Braibant S., Giacomelli G., Spurio M. Particles and Fundamental Interactions: An Introduction to Particle Physics
Подождите немного. Документ загружается.


412 13 Microcosm and Macrocosm
life.” The WMAP (see Sect. 13.5) measurement of the temperature differences
in the Cosmic Microwave Background radiation refers to this moment.
17. t
17
' 1 billion of years. The average particle energy is E
17
0:1 eV. The
galaxy formation starts. Galaxies and galaxy clusters form, then the first stars.
Clouds of matter were formed by fluctuations in the matter spatial distribution;
gravitation starts to form protostars. Over time, as the gas cloud compresses, the
temperature at the center increases until it becomes so high that thermonuclear
reactions can be initiated. Hydrogen is burned, and as a consequence, helium is
produced as “ash.” The starlight starts to illuminate the Universe. The average
wavelength of the cosmic microwave background became large, corresponding
to infrared radiation.
18. t
18
' few billion years. The first supernovae explode, launching a large
quantity of material containing heavy elements (synthesized within the stars)
in the interstellar space.
19. t
19
' 9 billion years. The Sun and its planets, including the Earth, start to
form by gravitational contraction. The material collected by our cloud mostly
contains hydrogen and helium, the material produced at the beginning of
the Universe; there are also significant quantities of materials such as iron,
synthesized in massive stars which have already exploded.
20. t
20
' 13:7 billion years. Approximately 1 million years ago (or less), the
Homo sapiens began to wonder how our Universe was made.
Current size of the Universe. In conclusion, it can be assumed that the Universe
has a radius of about 13.7 billion light years and consists of about 100 billion
galaxies; each galaxy is made of about 100 billion stars. Taking into account the
mass of each star, the observable Universe is made of about 10
80
protons. However,
this should account for less than 5% of matter and energy: most of the matter/energy
is still invisible to us. The number of cosmic background radiation photons is about
a billion times the number of protons.
One might ask: what existed before the Big Bang? There can be no answer that
physically makes sense because you cannot get any information for the time before
the Big Bang. However, the inflation mechanism might suggest that there are many
“parallel Universes.”
Expansion accelerated or decelerated. In 1998, two astrophysicist teams began
to study the motion of distant galaxies by observing the final stage of some of their
bright stars (type I supernovae). They found that these galaxies are moving away
from us more slowly than expected from the Hubble law [13S04]. The observed light
has left the galaxies a few billion years ago: looking further away in the Universe,
we observe objects at increasingly younger ages. In the spirit of the standard model
of the Big Bang, gravity slows the motion of bodies that are moving away from
each other. Therefore young “objects” should move faster away from us than older
objects. The results from type I supernovae indicate the opposite, namely, that the
Universe is now expanding faster than in the past. This is an outstanding result
whose consequences are not completely understood in the standard models of the
Microcosm and Macrocosm.
13.6 The Big Bang and the Primordial Universe 413
To obtain this situation, a field that tends to accelerate objects in the Universe
should probably be present. This (unknown) energy field would contribute to
approximately 70% of the Universe energy density. The name of quintessence or
dark energy is given to this energy field. In the future, if the type I supernovae
results are definitively confirmed by other independent methods, several concepts
about the Universe, particularly those relating to its future evolution, will probably
need to be revised.
The future of the Universe. What can be said concerning the future evolution of
the Universe? If the actual density of matter is smaller (or equal) than the critical
value, then the Universe will continue to forever expand. If it is larger, the Universe
would reach a maximum expansion and then have a contraction, leading to a final
implosion. The recent results from WMAP and others indicate that the actual density
of matter is exactly equal to the critical density, and that the Universe is flat. In the
far future, the stars, having finished their nuclear fuel, will go off one after the other,
and the Universe would become dark, without visible light. In the very far future,
one could have the proton decays and even later blacks hole “evaporation.”

Chapter 14
Fundamental Aspects of Nucleon Interactions
14.1 Introduction
The periodic table of elements (the Mendeleev table, Appendix A.1) is the most
extraordinary demonstration of the interconnection between the microcosm and
macrocosm, or between particle physics, astrophysics and cosmology. Following
the Standard Model of the macrocosm (Sect. 13.6), 3 min after the Big Bang, matter
in the Universe was primarily composed of hydrogen (92%), helium (8%) nuclei
(primordial nuclei) and electrons. Today, on Earth, every element of the periodic
table is present, from hydrogen .Z D 1/ to uranium .Z D 92/.Asweshallshow
in this chapter, all nonprimordial nuclei up to iron are formed inside stars, in the
processes of stellar nucleosynthesis. The release of these elements in the Universe
occurs through the gravitational collapse of massive stars, followed by the envelope
expulsion (supernova). Nuclei heavier than iron are formed through neutron capture
processes, followed by ˇ-decay. Most of the produced nuclei are radioactive. Only
those with long or very long lifetimes have survived. All the others were transformed
into stable nuclei.
