Bennemann K.H., Ketterson J.B. Superconductivity: Volume 1: Conventional and Unconventional Superconductors; Volume 2: Novel Superconductors
Подождите немного. Документ загружается.

14 High-T
c
Superconductivity 767
perconductors contain four or more constituents
in their chemical composition and their crystallo-
graphic structures, although being rather similar for
all of them, exhibit some intricate details whose im-
portance is still debated. It is for this reason that,be-
fore presenting and discussing a selection of physical
properties of high-T
c
superconductors, a section on
materials aspects including chemical compositions
and crystal structures is inserted at the beginning of
this chapter. An additional justification for this sec-
tion is the fact that more recently,solids with similar
structural properties have attracted a lot of attention
in other areas of condensed matter research, involv-
ing spin charge and orbital ordering phenomena [8].
With respect to physical properties in general and
to features of the superconductingstate in particular,
we shall concentrate on some typical aspects rather
than list and present many details. For instance, the
entire field of vortex physics that has emerged and
has attracted a lot of attention in connection with
high-T
c
superconductors, is discussed in a special
chapter of this treatise.
It was recognized very early [9] that the cuprate
materials, which exhibit the highest critical temper-
atures for superconductivity at present, cannot sim-
ply be regarded as common metals, because even the
normal state of these materials exhibits anomalous
features that are difficult to understand. Therefore,
some attention is also given to properties of the nor-
mal state above T
c
.
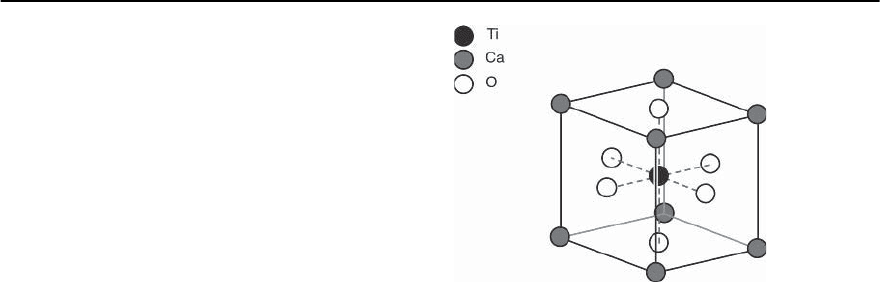
14.2 Typical Structural Characteristics
Although, as pointed out above, no consensus about
the real causes for superconductivityat elevated tem-
peratures has yet been achieved, all relevant materi-
als to be discussed here, with the exception of the
fullerenes and MgB
2
,insomewayshareacommon
structural feature and this is the unit cell of the per-
ovskite structureshown in Fig.14.2.This structureis
adopted by ABO
3
compounds in which A is a fairly
large cation and B, a metal element, helps to form a
three-dimensional array of corner-sharing BO
6
octa-
hedra. The undistorted version is cubic,as indicated
in Fig. 14.2, and only very few compounds for which
the ionic radii of theA atoms are of sufficientsize,are
Fig. 14.2. Schematic representation of the crystallographic
unit cell of CaTiO
3
(perovskite)
known to adopt the truly cubic perovskite structure.
Most of the so called perovskite materials crystallize
in a distorted version, the most common being an
orthorhombic distortion.Most of the fullerenes also
crystallize in cubic structures (fcc) but the occupa-
tion per lattice site is given by a rather large basis,
formed by the C
60
.
Duringthe time,when investigationsof supercon-
ducting materials were focused on either chemical
elements or, at most binary compounds and alloys, it
was argued [10] that a high symmetry of the crystal
lattice was favorable for achieving high critical tem-
peratures. Superconductivity of the cubic A15 com-
pounds with critical temperatures between 15 and
20 K was taken as the show case for this conjecture.
The most recent and also rather surprising excep-
tion from this trend is,no doubt,MgB
2
which adopts
a structure with hexagonal symmetry [11]. Its criti-
cal temperature, of the order of 40 K, is by now no
longer a top value in general, but for simple binary
compounds, it most certainly is.
14.2.1 BaPb
1−x
Bi
x
O
3
,BaPb
1−x
Sb
x
O
3
,BaBi
1−x
K
x
O
3
The structure of these non cuprate materials of the
type BaPb
1−x
Bi
x
O
3
and Ba
1−x
K
x
BiO
3
varies with the
parameter x and different varieties of distorted per-
ovskitetypearrangementsoftheatomsareobserved.
