Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

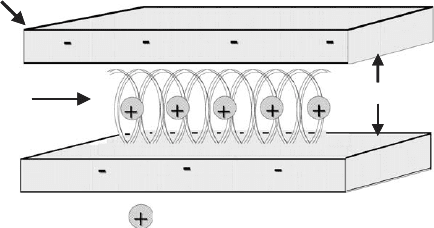
interlayer space depends on such factors as the nature of the exchangeable cation
and the water content (Hackett et al., 2000).
The technique of melt intercalation (Vaia et al., 1993 ) of polymers into phyllo-
silicates, is considered as one of the major practical steps forward in the field of
nanocomposite preparat ion. This procedure was successfull y applied to prepare PEO
nanocomposites (Vaia et al., 1995; Giannelis, 1996). PEO is intercalated in homo-
ionic alkaline cations exchanged montmorillonites after 6 h of treatment at 80 1C.
This temperature is only about 10 1C above the melting point of PEO, but is ap-
parently sufficient to ensure the mobility of the polymer chains so that they can
migrate into the interlayer space of the clay mineral.
The effectiveness of melt intercalation could be further improved by using mi-
crowave (MW) irradiation. The energy is mainly absorbed by the water molecules
coordinated to the interlayer cations. As a result the polymer and eventually the clay
mineral are heated, promoting rapid entry of the polymer into the interlayer space of
the montmorillonite. The MW-assisted melt intercalation procedure is successfully
applied for the first time to the preparation of montmorillonite–PEO nanocompos-
ites ( Aranda et al., 1998, 2003), saving time and energy compared to the conven-
tional method of oven-heating.
PEO is also adsorbed on sepiolite, either from a solution or from the melt by MW
irradiation. Only preliminary results were published (Ruiz-Hitzky, 2001). The salient
feature is that the polymer chains can partially penetrate into the structural tunnels
of 0.4 by 1.1 nm .This pr ocess apparently involves the irreversible displacement of
water molecules filling the tunnels of the natural sepiolite.
Until recently, the direct intercalation of polymers into kaolinite appears to be
unfeasible (Sanz and Serratosa, 2002). However, if the kaolinite layers are previously
expanded, using polar molecules like dimethylsulphoxide (DMSO), the interlayer
space becomes accessible to polar polymers like PEG (Tunney and Detellier, 1996).
The resulting kaolinite–PEG nanocomposites show Dd values of about 0.4 nm in-
dicating that monolayers of polyoxyethylene chains in planar zig-zag conforma tion
interlayer cation
intercalated
PEO-salt complex
layer silicate
8 Å
Fig. 10.3.3. Structural model of montmorillonite-PEO nanocomposites prepared from ace-
tonitrile solutions. After Ruiz-Hitzky and Aranda (1990).
10.3.3. Preparation of Clay Mineral– Polymer Nanocomposites 589
were intercalated. In contrast, a distorted helical structure for PEG is observed in
modified PEG intercalated in layered double hydroxides (LDH). This is attributed to
strong interactions between surface hydroxyl groups of LDH and the oxygen atoms
of the polymer (Leroux et al., 2003).
B. In Situ Polymerisation
This process consists of the intercalation of monomers as precursor species, followed
by their polymerisation inside the interlayer space of the pristine clay mineral.
The first polymerisation in the interlayer space of a clay mineral was reported by
Blumstein (1961) who demonstrated the possibility of achieving the homopolymer-
isation of unsaturated monomers, such as acetonitrile and methyl methacrylate,
previously intercalated in smectites. Numerous nanocomposite materials were pre-
pared by the so-called ‘in situ intercalative polymerisation’ (Kanatzidis et al., 1986).
For instance, the polymerisation of pyrrole and aniline in the interlayer space of a
clay mineral was reported by Cloos and co-workers (Cloos et al., 1979; Moreale
et al., 1985). In this process, interlayer exchangeable Cu
2+
cations in smectites pro-
mote the formation of the aniline radical (by oxidation), inducing its polymerisation
into polyaniline (PANI). The interaction of pyrrole with iron-rich smectites, con-
taining structural Fe(III), exchangeable Fe
3+
species or associated oxyhydroxyde Fe
species, spontaneously gives clay mineral–PPy nanocomposi tes Letaı
¨
ef et al., 2005).