In this chapter, we shall review the basic properties of nuclei, their structure
(distributions of electric charge and mass, volume) and the interactions between
nucleons (i.e., between neutrons and protons) to form the nuclei. The interaction
between nucleons cannot be described by a simple potential energy function. As
the molecules are formed by neutral objects (the atoms) and bound by residual
electromagnetic interactions, nuclei are formed in a similar way by objects which
are neutral for the strong interactions. Protons and neutrons have no color charge.
Nuclei are bound by a kind of residual strong interaction. Their interaction cannot
be formalized in a simple way from the mathematical point of view.
However, we shall see that a formula that parameterizes a fundamental quantity,
the nuclear binding energy, can be written. The higher the binding energy, the more
stable is the nucleus. The properties of the nuclear binding energy not only influence
the physics of nuclei, but also the structure and the stellar evolution.
S. Braibant et al., Particles and Fundamental Interactions: An Introduction to Particle
Physics, Undergraduate Lecture Notes in Physics, DOI 10.1007/978-94-007-2464-8
14,
© Springer Science+Business Media B.V. 2012
415

416 14 Fundamental Aspects of Nucleon Interactions
Table 14.1 Distribution of
stable nuclei as a function of
the atomic number Z
(number of protons), the
number of neutrons N and
the mass number
A D Z C N
Number of
AZND A Z stable nuclei
Even Even Even 157
Odd Even Odd 53
Odd Odd Even 50
Even Odd Odd 4
Total 264
It is not necessary to consider nuclear physics in terms of quarks and gluons,
even if protons and neutrons are made of quarks. In classical nuclear physics, the
existence of quarks can be ignored as well as the existence of meson and hadron
resonances. A nucleus consists of nucleons that somehow behave as almost free
particles, although they are in a high density medium (about 10
38
nucleons/cm
3
).
The average kinetic energies of nucleons in the nucleus are of the order of 20 MeV
(Sect. 14.3.1), which is considerably smaller than the energy scales of elementary
particles. The hadron composition in terms of quarks and gluons should only be
taken into account in a deeper description of nuclear physics. Moreover, under
particular conditions of energy density, such as those that can be reached during
the collision between two very high energy heavy nuclei, quarks could become free
and nuclear matter should behave as a plasma of quarks and gluons.
The chemical elements existing in nature are a finite number: they are those
that appear in the periodic table of elements. Each element has a nucleus with a
definite electric charge (i.e., number of protons). In the laboratory, it was possible
to artificially create some nuclei, called transuranic. In the Mendeleev table, their
position is higher than that occupied by uranium. However, these artificial nuclei
have relatively small lifetimes. The stable nuclei observed in Nature are 264; the
number of those unstable is larger than 1,500. Their number is increasing every year,
as more refined experimental techniques allow one to observe unstable nuclei with
shorter and shorter lifetimes. The nuclei can be classified in terms of the number
of protons Z (the atomic number), the number of neutrons N and the number of
nucleons A (A D Z C N D Z protons plus N neutrons) (the mass number). By
sorting the nuclei on the basis of Z and N , the stable nuclei are distributed as shown
in Table 14.1. The largest number of stable nuclei occur when both Z and N are
even. The number of nuclei with Z even and N odd is approximately equal to that
with Z odd and N even. The content of Table 14.1 is evidence that the nuclear force
is independent of whether the nucleons are protons or neutrons.
The fundamental properties of nuclear physics are more complicated than those
of atomic physics. Our knowledge of nuclear interactions is, in any case, advanced
enough to conclude that the chemical elements everywhere in the Universe are the
same as those found on Earth. It does not exist, therefore, some elusive stable
element with physics properties unknown on Earth (e.g., the unobtanium on the
Pandora planet of the Avatar movie).