The Pb/Bi alloy series exhibitsa number ofstructural
phase transitions,starting with an orthorhombic lat-
tice for metallic BaPbO
3
[12], changing to tetragonal
at x ∼ 0.1, back to orthorhombic for x ∼ 0.35 [13]
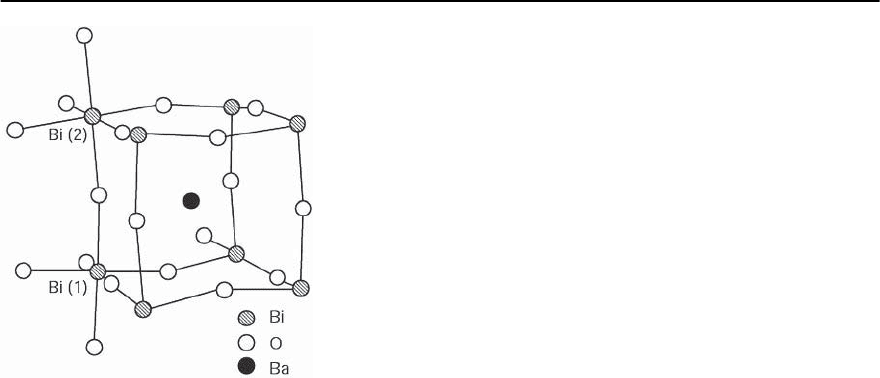
768 H.R.Ott
Fig. 14.3. Schematic representation of the crystallographic
unit cell of BaBiO
3
, as reported in [14]
and finally distorting to a monoclinic lattice struc-
ture at x ∼ 0.9 [14]. In the range of 0.1 < x < 0.3,
also the Pb/Sb alloys adopt a tetragonally distorted
perovskite-type structure. BaBiO
3
is electrically in-
sulating. Its crystallographic unit cell is shown in
Fig. 14.3, revealing the distorted perovskite-type ar-
rangement of the atoms by the quasi-octahedral co-
ordination of oxygen atoms around the Bi atoms on
inequivalent sites. On site B(1), one of the Bi-O dis-
tances is much shorter than on site B(2) and this
freezing of a breathing mode is thought to be the rea-
son for the insulating ground state. The substitution
of Ba with K atoms in BaBiO
3
to form Ba
1−x
K
x
BiO
3
,
eventually triggers a transition to a cubic crystal lat-
tice and a metallic ground state [15,16]. It is obvious
that for these oxides, structural and electronic prop-
erties are intimately coupled, a feature that is also
observed for the cuprate materials to be discussed
below.
14.2.2 Copper Oxide Superconductors
The crystal structures of all superconducting Cu ox-
ides are more or less evidently related to the per-
ovskite structure. These Cu compounds belong to a
large class of mixed-valency Cu oxides where the Cu
cations may adopt different ionic configurations (2+
or 3+). In some cases rather the concept of interme-
diate valence (between 2+ and 3+) seems appropri-
ate. The three-dimensional character of the original
perovskite structure,which may also be viewedas be-
ing built by a stacking of AO and BO
2
planes, is lost
because these planes are now stacked in different se-
quences,leading to a more or less sizeable anisotropy
between the directions parallel and perpendicular to
these planes. A fairly transparent case in this respect
is the parent compound of the material where su-
perconductivity of the cuprates was discovered by
Bednorz and M¨uller [1].
La
2−x
A
x
CuO
4+ı
(A = Sr, Ba)
An early study [17] of substances of this type with
A = Ca, Sr, Ba and Pb revealed the metallic conduc-
tivity for these materials in the form of a decreas-
ing electrical resistivity with decreasing temperature,
i.e.,∂/∂ T > 0. For unknown reasons,this study was
limited to temperatures above 200 K,but it contained
also important information concerning the crystal
structure of this series of compounds. Later a more
detailed investigation [18] confirmed and extended
these findings. As is already legend by now, it was
this type of materials where Bednorz and M¨uller [1]
found the first evidence for the onset of supercon-
ductivity between 30 and 40 K.