Other polar species like thiophene and its derivatives can also form nanocom-
posites by interlayer polyme risation induced by Cu
2+
and other transition metal ions
in smectites (Cloos et al., 1973; Soma et al., 1987). Not every polar monomer gives
nanocomposites by this process. For example, vinylcarbazole gives clay–polymer
compounds after treatment of a smectite by the monomer in benzene solut ion (or in
presence of the molten polymer, at 65 1C) (Biswas and Ray, 1998). The authors
indicate that the formation of the poly(N -vinylcarbazole) (PNVC) is directly induced
by the action of the clay mineral involving cationic proton species, without any
addition of polymerisation initiators. Similarly, Lewis or Brønsted acid sites in clay
minerals such as montmorillonite and kaolinite can induce polymerisation of dif-
ferent vinyl monomers such as styrene (Solomon, 1968; Solomon et al., 1971; Haw-
thorne et al., 1974). These reactions occur by simply heating a blend of the clay
mineral and the monomer in soft conditions, giving in this case non-intercalated clay
mineral–polymer materials (micro-composites). Further, XRD shows a very weak
peak at 1.46 nm assigned to the intercalated PNVC in additio n to the peak at 1.0 nm
characteristic of the pristine clay mineral. These results are not conclusive for the
formation of true nanocomposites.
AN can also easily intercalate into smectites where it is directly associated with the
interlayer cations such as Li
+
or Na
+
, forming complexes through –CRN?cation
(ion-dipole) interactions (Blumstein et al., 1974; Bergaya and Kooli, 1991; Sanz and
Serratosa, 2002). Heat treatment or gamma-irradiation induces the polymerisation
of AN in the interlayer space, giving PAN. Similarly , AN is incorporated into the
Chapter 10.3: Clay Mineral– and Organoclay– Polymer Nanocomposite590
channels of sepiolite replacing zeolitic water and interacting with coordinated water
molecules by hydrogen bonding (Ferna
´
ndez-Saavedra et al., 2004). In this case the
polymerisation of AN to PAN is achieved using a radical initiator such as 2,2
0
-
azobisisobutyronitrile.
In contrast to hydrophilic monomers, which polymerise in the interlayer space,
low polarity monomers such as styrene, can only polymerise in the interlayer space if
the clay mineral was pr eviously modified. This will be discussed later in this chapter.
The preparation of sepiolite–polymer nanocomposites based on isoprene and
styrene entering into the tunn els of this clay mineral was reported (Inagaki et al.,
1995). On the basis of adsorption isotherms, the authors propose that the monomers
are adsorbed on both the external surface and in the tunnels of the mineral. The
strong Brønsted acid sites of sepiolite being assum ed to catalyse the process of the in
situ polymerisation. However, the occurrence of acid sites in sepiolite is uncertain
because adsorbed basic species such as pyridine are not protonated (Ruiz-Hitzky,
2001; Kuang et al., 2003). The colour changes observed after adsorption of isoprene
suggest instead that the transition metals such as iron, are involved in the polym-
erisation reaction.
Other molecules such as pyrrole and thiophene polymerise incompletely when
adsorbed on sepiolite (Inagaki et al., 1995). The formation of polymers from such
monomers, however, is of potential interest for the development of conducting
nanowires, having the size and shape imposed by the geometry of the sepiolite.
Under drastic conditions, it is possible, to polymerise unsaturated monomers such as
ethylene in the tunnels of sepiolite (Sandi et al., 1999). If the sepiolite–PE nano-
composites are subsequently carbonised by pyrolysis, carbon nanofibres useful for
solid-state lithium batteries, can be produced. Similarly, carbonisation of sepio-
lite–PAN nanocomposites can give rise to sepiolite-carbon materials useful as elect-
ro-active components in electrochemical devices. The elimination of the sepiolite
moiety by treatment with HCl and HF acids gives rise to carbon nanofibres of ca.
1 mm by 20–30 nm (Ferna
´
ndez-Saavedra et al., 2004).