The physics laws are universal; from nuclear physics, we know that the nuclei
(and thus the atoms) on Earth are the same as those that found elsewhere in the
14.2 General Properties of Nuclei 417
Universe. From nuclear, atomic physics and astrophysics we also know that the
chemical composition of planetary systems may not be very different from that of
our Solar System. We do not know indeed if there is intelligent life beyond our Solar
System, anywhere else in the Universe. We are also unaware of the biology of this
hypothetical intelligent species, nor of their reproduction modality. Reproduction
can occur similar to life on Earth, with the union of two individuals of different
sexes: having seen the success and repeatability of natural laws, this assumption
might be plausible. In this case, the hypothetical aliens will probably exchange a
golden gift and not an iron object as a token of love. Also, in this planetary system,
the ratio between the number of gold nuclei with respect to iron nuclei will be
10
6
–10
5
, and gold will be a precious good. If in addition, there is on the gift a
brilliant transparent stone made of carbon atoms in a particular lattice arrangement,
we think that the hypothetical individuals of this planet will have a good chance of
successfully transmitting their genes .
14.2 General Properties of Nuclei
In 1911, Rutherford, studying the transmission of alpha particles (He nuclei) in
a thin layer of gold (Au), realized that they were also scattered at large angles
(up to 180
ı
, i.e., backward). At that time, the Thomson model of the atom was
generally accepted: it assumed that the electrons are drowned in a positive charge
distributed throughout the volume of the atom. Such a charged sphere is not able to
significantly deviate a particle with a mass equal to 7,300 electron masses. To obtain
deviations such as those observed, it must be hypothesized that the positive charge
is concentrated in a much smaller volume.
The interpretation of emission spectra of atoms and the Rutherford experiment
are at the base of the Bohr–Sommerfeld atomic model:
• The atom consists of a nucleus of charge C Ze.
• Z electrons, each with a charge e, are bound to the nucleus by the Coulomb
potential.
• The nucleus mass is much greater than the electron mass.
• The electric charge of the nucleus is concentrated in a region of space much
smaller than the size of the atom.
After the discovery of the neutron (1932), it was realized that the nuclei are bound
states of subconstituents nearly equal in mass, with N neutrons and Z protons.
The interaction between the nuclear constituents has characteristics very different
from the electromagnetic interaction. In particular, the forces holding the nucleus
together are called nuclear; nuclear forces do not depend on the electric charge and
they have an interaction range of about 10
15
m. The order of magnitude of the
nuclear binding energy is the MeV; it is straightforward to estimate that to maintain
two protons at a distance of r 1 fm against the Coulomb repulsion, an energy of
U>e
2
=r 1 MeV is needed. Quantities that characterize the atomic nuclei and

418 14 Fundamental Aspects of Nucleon Interactions
S
ΔV
E
B
B
Detector
Fig. 14.1 Operation principle of a mass spectrometer. The ionized nuclei are emitted from the
source S; they enter, through a collimator, in a region with electric field E and magnetic field B.
Because of E, the ion is subject to an upward force, whose module is ZeE. Meanwhile, due to the
magnetic field, it is deflected downward with a force ZevB (c.g.s. units). The second collimator
selects only those particles for which the two forces cancel each other (those for with v D E=B).
After the second collimator, only the magnetic field B is present. The Lorentz force deflects the
ions with a curvature radius R which can be experimentally measured
give information on their structure are: mass, radius, spin; the electric charge, the
magnetic dipole moment, the electric quadrupole moment.
Nuclei are indicated with the symbolic name of the element X (e.g., H for
hydrogen, Fe for iron). The atomic number Z (and therefore the electric charge
of the nucleus) is written as a subscript on the left. The mass number A is placed on
the top left:
A
Z
X: (14.1)
Electric Charge of Nuclei. The electric charge of nuclei is measured by studying
the X-ray emission spectra of electrons in inner orbitals (K orbital); these electrons
are not affected by the electromagnetic shielding effect by the electrons arranged
in the outer orbitals. In 1913, Moseley established a relationship between the
frequencies of X-rays and the atomic number, that is,
h D
3
4
R
y
.Z 1/
2
(14.2)
where R
y
D m
e
c
2
˛
2
=2 D 13:6 eV is the Rydberg constant. Moseley’s law ordered
all known elements in the Mendeleev table, showing that the nuclear electric charge
is a multiple of the elementary electric charge e.
