Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

magnetic separation; (v) flotation and selective flocculation; (vi) drying and calci-
nation; and (vii) chemi cal modification and activation. A typical dry processing plant
for kaolin or bentonite is shown in Fig. 10.1.10. Once the basic composition of a raw
material was understood, a series of processing steps may be introduced. For high-
quality clays the processing may be relative ly complex.
B. Processing of Kaolins
Common clays for production of tiles, bricks and ceramics are generally used with-
out any processing. In some cases the largest particles are removed by sim ple sed-
imentation techniques without pre-purification to obtain homogeneous ceramic
bodies. Antique ceramists used advanced processing of common clays to obtain the
refined clays for shaping and the slips for decorat ion. The red/black decoration
(‘Glanzton’ technique) of the famous Antique vases with the appealing gloss was
produced by using clay masses and slurries with different contents of kaolinite and
illite (Hofmann, 1962b; Noll, 1982, 1991).
Kaolins for the paper industry now require sophisticated and extensive processing
technologies (Table 10.1.4). Fig. 10.1.11 summarizes the applied technologies that
may be used to refine and improve the industrial properties of kaolins. Fig. 10.1.12
shows a US kaolin mining and degritting operation from which the degritted kaolin
is piped up to 40 km to a processing plant (Fig. 10.1.13) that may typically produce
over 1 million tons of processed kaolin.
C. Raw and Activated Bentonites
Bentonites are fine-sized materials, and hence are generally used without significant
size fractionation (Figs. 10.1.14 and 10.1.15). As certain pr operties of bentonites can
change between neighbouring pits or between individual layers within a pit, blending
is common ly used to maintain a consistent product with standard properties.
Bentonites may be used in the raw form (as mined) or they may be activated (Tables
10.1.2b and 10.1.3). Alkali or soda-activation, using sodium carbonate (Na
2
CO
3
),
transforms the calcium (magnesium) bentonites into sodium-calcium forms. The
bentonite with water contents of 35–40% (w/w) is usually kneaded or milled with
1–5% (w/w) sodium carbonate and homogenized (Fig. 10.1.16). This process of soda-
activation was first proposed by Hofmann and Endell (1935). Fluid dispersions are
usually avoided because sodium bentonite dispersions are extremely difficult to filter
due to their high viscosities (see Chapter 5). Nevertheless, smaller amounts of high-
quality sodium bentonites, or especially hectorites for certain applications, are pro-
duced in dispersions after careful selection of the raw bentonite. The quality of the
products can be improved by special processing of the raw bentonite (fractionation or
hydrocycloning, mechanical treatments, reaction with heated water vapour, etc.).
During soda-activation a large part of the interlayer calcium ions is precipitated
as calcium carbonate. As the calcium ions are still present in the system when this
Chapter 10.1: Conventional Applications518
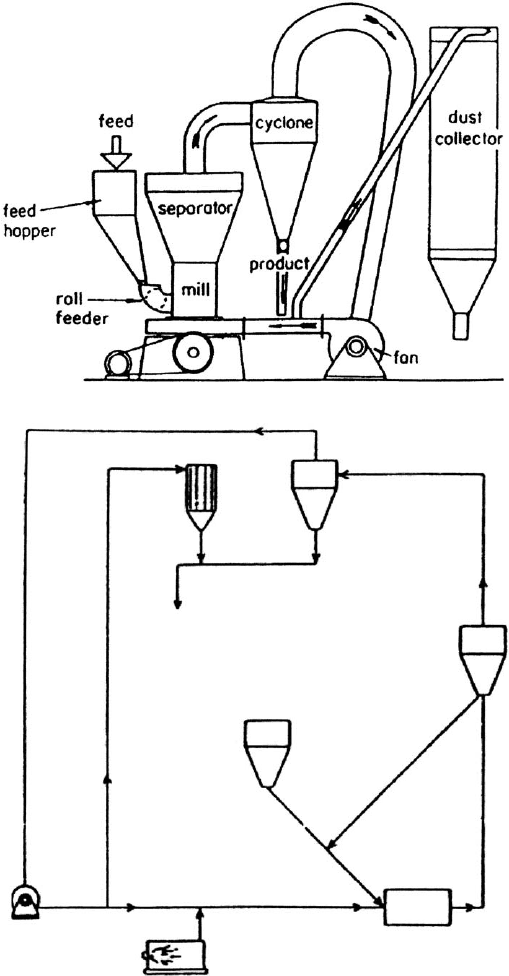
dust collector
product collector
product
classifier
feed
ov
ersize
mill
hot air
furnace
exhaust fan
vent
(a)
(b)
Fig. 10.1.10. Dry processing for kaolin or bentonite. (a) Mill, cyclone, and dust collection
circuit, (b) hot air circuit for drying in grinding mill.