C. Template Synthesis
This recent procedure (Carrado and Xu, 1998; Carrado, 2000; Carrado et al., 2000)
is based on the in situ hydrothermal crystallisation of gels from a mixture of silica
sol, magnesium hydroxide, LiF and selected water-soluble polymers in solution (Fig.
10.3.4). The synthetic clay mineral formed under these conditio ns is considered as a
poorly ordered fluorohectorite.
By this method, polymers like PANI, PAN and PVP give hybrid materials with a
controllable silicate/polymer ratio. This value determines both the structural ar-
rangement and properties such as the electrical conductivity. The clay mineral–PANI
nanocomposites prepared following this procedure are semi-delaminated, showing
clay/polymer ratios smaller than those of their counterparts prepared by in situ
polymerisation. One interesting characteristic of these nanocomposites prepared by
10.3.3. Preparation of Clay Mineral– Polymer Nanocomposites 591
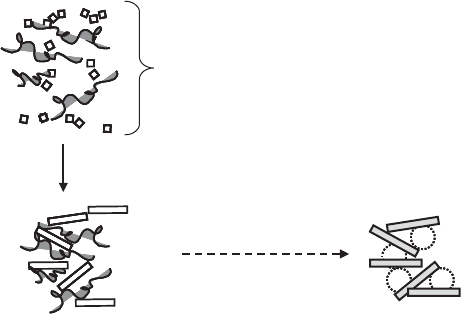
this method is their ability to form well-dispersed systems in an aqueous medium
without any loss of the polymer back into the solution (Carrado et al., 2000). Un-
fortunately, this method does not work with certain polymers such as PEO.
The compounds obtained by template synthesis are of great interest in view of the
preparation of inorganic porous materials (Fig. 10.3.4). When the PVP is removed
from the nanocomposite by calcination (at 500 1C in air), the rearrangement of
individual clay mineral layers gives nanoporous materials with a narrow distribution
of mesopores in the range of 4 to 10 nm (Carrado, 2000); in general, the pore size of
such materials is related to the MW of the polymer involved (Carrado and Xu,
1999). This is then a very interesting method to design inorganic materials with pre-
determined mesoporous dimensions representing new applications of clay–polymer
nanocomposites. An alternative approach to preparing of clay mineral–silica nano-
composites from organoclays uses the sol-gel processes involving the controlled hy-
drolysis of alkoxysilanes such as tetramethylorthosilicate (TMOS) (Lataı
¨
ef and
Ruiz-Hitzky, 2003). In this case the exfoliation of montmorillonite occurs as a con-
sequence of the form ation of silica, which is in fact an inorganic polymer.
10.3.4. HOST-GUEST COMPATIBILITY
Most polar monomers or polymers can be intercalated in the pristine clay mineral.
Because clays are hydrophilic, polymers of low polarity are in general incompatible
with unmodified raw clays. The less polar compounds can be intercalated only if
the clay minerals are previously modified by exchange with alkylammonium ions
polymer-clay
nanocomposite
SiO
2
+ Mg (OH)
2
+ LiF + polymer
(GEL)
100ºC, 2 days
calcination
(500ºC)
mesoporous solid
Fig. 10.3.4. Template synthesis of clay-polymer nanocomposites as intermediates for pre-
paring mesoporous solids. After Carrado (2000).
Chapter 10.3: Clay Mineral– and Organoclay– Polymer Nanocomposite592

(organoclays) or by grafting with suitable species. For intercalation of non-polar
polymers, it is necessary to render the mineral surface organophilic, or modify the
polymers.
A. Organoclays
The principal method used to modify the interlayer space of smectites is based on
cation exchange with alkylammonium ions (Jordan, 1949a, 1949b). Such a procedure,
well known since several decades, was thoroughly investigated by numerous research-
ers (Theng, 1974; Lagaly, 1986; Raussell-Colom and Serratosa, 1987; Yariv and Cross,
2002; Ruiz-Hitzky et al., 2004), and the resulting materials, the so-called organoclays,
are used commercially in many industries under the generic name of ‘Bentones’. The
alkylammonium species used to prepare organoclays are quaternary ammonium
compounds containing alkyl, phenyl, benzyl and pyridyl groups (Table 10.3.2).