Ter nary La
2
CuO
4
, an insulating antiferromagnet,
crystallizes in a tetragonal K
2
NiF
4
-type structure
but at lower temperatures adopts an orthorhombi-
cally distorted version of this structure, induced by a
cooperative alternating tilting of the CuO
6
octahedra
about the [110] tetragonal axis, as shown in Fig. 14.4
[19, 20]. For x = 0 the tetragonal to orthorhombic
transition occurs at approximately 530 K. The on-
set temperature of the orthorhombic distortion T
d
can be reduced by partly replacing La by an alkaline
earth element A and above a critical concentration
of x ∼ 0.2, the tetragonal K
2
NiF
4
-type structure is
stable down to very low temperatures [21, 22]. The
tetragonal arrangement, also denoted as T-structure
and shown in Fig. 14.5,may be regarded as a stacking
of different planes that are also contained in the orig-
inal perovskite structure. Between two CuO
2
(BO
2
)
planes, two LaO (AO) planes instead of only one
are inserted,hence weakening the three-dimensional
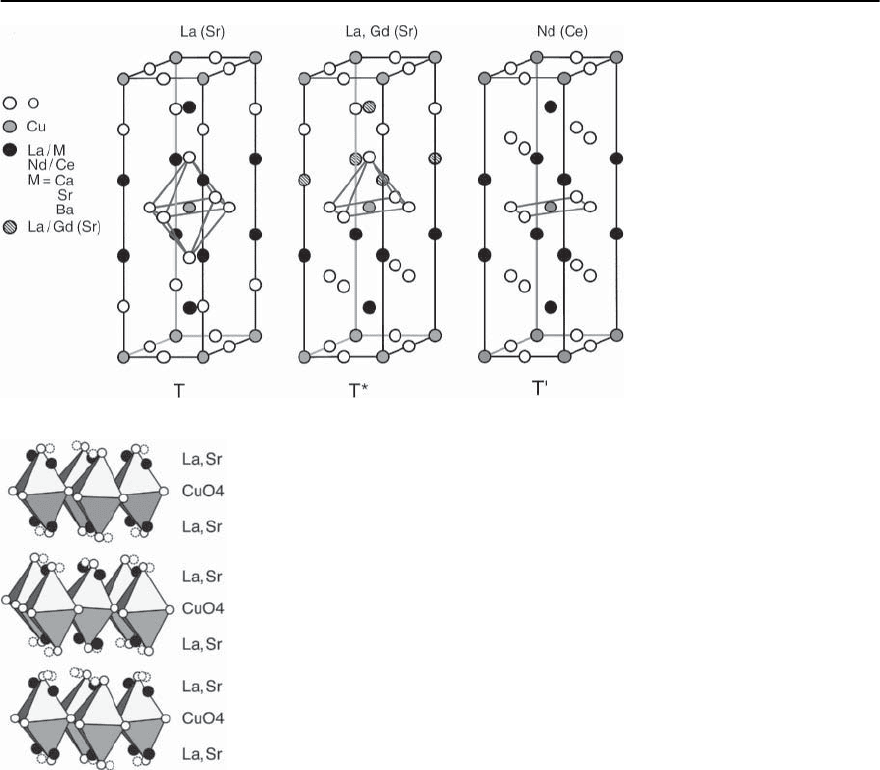
14 High-T
c
Superconductivity 769
Fig. 14.5. Schematic representa-
tion of the tilting of the oxygen
octa-hedra in La
2−x
Sr
x
CuO
4
(see [36])
Fig. 14.4. Schematic representation of the tilting of the oxy-
gen octahedra in La
2−x
Sr
x
CuO
4
(see [36])
character of the structure.The essential subunitsare
clearly the CuO
2
planes, a major characteristics of
all known cuprate superconductors. For A = Ba, the
appearance of a low-temperature tetragonal phase
has been reported for x values around 0.125 [23].
As is well known, enhancing x eventually leads to
a metallic behavior and superconductivity. Optimal
conditions for superconductivity are reached for ma-
terials exhibiting the orthorhombiccrystal structure
at T ∼ 4T
c
. Structural effects are particularly pro-
nounced in La
2
CuO
4+ı
, containing excess oxygen on
interstitial sites.Also oxygen rich material undergoes
a transition from a tetragonal high-temperature to
an orthorhombic low-temperature phase [24]. The
transition temperature T
d
is only weakly reduced
upon increasing ı. For low oxygen surplus, a macro-
scopic phase separation phenomenon is observed be-
low room temperature. The material separates into
an oxygen-rich metallic phase and an oxygen-poor
insulating phase with an antiferromagnetically or-
dered ground state [24].
Ln
2−x
Ce
x
CuO
4−ı
(Ln = Pr, Nd, Sm)
Cu-oxides with a 214-type composition as men-
tioned above also form if La is replaced by rare-earth
or lanthanide (Ln) elements.These compounds,how-
ever, adopt a somewhat different crystal structure
[25], the so called T’ structure and they keep the
tetragonal structure down to low temperatures. It is
depicted in Fig. 14.5. Here, the oxygen environment
of each Cu atom in the form of a planar square is
distinctly different from that of an octahedron in the
T structure. As will be discussed later, the ternary
compounds are again insulating antiferromagnets.
Both the Cu spins and the localized Ln moments are
involved in magnetic-orderingphenomena,at differ-
ent temperatures, however.Metallic behavior and su-
perconductivity is obtained by a partial replacement
of the trivalent Ln element by tetravalent Ce and, in
addition, a slight reduction of the oxygen content.