10.1.2. Processing Industrial Clays 519
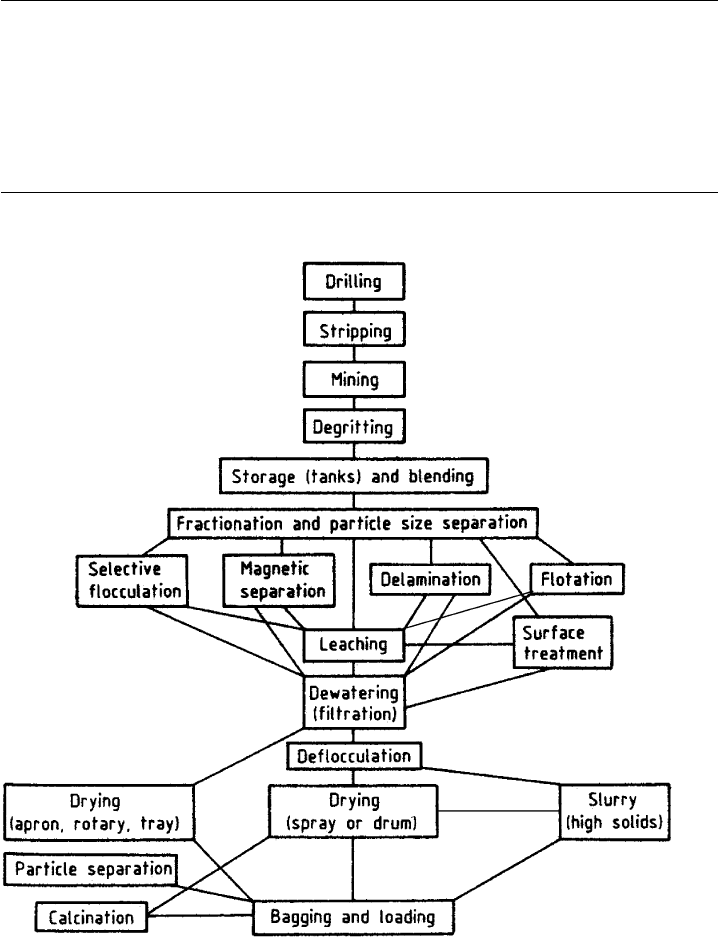
Fig. 10.1.11. Flow sheet showing the various stages and complexities in the modern wet
processing of kaolin.
Table 10.1.4. Applied technologies used in kaolin processing
Delamination
High gradient magnetic separation
Selective flocculation
Column flotation
Ozone bleaching
Ultrafine particle size separation
Surface modification
Calcination
Chapter 10.1: Conventional Applications520

bentonite is dispersed in water, the degree of delamination is not optimal, and the
colloidal properties depend on the amount of soda added (Fig. 10.1.17) (see Chapters
4 and 5). The degree of thixotropy (hysteresis of the flow curves) reaches a maximum
as a function of the amount of soda added. The yield value also increases to a
Fig. 10.1.13. A US kaolin processing plant, Georgia, USA.
Fig. 10.1.12. Mining and degritting operation in a kaolin mine, Georgia USA.
10.1.2. Processing Industrial Clays 521

Fig. 10.1.14. Bentonite pit in Bavaria, Germany (Courtesy N. Schall, Su
¨
d-Chemie AG,
Germany).
Fig. 10.1.15. Storage of raw bentonite (Courtesy N. Schall, Su
¨
d-Chemie AG, Germany).
Chapter 10.1: Conventional Applications522

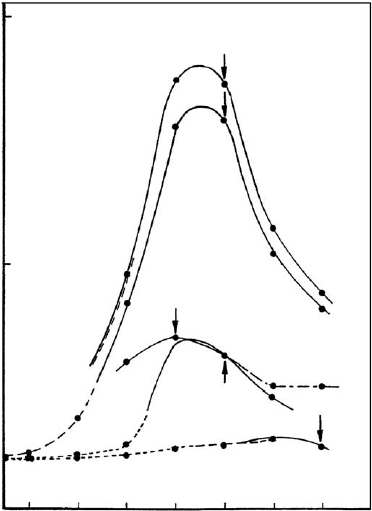
maximum at an optimum addition of soda (Fig. 10.1.18). The properties of bent-
onites respond differently to soda-activation. This is not only due to differences in
particle size distribution betw een the raw bentonites but also because of differences
in layer arrangement within individual mo ntmorillonite particles (see Chapter 5).
