids content and hence water activity.
.
0050Any other relevant processing conditions – holding
of syrup at ambient/raised temperature, use of
homogenization, filtration, etc.
.
0051Type of packaging to be employed.
.
0052Desired shelf-life.
.
0053Microbiological conditions (and history) of the
filling line and ancillary equipment to be employed.
(See Pasteurization: Principles.)
0054Hot-fill This can only be used for finished still
products or intermediate raw materials when being
packed for storage and shipment. The product/mater-
ial is heated in a heat exchanger to a temperature of
85–90
C; filled into the pack (glass, cans, etc.) so that
the temperature of the contents is in excess of 85
C;
sealed via capping or seaming; inverted for a min-
imum of 1 min; and cooled as quickly as possible.
Any flaws in the capping/seaming will result in con-
taminated cooling water or air entering the package
and this will cause spoilage.
0055The disadvantage of this system is the large amount
of heat that is put into the product/material during
the whole process, which results in flavor, color, and
clouding degradation on storage, producing a reduc-
tion in total shelf-life on the product/material.
0056In-pack (or tunnel) pasteurization In its simplest
form, this type of pasteurization involves immersing
the filled, capped, or seamed pack of either still or
carbonated product in a tank filled with hot water at
60–90
C in a removable basket and holding at the
selected temperature for the required period of
pasteurization holding time. In reality the packs are
placed in water at a temperature lower than that
required and the temperature of the water is raised
until the specified temperature has been reached.
0057Examples of pasteurization conditions for this type
of processing are:
5356 SOFT DRINKS/Production