In some cases, a mixture of alkyl- and alkenylammonium ions is used. The most
important application of these organoclays is to control the rheological behaviour of
dispersions in organic solvents where they function as thickeners and thixotropic
agents (Cody and Kemnetz, 1987; Finlayson et al., 1987 ).
Sepiolite has a much lower cation exchange capacity (CEC) than smectites (about
15 vs. about 100 cmol/kg). Nevertheless, treatment with quaternary ammonium salts
also produce organophilic solids (Alvarez et al., 1987). As in the case of smectites
additional surfactant ion pairs (cations plus the counterions) can be adsorbed (see
Chapter 7.3). These types of materials also show an excellent compatibility with low
polar organic media, and are used as paint thickeners and other industrial appli-
cations (Alvarez et al., 1984, 1985).
Several interlayer structures were proposed for alkylammonium-exchanged
phyllosilicates, mainly smectites and vermiculites. The alkyl chains are generally
lying flat on the clay mineral surface as mono- or bilayers. Other arrangements are
the pseudo-trimolecular and paraffin-type arrangements (Lagaly, 1986; Ruiz-Hitzky
et al., 2004). The factors controlling the interlayer arrangement of the alkyl chains
Table 10.3.2. Quaternary ammonium cations frequently used to prepare organoclays
Quaternary cation Abbreviation Formula
Tetramethylammonium TMA (CH
3
)
4
N
+
Trimethyl phenylammonium TMPA C
6
H
5
N
+
(CH
3
)
3
Benzyl trimethylammonium BTMA C
6
H
5
CH
2
N
+
(CH
3
)
3
Hexadecylpyridinium HDPY C
6
H
5
N
+
(C
16
H
33
)
Benzyl dimethyl tetradecylammonium BDTDA C
6
H
5
CH
2
N
+
(C
14
H
29
)(CH
3
)
2
Hexadecyl trimethylammonium HDTMA C
16
H
33
N
+
(CH
3
)
3
Dioctadecyl dimethylammonium DODMA (C
18
H
37
)
2
N
+
(CH
3
)
2
10.3.4. Host-Guest Compatibility 593
are discussed in detail in Chapter 7.3. The surface properties of the organoclays
predetermines their potential uses.
The most important new application of organoclays today is the preparation of
various types of nanocomposites. As shown above, PAN is not intercalated by un-
treated clay minerals but vermiculite exchanged with n-butylammonium is able to
form nanocomposites after treatment with PAN in dimethylformamide (Aviles et al.,
1993; Pe
´
rez-Rodrı
´
guez and Maqueda, 2002). Alternatively, this type of nanocomposite
was prepared by intercalation of AN monomer into untreated clay mineral, followed
by polymerisation using a radical initiator such as benzoyl peroxide at 50 1C during
24 h (Pe
´
rez-Rodrı
´
guez and Maqueda, 2002). As already indicated, PAN is a polymer
of interest as it may form carbon fibres by an adequate thermal treatment. When
heated, clay mineral–PAN nanocomposites give graphitised carbonaceous clay ma-
terials, which are transformed at high temperature into sialons, after carboreduction of
the silica and other metal oxides composing the silicate (Aviles et al., 1993, 1994;
Pe
´
rez-Rodrı
´
guez and Maqueda, 2002).
Low polarity polymers such as PS, in the molten state, can be directly intercalated
in alkyl-ammonium-exchanged smectites. Typical experimental conditions (Vaia
et al., 1993) require heating at 1651C in vacuum for more than 24 h. The resulting
organosmectite–PS nanocomposites show a basal spacing of 3.2 nm, which decreases
to the value of the pristine organosmectite (2.52 nm) after toluene extraction. This
type of nanocomposites displays a heterogeneous structure with well ordered inter-
calated layers and some disordered layers. This structure depends on processing
conditions. Ultrasonication and other experimental procedures were proposed to
obtain more homogeneous systems (Vaia et al., 1993).