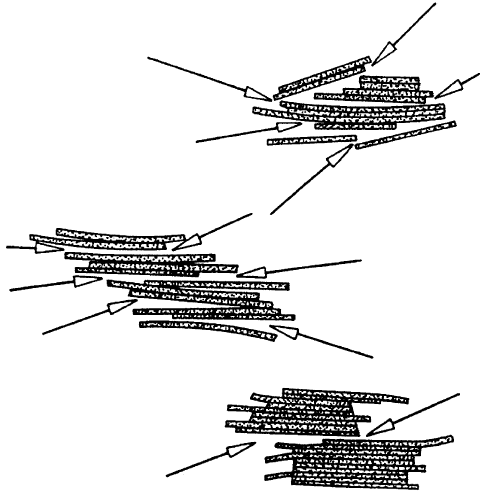
Domains of silicate layers with a certain degree of ordering are separated by areas of
loose contact s (‘breaking points’), deno ted by arrows in Fig. 10.1.19. Depending on
the distribution and the extent of these defects in the parent montmorillonite, soda-
activation yields different size distributions of the delaminated particles .
The strong dependence of rheological properties on the Na
+
/Ca
2+
ratio is illus-
trated in Fig. 10.1.20 (see also Chapter 5). Varying the Na
+
/Ca
2+
ratio is, therefore,
an important way to optimize and tailor the propert ies of soda-activated bentonites
for the intended applications.
Many applications, listed in Tables 10.1.2b and 10.1.3, require hydrophobized
bentonites. To this end, the bentonite is usually reacted with quaternary alkylam-
monium salts such as dialky l dimethylammonium (I), dialky l benzylmethylammo-
nium (II), and alkyl benzyldimethylammonium salts (III),
I II III
N
CH
3
CH
3
R
R
CH
2
N
CH
3
R
R
CH
2
N
CH
3
R
H
3
C
Fig. 10.1.16. Activation of bentonite by kneading with soda (Courtesy N. Schall, Su
¨
d-Chemie
AG, Germany).
10.1.2. Processing Industrial Clays 523
The technical products contain alkyl chains (R) of different lengths. For instance,
a technical dioctadecyl dimethylammonium chloride had the composition (% w/w):
1.5 C
14
, 0.8 C
15
, 27.5 C
16
,2C
17
,67C
18
, 1.2 C
20
(Favre and Lagaly, 1991). Ditalloyl
ammonium salts (derived from by-products of cellulose production from deal and
pine wood) are mixtures of dialkyl dimethylammonium surfactants with varied alkyl
chain length s, for instance 65% C
18
, 30% C
16
and 5% C
14
.
The amount of alkylammonium ions added corresponds to the cation exchange
capacity (CEC) of the clay, and the exchange is nearly quantitative. Products with
smaller amounts of alkylammonium salts can also be obtained.
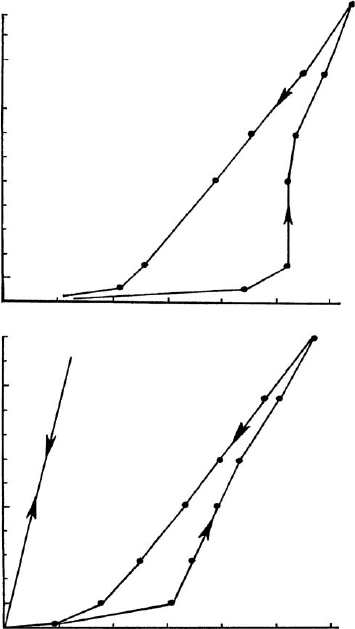
rate of shear (s
-1
)
100
100
50
0
A
C
500
100
shear stress (mPa)
100
50
0
B
500 700
Fig. 10.1.17. Soda-activation of bentonites. Influence of soda addition on the flow behaviour
(shear rate vs. shear stress) and thixotropy. 2% dispersions of Amory bentonite (Mississippi,
API 22b, Wards). A 0.5 mmol Na
2
CO
3
/g bentonite, B 2.5 mmol Na
2
CO
3
/g bentonite,
C 5 mmol Na
2
CO
3
/g bentonite.
Chapter 10.1: Conventional Applications524
In most cases the products are not washed after cation exchange so that some free
(unreacted) alkylammonium salts may still be present. These free salt influences the
properties of the product such as ion-exchange and rheological behaviour (see Fig.
5.43, Chapter 5). The ratio alkylammonium salt/CEC and the treatment of the
organo-bentonite after the exchange reaction are parameters that can be used to
tailor these products.