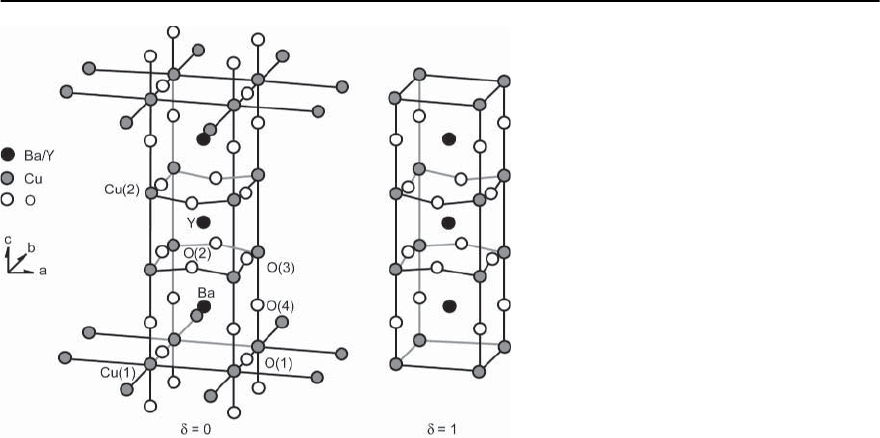
770 H.R.Ott
Fig. 14.6. Schematic representation of the unit
cells of the crystal structure of YBa
2
CuO
7−ı
for
(a) ı =0,and(b) ı =1
Ln
2−x−y
Ce
x
Sr
y
CuO
4−ı
Yet another type of Cu-O coordination is obtained
in this type of compounds where part of the Ln sites
areoccupied by Ce and Sr [26].The resulting unitcell
of the structure is shown in Fig. 14.5. A remarkable
featureof this T*-structureisthe lack of an inversion
center, obviously implying polarity but, nevertheless,
with the proper values of x,y, and ı, superconductiv-
ity may still be achieved.The Srcontent mustbe large
enough in order to allow for a sequential ordering
(Sr,Ce) and (Nd,Ce) planes as indicated in Fig. 14.5.
MBa
2
Cu
3
O
7−ı
,MBa
2
Cu
4
O
8
Structurally,these two types of compounds, where M
may be Yttrium or any element of the rare-earth se-
ries except Ce and Tb, are related but chemically,
the second type of materials is much more stable
than the first. Again the structures may be viewed
as stackings of different layers with different chemi-
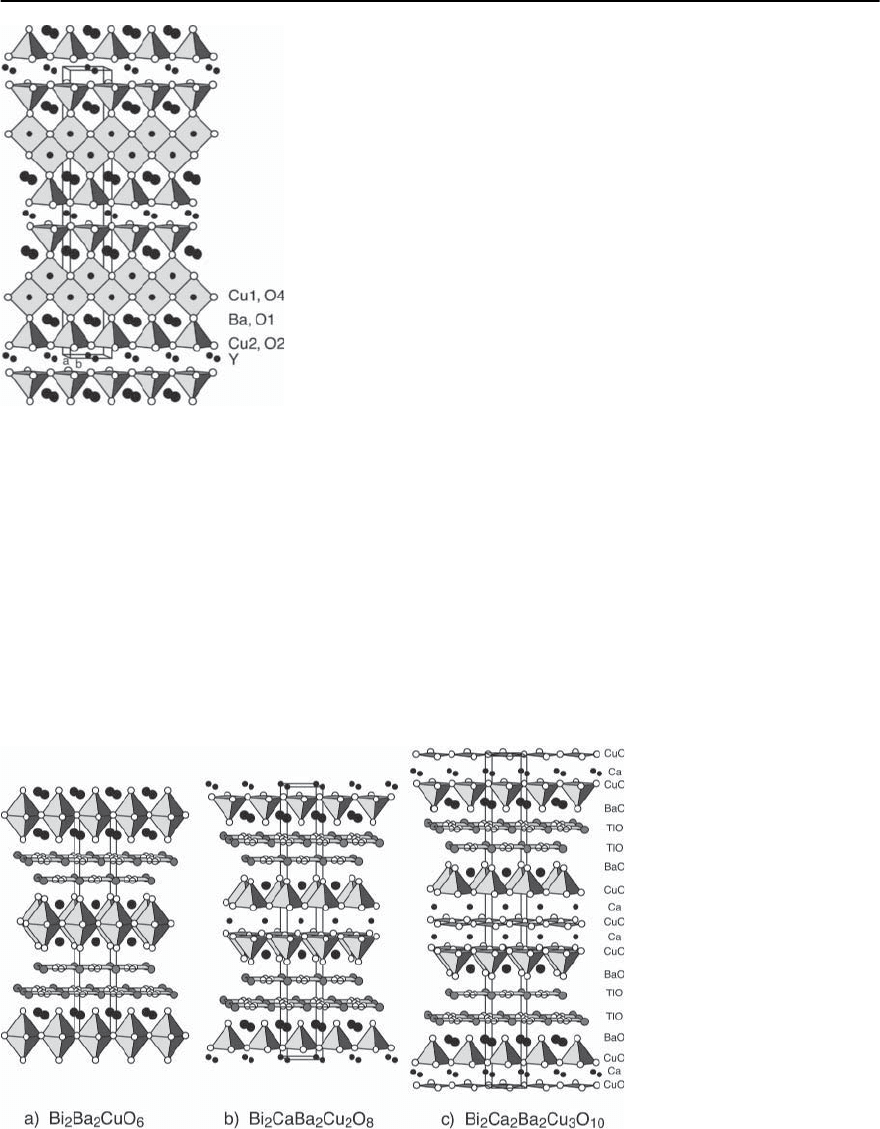
cal compositions. For YBa
2
Cu
3
O
7−ı
(YBCO-123),the
stacking sequence in the crystallographic unit cell is
Y-CuO
2
-BaO-CuO
x
-BaO-CuO
2
-Y, reminiscent of the
perovskite structure [27]. The resulting orthorhom-
bic unit cell is shown in Fig. 14.6a for the ideal case
where ı = 0, i.e., x = 1. We note two inequivalent
Cu sites which can most easily be distinguished by
their different oxygen environments. In detail the M
atoms separate two identical blocks which contain
two CuO
2
planes with the Cu(2) sites,two BaO planes
and a plane formed by Cu-O chains along the b di-
rection of the orthorhombic structure involving the
Cu(1) sites. As may be seen from Fig. 14.6a, the oxy-
gen coordination of the two Cu sites is pyramidally
for the Cu(2) sites and linear for the Cu(1) sites. It is
the missing oxygen between the chains which gives
rise to the orthorhombic distortion of the lattice.