Organoclay–PS materials have also been prepared by a novel procedure consist-
ing of modifying the clay mineral (Na
+
–montmorillonite) by ion-exchange with an
ammonium salt containing a styrene group such as CH
2
QCH–C
6
H
4
CH
2
(CH
3
)
3
N
+
Cl
(Moet and Akelah, 1993). In this case, the PS remains partially
attached to the exchangeable cation s of the clay mineral, and only the excess polymer
can be extracted with appropriate organic solvents. The insertion of grafted PS
between the clay mineral layers is clearly monitored by XRD. The basal spacing
of the exchanged clay mineral (1.5 nm) increases to 2.45 nm after react ion with
styrene.
Clay mineral–nylon nanocomposites were prepared at the Toyota Central Re-
search Laboratories (Fukushima and Inagaki, 1987; Fukushima et al., 1988; Usuki
et al., 1993). The original method involves three steps: (i) ion exchange of Na
+
for
protonated 12-aminolauric acid
+
NH
3
–(CH
2
)
11
–COOH; (ii) intercalation of epsilon-
caprolactam into the modified clay mineral; and (iii) thermal polymerisation of the
monomer, generating nylon-6 nanocomposites. These materials show interesting
mechanical properties. Although they are not the first clay–polymer nanocomposite,
they are the first to were used commercially. Since the first publication in 1993, the
preparation method for clay–nylon nanocomposites was further improved by adding
small amounts of 6-aminocaproic to accelerate the polymerisation process
Chapter 10.3: Clay Mineral– and Organoclay– Polymer Nanocomposite594
(Usuki et al., 1993). Researchers at Toyota laboratory made various attempts to
prevent the partial co-polymerisation of the epsilon-caprol actam with the interca-
lated 12-aminolauric acid. They found that the epsilon-caprolactam can be directly
intercalated in the unmodified clay mineral at 2001C. The lactam is then reacted with
the 6-aminocaproic acid, yielding also a nylon-6 nanocomposite (in situ polymer-
isation) (Kojima et al., 1993a).
Numerous papers were published, showing that the choice of the clay modifier is
important in reaching optimum mechanical properties and nowadays, commercial
nylon nanocomposites exist, wherein the 12-aminolauric acid was eventually replaced
by alkylammonium cations (Vaia et al., 1993; Krishnamoorti et al., 1996; Fornes et al.,
2002). Further, it was reported that clay–nylon nanocomposites can be synthesised in
one step, instead of the original three steps process (Kojima et al., 1993b).
B. Grafting of Clay Minerals
Silane coupling agents were extensively used to prepare micro-composites based on
clays, silica, fibreglass, etc. These agents can also be used effectively to prepare
nanocomposites, since they make the clay mineral surfaces organophilic. Organosi-
lane coupling agents contain RSi–X groups (X ¼ OR, Cl) and are able to react
with silanol groups on the clay mineral surface, givi ng stable siloxane bridges.
½surfaceRSi2OH þ X2 SiR½R
1
R
2
R
3
!
½surfaceRSi2O2SiR½R
1
R
2
R
3
þHX (1)
Smectites and vermiculites have a low content in silanol groups because these groups
only occur at particle edges. In contrast, sepiolite is rich in such reactive hydroxyls,
due to the discontinuity of the silicate layers (Ahlrichs et al., 1975). The silanol
groups located on the external surface, at the edges of the struc tural channels, are
directly accessible to reagents. After grafting with organosilanes (Ruiz-Hitzky and
Fripiat, 1976), the hydrophilic surface becomes organophilic, and sepiolite can be
easily dispersed in low-polarity compounds including polymers (Ruiz-Hitzky, 1974).
The stability of these organic derivatives is excellent; the attached groups are only
eliminated after heating at elevated temperatures by combustion or pyrolysis, re-
spectively in the presence or absence of oxygen.
Organosilanes containing unsaturated groups (vinyl and methacryloxi) or thiol
functions (3-propylmercapto) give organic derivatives of sepiolite capable of further
co-polymerisation reactions. Nanocomposites in which the polymer is covalently
bonded to the modified clay mineral were prepared (Ruiz-Hitzky, 1974).