Bleaching earths are obtained by treating bentonite with acids in such a way that
the layer structure is largely decomposed (see Chapter 7.1). Typically, the bentonite
is reacted with hydrochloric acid (up to 17 mmol/g bentonite) at 90–100 1C for a few
hours, then washed to reach pH 4, dried, milled and sieved (Fig. 10.1.21). Con-
centration of the acid, temperature and activation time determine the properties of
the prod uct (Fahn, 1973 ). The specific surface area and bleaching activity usually
0 0.05
0.25 0.5 1 2.5 5 10
soda addition (mmol/
g
bentonite)
maximum thixotropy
Dakota
Cameron
Bavaria
Otay
Amory
1
0
0.5
shear stress at a shear rate of 100 s
-1
Fig. 10.1.18. Rheological properties of 2% dispersions of different bentonites. The curves
show the effect of soda addition on shear stress measured at a rate of shear of 100 s
1
. Arrows:
denote maximum thixotropy. Newtonian flow, – d – d – (pseudoplastic), ——
thixotropic, – – – antithixotropic. Bentonite from Amory, Mississippi (API 22b, Wards);
Bavaria (Schwaiba, Su
¨
d-Chemie, Germany); Cameron (Arizona, API 31); Dakota; Otay
(California, API 24). From Jasmund and Lagaly (1993).
10.1.2. Processing Industrial Clays 525
show maximum values at distinct activation conditions whereas the pore volume and
the silica content increase continuously (Fig. 10.1.22)(Fahn, 1973; Christidis et al.,
1997). The large volumes of acidic waste waters, containing aluminium salts, that are
produced can give rise to environmental problems. Clays of certain localities exhibit
similar adsorption properties without acid-activation. Before bleaching earths were
produced at an industrial scale, fuller’s earth was used for oil bleaching (Bene ke and
Lagaly, 2002 ).
10.1.3. LABORATORY EVALUATION OF CLAY SAMPLES
Any clay material may be recognized by some plasticity in its raw stat e. Some clay
materials may meet the required specifications for industrial uses with no processing
or blending with other raw materials. Other clays may be components in simple or
complex blends of various raw materials. Other clay raw materials require simple or
complex processing to remove contaminants or non-clay minerals.
The assessment of an undeveloped clay resource requires a series of screen-
ing techniques by which the clay technician can progressively move towards the
Fig. 10.1.19. Montmorillonite particles with inherent ‘‘breaking points’’ (arrows). From Jas-
mund and Lagaly (1993).
Chapter 10.1: Conventional Applications526
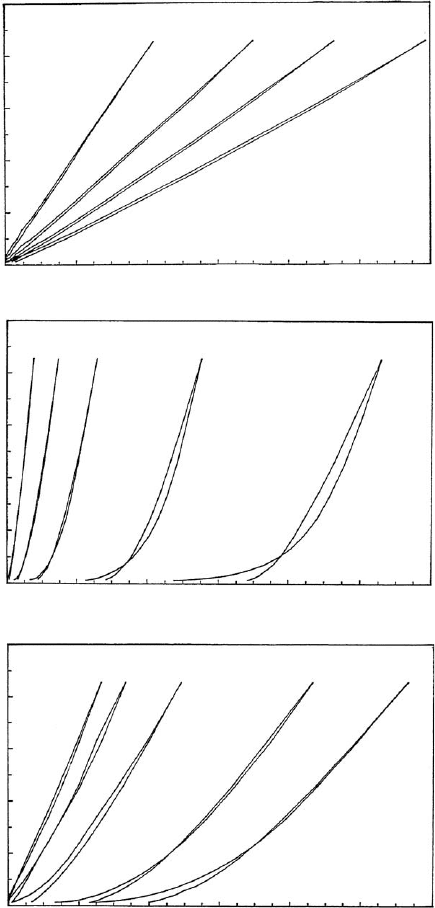
0.5 %
0 100 200 300 400
shear stress (mPa)
150
75
0
rate of shear (s
-1
)
1.5 % 2 %
2.5 %
1 %
1.5 % 2 % 0.5 2.5%
0 600 1200 1800 2400 3000 3600
shear stress (mPa)
150
75
0
rate of shear (s
-1
)
0.5 % 1 % 1.5 % 2 % 2.56 %
shear stress (mPa)
75
150
0
rate of shear (s
-1
)
0 120 240 360 480 600 720
(a)
(b)
(c)
Fig. 10.1.20. Flow curves of sodium bentonite (Bavaria, Germany), (a) in water, (b) in
0.01 M CaCl
2
, (c) in 0.025 M CaCl
2
. Bentonite content 0.5–2.5% (w/w), pH ¼ 6.5, 20 1C. From
Permien and Lagaly (1994).
10.1.3. Laboratory Evaluation of Clay Samples 527