This low temperature orthorhombic structure devel-
ops out of a tetragonal high temperature structure
at about 750
◦
C by a rearrangement of atoms within
the oxygen sublattice. This rearrangement can be re-
versed at low temperatures by reducing the oxygen
content of the material. For ı =0.6, the structure
is again tetragonal also at low temperatures. Upon
depletion, oxygen vacancies are formed within the
Cu-O chains and they redistribute in a fashion such
that on the average, oxygen atoms occupy sites along
the a and b directions with equal probability [28].For
ı = 1, i.e., x = 0, the former Cu-O chains are fully
depleted of oxygen. The resulting structure is shown
in Fig. 14.6b. This variation of the oxygen content
not only influences the structure of the crystal lattice
but also many other properties of different ground
states, as we shall see below. A particular aspect of
14 High-T
c
Superconductivity 771
Fig. 14.7. Schematic representation of the unit cell of the
crystal structure of YBa
2
Cu
4
O
8
(see [36])
the crystal structure in this YBCO-123 series is the
formation of superstructures in different regions of
oxygen content, leading to inhomogeneities and dif-
ferent orthorhombic phases at different values of ı.
Since a detailed discussion of these aspects is beyond
the scope of this review, we refer to relevant work in
the literature [29]. The unit cell of the MBa
2
Cu
4
O
8
(YBCO-124) compounds[30,31] is shown in Fig.14.7.
As mentionedabove this type of compoundis chemi-
cally less fragile than theYBCO-123 variety and crys-
tals may be grown without twin boundaries, a ma-
jor problem in the growth of YBCO-123 compounds.
The insertion of a second Cu-O chain element,form-
ing Cu-O ribbons rather than chains, enhances the
anisotropy, especially within the planes perpendicu-
lar to the c axis. In this Y–Ba–Cu–O quaternary sys-
tem,an additional stable compound with yet another
although related structure and composition, namely
Y
2
Ba
4
Cu
7
O
14+ı
,in short YBCO-123.5,has been iden-
tified [32,33].The corresponding unit cell is obtained
by stacking theunitcellsof YBCO-123andYBCO-124
on top of each other.Concerning the Cu-O structural
elements, the unit cell of the atomic arrangement
thus contains, apart from the notoriousCu-O planes,
both Cu-O ribbons and Cu-O chains.
A
m
M
2
R
n−1
Cu
n
O
3n+m+1
A fairly large number of high-T
c
cuprate supercon-
ductors may be classified according to the schematic
chemical composition given in the subtitle.Thistype
of compounds form with A = Hg, Tl or Bi, M = Sr
or Ba and R = Ca or a heavy rare earth. These ma-
terials are of particular importance because some of
them exhibit the highest critical temperatures that