Direct reaction of organochlorosilanes with vermiculite gives essentially organ-
osiloxanes where the silane is not grafted to the silicate (Arago
´
n de la Cruz et al.,
1973). In the absence of reactive silanol groups on the surface, the RSi–Cl groups of
10.3.4. Host-Guest Compatibility 595
the reagent are quickly hydrolysed by the water molecules directly coordinated to the
interlayer cations.
X2SiRðRÞ
3
þ H
2
O !½ðRÞ
3
RSi2OH!ðRÞ
3
RSi2O2SiRðRÞ
3
(2)
Unstable intermediates are formed which polycond ense to siloxanes.
Nanocomposites were prepared by co-hydrolysis involving silicates, organosilanes
and strong acids. The clay minerals involved are chrysotile, sepiolite, and vermiculite
(Fripiat and Mendelovici, 1968; Zapata et al., 1972; Ruiz-Hitzky and Van Meerbeek,
1978; Van Meerbeek and Ruiz-Hitzky, 1979). The extraction of octahedral cations
such as Mg
2+
ions by acid attack yields silica containing fresh silanol groups,
RSi2O2Mg2O2SiR þ½H
þ
=H
2
O!½surfaceRSi2OH þ Mg
2þ
(3)
which reacts with the organosilanols produced by hydrolysis of the chloro- or
alkoxy-organosilanes as shown in Eq. (1). Chrysotile and sepiolite preserve their
morphology even after complete removal of the octahedral sheet. When this sheet is
eliminated the particles disintegrate, and individual inorganic-organic micro- or even
nanofibres are produced. When the surface was made compatible, nanocomposites
can be prepared with many polymers.
Surface-grafting with vinylsilanes further allows co-polymerisation with various
unsaturated monomers such as methylacrylate, methylmethacrylate, n-butylacrylate,
vinylacetate and styrene (Zapata et al., 1973). The resulting nanocomposites show
improved mechanical properties; for example, the dynamic modulus is nearly 100
times higher than the neat polymer (Zapata et al., 1973). These and other grafted
phyllosilicates, and especially their blending with pol ymers merit thorough inves-
tigation.
After reaction of sepiolite with vinylsilanes, the unsaturated groups are homog-
enously distributed. This is visualised by TEM by reacting the vinyl groups on the
sepiolite with OsO
4
(Barrios-Neira et al., 1974), confirming the arrangement of the
grafted species to silanol sites along the edge of the structural channels.
Clay minerals such as sepiolites and vermiculites can also be grafted using co m-
pounds of various functionalities such as isocyanates and epoxides (Table 10.3.3).
Isocyanates react with silanol grou ps forming silyl-urethane bridges:
RSi2OH þ R2NCO ! R Si2O2CO2NH2R (4)
as demonstrated by IR spectroscopy (Ferna
´
ndez-Herna
´
ndez and Ruiz-Hitzky,
1979).
The stability of the grafted species depends on the nature of the alkyl groups (R),
which stabilise the bridges by their positive inductive effect whereas phenyl groups
Chapter 10.3: Clay Mineral– and Organoclay– Polymer Nanocomposite596

give less stable bridges. A competitive reaction is the hydrolysis of both the is-
ocyanate reagents and the silylurethane bridges.
2R2NCO þ H
2
O !ðNH2RÞ
2
CO þ CO
"
2
(5)
RSi2O2CO2NH-R þ H
2
O ! RSi2OH þ CO
"
2
þ R2NH
2
(6)
The formation of N,N
0
-disubstituted ureas (R–NH)
2
CQO, by reaction of isocyanates
with water molecules adsorbed on the surface of clay minerals is a further process that
predominates when R is a phenyl group. When sepiolite is reacted with phenyl is-
ocyanate diphenyl urea is almost quantitatively formed (Ferna
´
ndez-Herna
´
ndez and
Table 10.3.3. Organic derivatives of silicates by grafting of isocyanates and epoxides
Function Grafted groups Reagent Substrate
Isocyanates
Butyl isocyanate Sepiolite
,y
Phenyl isocyanate Sepiolite
,y
R–CQNQO RSi–O–CO–NH–R Hexamethylene
di-isocyanate
Sepiolite
y
Vermiculite
z
2,4-Toluene di-
isocyanate
Sepiolite
y
Vermiculite
z
Epoxides
1,2-Epoxybutan Sepiolite
y,z,J
1,2-Epoxyethyl
benzene
(epoxystyrene)
Sepiolite
y,z,J
CH
2
- CH-R
O
RSi–O–CH(CH
2
OH)–R 1-Allyloxy-2,3-
epoxypropane
(allylglycidyl
ether)
Sepiolite
y,z,J
2,3-Epoxypropyl
methacrylate
Sepiolite
J
3-Vinyl-7-
oxabycycle (4,1,0)
heptane
Sepiolite
Ferna
´
ndez-Herna
´
ndez and Ruiz-Hitzky (1979).