Mass of the nuclei. The mass of the nuclei is determined by measuring their
trajectory in an electric and a magnetic field. The mass spectrometer, firstly
developed by Aston in 1920 (Nobel laureate in 1922), was gradually improved to
achieve a precision measurement of M=M 10
6
. The operating principle is
shown in Fig. 14.1. Ions emitted from the source S are accelerated by an electric
field and introduced in the area between two collimators where there is an electric
field E orthogonal to a magnetic field B.BothE and B are also orthogonal to the

14.2 General Properties of Nuclei 419
ion trajectory in order to only select nuclei with electric charge q D Ze and speed
v D E=B; after the second collimator, there is only the magnetic field. The mass M
of the nucleus is determined by measuring the curvature radius R of the trajectory
curved by the Lorentz force (qvB D M v
2
=R)
M D
qB
v
R D
qB
2
E
R: (14.3)
To reduce systematic uncertainties, the measurements are usually done by compar-
ison amongst nuclei that have very small mass difference. An instructive online
applet of a mass spectrometer can be found in [14w1].
The mass spectrometer is also used to separate isotopes of the same element and
to measure their relative abundance. Carbon, for example, exists in Nature as two
isotopes with relative abundance of 98.89%
12
6
C
and 1.11%
13
6
C
. The atomic
weight of natural carbon corresponds to the mean value
A D12.01. For historical
reasons, the atomic mass unit (AMU), denoted as u, is often used in nuclear physics.
It is defined by the relation 12u D mass of the isotope 12 of carbon. In these units,
the mass of the hydrogen atom is
M
1
1
H
D 1:007825 u D 938:783 MeV/c
2
:
The conversion factor between the units is
1 u D 931:494 MeV/c
2
: (14.4)
The number at the bottom of each element of the Mendeleev table in Appendix A.1
shows the value of the nuclear mass in AMU. For practical reasons, the proton rest
mass (m
p
D 938:271MeV/c
2
)isalsobeusedasmassunit.
14.2.1 The Chart of Nuclides
Nuclei with the same value of Z and different values of A have the same atomic
properties: chemical reactions depend almost exclusively on Z. For this reason,
nuclei with the same number of protons are called isotopes (because they occupy
the same position in the Mendeleev table of elements). Nuclei with the same value
of A and different values of Z are called isobars (because they have approximately
equal mass). In terms of variables .N; Z/, the stable nuclei are concentrated in a
narrow band, called stability valley (Fig.14.2), indicating a correlation between the
electric charge and the number of constituents.
The bond due to the strong interaction between .n–n/ and .p–n/ pairs is
identical. It is different for .p–p/ pairs because the protons repel electromagnet-
ically. For low values of A, nuclei have N D Z; starting from A ' 40, the fraction
of neutrons increases.
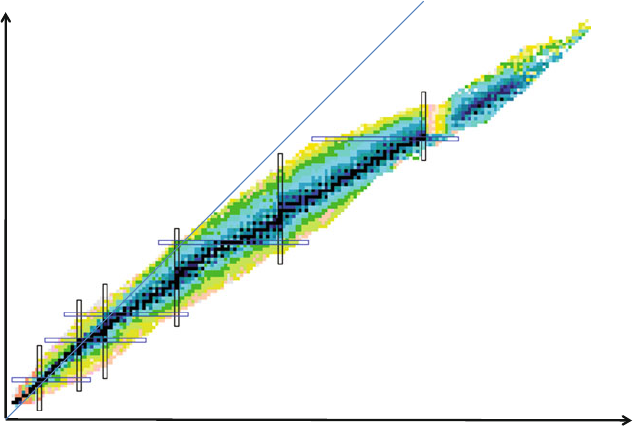
420 14 Fundamental Aspects of Nucleon Interactions
number of neutrons, N
number of protons, Z
Z=N
N=20
Z=20
N=8
Z=8
N=28
Z=28
N=82
N=50
Z=50
z=82
N = 126
Fig. 14.2 Chart of nuclides. The nuclide lifetime increases as the darkness of the square. The
stable nuclei are shown in black in the middle. The bars show the nuclei with magic numbers,
Sect. 14.3.3 (Adapted from the interactive and color chart, which is available at the Brookhaven
National Laboratory website [14w2])
14.2.2 Nuclear Binding Energy
The equivalence between mass and energy (E Dmc
2
) is not appreciable in atomic
physics. The reason is that the binding energies (of the order of eV, i.e., 2 10
5
m
e
)
are much smaller than the masses of the particles. The relation E D mc
2
becomes important in nuclear physics, where the energies involved may represent
a significant fraction of the rest masses of the nucleons. For this reason, it is
sometimes convenient to express both energies and masses in natural units „D
c D 1 (see Appendix A.2), that is in MeV.