have been achieved until today. For A = Hg, m may
be 1, for Bi only 2 and for Tl 1 or 2. Although Bi-
Fig. 14.8. Schematic represen-
tation of the crystal structure
of some Bi-based Cooper ox-
ide superconductors: Bi-2201,
Bi-2212 and Bi-2223 (see [36])
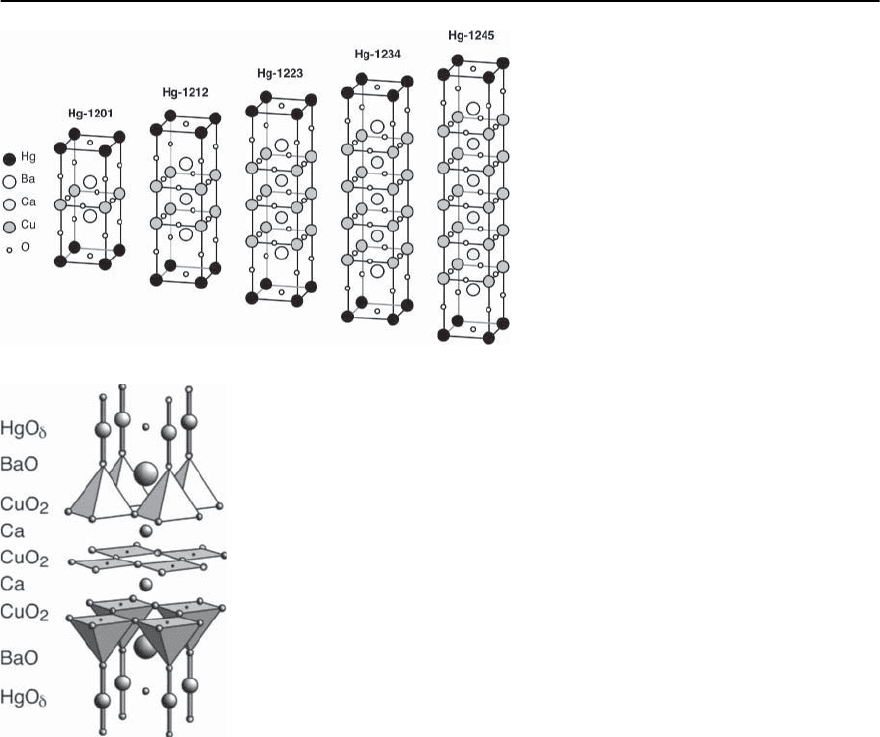
772 H.R.Ott
Fig. 14.9. Schematic representation of the crys-
tallographic unit cells of Hg-12(n-1)n copper
oxides
Fig. 14.10. Schematic representation of the crystallographic
unit cell of HgBa
2
Ca
2
Cu
3
O
8+ı
based cuprates have been synthesized with the 1222-
type structure, all of them turned out to be non su-
perconducting above 1.5 K [34]. In most cases the
parameter n varies from 1 to 4 but may also adopt
higher values.The parameter n indicates the number
of CuO
2
planes within one unit cell, separated from
each other by n-1 Ca layers, and as such it is related
with some physical properties to be discussed below.
Thestructuresofthesecompoundsmaybeviewedas
sequential stackings of elements of the perovskite as
well as the NaCl structure [35].Very often, crystallo-
graphic shears,i.e., shifts between adjacent layers are
introduced in the stacking sequences. A nice com-
pilation of possible atomic arrangements in the Tl-
based and Bi-based compounds is given, e.g., in [36]
and therefore we present only three examples of the
physically most important Bi compounds in Fig. 14.8
(on page 771). It is also possible to partially replace
some of the above mentioned constituents by other
elements. One such example is Pb substituting for
Bi, which in some cases provides a way to stabilize
a favorable phase, such as the Bi-2223 phase [37].
The latest progress in raising T
c
has been made in
the series HgBa
2
Ca
n−1
Cu
n
O
3n+2
[38] and it also has
been possible to synthesize thin layers of material
where n ≥4 [39]. A schematic representation of the
stacking with increasing n is shown in Fig. 14.9 and
a somewhat more detailed drawing of the unit cell of
HgBa
2
Ca
2
Cu
3
O
8+ı
,the material for which the highest
values of T
c
have as yet been observed, is presented
in Fig. 14.10.
Ca
1−x
Sr
x
CuO
2
The structure of this type of compound is of interest
here, because it may be viewed as the result of en-
hancing the number n of CuO
2
layers per unit cell
in the compounds discussed in the previous section
towards infinity. Taking into account the n −1Ca
interlayers, for large values of n,thestoichiometry
will approach that of CaCuO
2
. With respect to the
perovskite structure, the (Ca/Sr) layers may be re-
garded as AO layers from which all the oxygen atoms
have been removed. CaCuO
2
, a hypothetical tetrago-
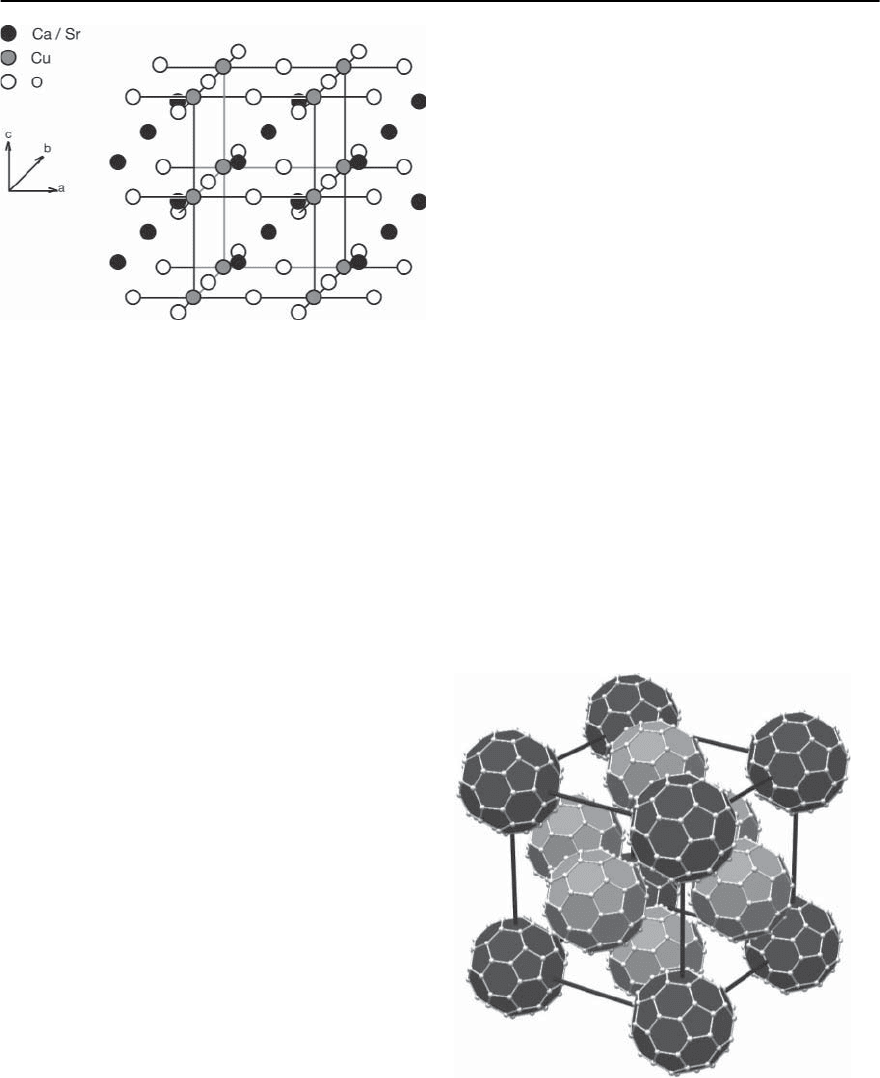
14 High-T
c
Superconductivity 773