y
Ruiz-Hitzky et al. (1979).
z
Siffert and Biava (1976).
y
Casal and Ruiz-Hitzky (1977).
z
Casal and Ruiz-Hitzky (1984).
J
Casal et al. (1980).
10.3.4. Host-Guest Compatibility 597
Ruiz-Hitzky, 1979). By contrast, alkyl isocyanates, like butyl isocyanate, give stable
grafted sepiolite compounds.
The reaction of di-isocyanates, such as hexamethylene di-isocyanate and 2,4-
toluylene di-isocyanate, with vermiculite were also reported (Siffert and Biava,
1976). By heating the clay mineral to remove the associated water before starting the
chemical reaction, urea formation is avoided. The yield of grafted species depends on
the temperature of the pre-treatment. The potential interest of these grafted minerals
is to introduce free –CQNQO functions on the mineral, which are able to react
further with polyols and polyesters in view of preparing inorganic-organic polycon-
densates. Nanocomposites of isocyanate grafted silicates and polyurethane foams
have promising industrial applications (Casal et al., 1980).
Grafting of 1,2-epoxides to silanol groups present on mineral surfaces was well
established by IR spectroscopy. The epoxy functions are opened at 80–100 1C, in
vapour phase or in solution using aprotic solvents. The coupling reaction of the
epoxide group with the RSi–OH groups in sepiolite (Casal and Ruiz -Hitzky, 1977,
1984) is shown below :
CH
2
- CH-R
O
Si-O-CH (CH
2
OH) - R
Si-OH +
ð7Þ
In this case the organic R groups are linked to the surface through RSi–O–CR
bonds. The reaction takes place only in the absence of physically adsorbed water.
Vapour phase reactions can be made easily under dynamic vacuum. Reactions in
solution are carried out after elimination of ad sorbed water molecules, using a Dean-
Stark system to separate the azeotrope during the grafting reactions.
IR and chemical analysis show that the extent of grafting, related to the same
available surface area, is greater for sepiolite than for silica (Casal and Ruiz-Hitzky,
1977, 1984). This is attributed to the better ordering of RSi–OH groups on the clay
mineral compared to amorphous silica.
The reaction of allylglycidyl ether with sepiolite in organic solvents gives a
monomolecular surface coverage even when the concentrations of the starting epox-
ide are low (Casal and Ruiz-Hitzky, 1977, 1984). The treatment of clays by epoxides
with unsaturated groups such as allylglycidyl ether, 2,3-epoxypropyl methacrylate or
3-vinyl-7-oxabicycle (4,1,0) heptane (Casal et al., 1980) give rise to materials that can
be used as reinforcing fillers for elastomers and thermoplastics. The grafted groups
are very stable, and are eliminated only by burning. However, the Si–O–C bonds
could be hydrolysed by hot water. Seconda ry reactions giving carbonyl compounds
by the catalytic activity of silico-alumina centres can be produced in the reactions of
epoxides with clay minerals (Ruiz-Hitzky and Casal, 1985).
The opening of epoxy rings can be applied to forming smectite–polyether nano-
composites. This chemistry is therefore useful for the preparation of thermoset
nanocomposites that have potential applications (Wang et al., 2000).
Chapter 10.3: Clay Mineral– and Organoclay– Polymer Nanocomposite598