In the formation of a bond (atomic or nuclear), energy is gained because a
more stable system is obtained. The energy released should be compensated by
the decrease of the final mass, with respect to the sum of the initial masses of
the elements. For example, the bound state with the smaller nuclear mass is the
deuterium nucleus (deuteron); the deuteron is a hydrogen isotope made by one
proton and one neutron (Z D 1; A D 2). In this case, the mass is decreased by
2.224MeV; it is a small amount compared to m
p
C m
n
, but not quite negligible
(0.2% m
p
).

14.2 General Properties of Nuclei 421
U
235
U
238
Fe
56
O
16
C
12
He
4
Li
6
Li
7
He
3
H
3
H
2
H
1
9
8
7
6
5
4
3
2
1
0
0 30 60 90 120 150 180 210 240 270
Mass number A
Average binding energy per nucleon (MeV)
Fig. 14.3 The measured binding energy (BE) per nucleon of stable nuclei measured as a function
of A. The peaks correspond to particularly stable nuclei. The curve has a maximum at A 60
The binding energy (BE) is defined as the difference between the mass of the
nucleus and the sum of the masses of the constituent nucleons:
M
nucleus
D
A
X
kD1
m
k
BE D .Zm
p
C Nm
n
/ BE: (14.5)
The helium nucleus
4
2
He (also called ˛ particle) is a particularly stable configuration
whose binding energy is equal to 28.298 MeV. The binding energy of nuclei with
small A is not a regular function. For A>12, the binding energy is approximately
proportional to the number of nucleons (Fig. 14.3), with
BE
A
8 MeV/nucleon: (14.6)
This relation has a justification in the framework of the nuclear drop model (see
Sect. 14.3.2).
14.2.3 Size of the Nuclei
The term nuclear radius needs to be correctly defined through the operational
specification of what is measured and of the measurement method. Information

422 14 Fundamental Aspects of Nucleon Interactions
on spatial distribution of nuclear matter andonthenuclear interaction range are
obtained with different methods: diffusion experiments (with ˛ particles, neutrons,
protons, electrons); spectroscopy of nuclear levels; analysis of nuclear binding
energies; study of nuclear decays. In this section, we shall mainly focus on the
scattering of particles on nuclei.
In Rutherford scattering (Sect.4.7.1), the closest approach of a particle depends
on the scattering angle . Rutherford and Chadwick already noted a large deviation
from the expected Coulomb cross-section for a point-like charge when the trans-
ferred momentum is high, i.e., when the distance of closest approach is comparable
with the range of nuclear forces (see Sect. 7.1.1).
Since the first measurements, it was found that the nuclear radius R is propor-
tional to the cubic root of the atomic weight A:
R D R
0
A
1=3
R
0
' 1:2 10
13
cm: (14.7)
Using particle accelerators, it was possible to reach higher transferred momenta,
and to study the structure of nuclei and nucleons in more detail (see Chap. 10).
The information obtained depends on the type of particle used as a probe. ˛
particles and protons are subject both to the Coulomb interaction and to the nuclear
interaction. The neutrons are subject to the nuclear interaction only (the magnetic
dipole interaction is negligible at low energy).
Distribution of the electric charge density. The electrons do not have nuclear
interactions and electron scattering experiments give detailed information on the
electric charge distribution, on the nucleus electromagnetic radius and on their mag-
netization. Using high energy electrons, E
e
D 100–1,000MeV, the electromagnetic
form factors of nuclei (Sect. 10.4) were measured. From the form factors, the electric
charge density .r/ and the nucleus magnetization M.r/ can be deduced. The
elastic cross-section of electrons on nuclei decreases as the transferred momentum
increases. This is roughly compatible with the expected behavior from a uniform
charge distribution in a sphere of radius R. A more accurate parameterization of the
electric charge density inside the nucleus is obtained with a spherically symmetric
distribution, that is,
.r/ D
0
1 C e
.rR/=t
: (14.8)
This is known as the Woods–Saxon distribution. It depends on two parameters:
R represents the value of the radius where the charge density is .r/
0
=2; t
measures the thickness of the region where the charge density decreases rapidly.
For nuclei with large A, one approximately has
R D .1:18A
1=3
0:48/ fm;tD 0:55 fm:
Distribution of nuclear matter. Neutron–nucleus scattering experiments are used
to study the distribution of nuclear matter and of the nucleus mean square radius.