Fig. 14.11. Schematic representation of the crystal structure
of Ca
1−x
Sr
x
CuO
2
(see [40])
nal compound does not form but it has been found
that small amounts of Sr on Ca sites can stabilize
the structure, which is shown in Fig. 14.11, and sin-
gle crystals may be grown [40]. Subsequently it has
been found that a high pressure synthesis helps to
extend the compositional range of stability consid-
erably [41].
General Remarks
This brief overview of some crystal structures of rel-
evant cuprate materials demonstrates quite clearly
that one ingredient, namely the CuO
2
planes, are
a common building element for all the structures
presented in the previous subsections. Even before
discussing the physical properties of these materials
we note that the electronic features are, to a large
extent, dominated by these CuO
2
planes. They are
separated from each other by additional building
blocks that serve both to stabilize the structure and
as charge reservoirs, which control the number of
itinerant charge carriers in the planes containing Cu
atoms.In this sense, the structures of the compounds
mentioned in the first three subsections contain one
CuO
2
plane per unit cell. Two CuO
2
planes per unit
cell are the hallmark of the compounds mentioned
in the fourth subsection. Structurally the most sim-
ple member of this type of double-layer compounds,
but not mentioned in detail here,is La
2−x
Sr
x
CaCu
2
O
6
[42]. The compounds with the possibility of accom-
modating more than two CuO
2
planes per unit cell
are mentioned in Sect.14.5.Another important com-
pound,with all the features of the m =2andn =2Tl-
based or Bi-based compounds,except foran inserted
oxygen depleted Cu layer, is Pb
2
Sr
2
YCu
3
O
8
[43].
14.2.3 Fullerites, Fullerides
Fullerites are solids composed by a regular arrange-
ment of C
60
molecules, which belong to a large class
of fullerenes,i.e.,stable molecules formed by a closed
carbon network (C
60
,C
70
,C
540
,etc.)[44].Forourpur-
poses, only C
60
molecules are of interest, because up
to now, bulk superconductivity has only been ob-
served in solids formed by them. A pure C
60
solid
is, under normal circumstances, an electrical insula-
tor [45]. It adopts an fcc structure which, with de-
creasing temperature, transforms to a simple cubic
structure, at 260 K, accompanied by an orientational
order of the individual C
60
molecules [46]. Metal-
lic C
60
-based compounds, the fullerides, may be ob-
tained by doping [47] and the most relevant ma-
terials in our context are A
3
C
60
,whereAisK,Rb,
Cs or some combination of these three alkali ele-
ments and Na
2
AC
60
,where A may again be one of the
other alkali elements or a combination of them [45].
The structure of these materials is of fcc type with
Fig. 14.12. Schematic representation of the unit cell of
fcc C
60
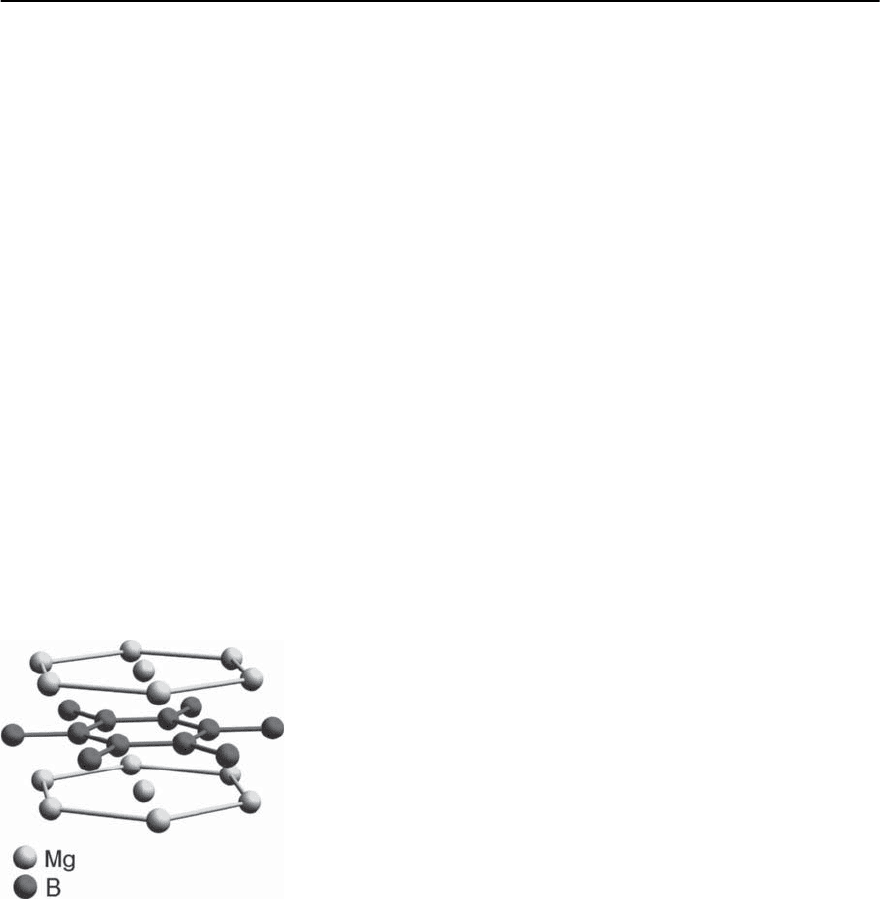
774 H.R.Ott
varying space groupsymmetries,however.Non cubic
structures of superconducting fullerides have been
reported for the cases of Cs
3
C
60
and NH
3
K
3
C
60
under pressure. The compounds with chemical com-
position of the type A
4
C
60
usually adopt a bct cubic
structure and are insulators. A schematic drawing
of the cubic structure of the A
3
C
60
type materials is
shown in Fig.14.12.Moreon structuralaspects of C
60
material may be found in [48].
14.2.4 MgB
2
Although this compound has been known for a long
time, it obviously had never been tested for super-
conductivity until late in 2000. The discovery of its
unexpectedly high critical temperature of the order
of 40 K by Akimitsu and co-workers [11] came as
a real surprise. MgB
2
crystallizes in the hexagonal
AlB
2
-type structure, shown in Fig. 14.13, which ex-
hibits clear 2D-type features. The B atoms form lay-
ers that are equivalent to the layered structure of
graphite. It has been suggested [49] that this appar-
ent two-dimensionality might be the key to the high
value of T
c
of this material, reminiscent of the case
of the cuprates.
Fig. 14.13. Schematic representation of the crystallographic
unit cell of MgB
2
14.3 Occurrence of Superconductivity
For all thematerials thatin this review are considered
in relation with high-T
c
superconductivity, a super-
conducting ground state is achieved only in limited
regions of chemical composition. In this section, we
briefly discuss the relation between chemical compo-
sition and the observation of superconductivity, in-
cluding the established critical temperatures T
c
, for
a few instructive cases.
14.3.1 BaPb
1−x
Bi
x
O
3
,Ba
1−x
K
x
BiO
3
Superconductivity in the Pb/Bi compound series [50]
is restricted to values of x that are close to 0.25 where
a maximum critical temperature of approximately 12
K isobserved [51,52].Thisvalueof T
c
is unexpectedly
high and remarkable becauseof the absence of any el-
ement of the d-transition range in the chemical com-
position. Figure 14.14 demonstrates that, although
partial superconductingtransitions, measured mag-
netically and resistively, have been observed around
x=0.1 and x = 0.3, narrow and complete transi-
tions are monitored only for x = 0.25 [52].It is there-
fore quite obvious that the optimal conditions for
superconductivity are reached for BaPb
0.75
Bi
0.25
O
3
and any deviation from this composition leads to a
rapid deterioration of the superconducting proper-
ties and, in addition, favors inhomogeneities in the
chemical composition. It is conceivable that the oc-
currence of superconductivity is extremely depen-
dent on the doping level and that compositional in-
homogeneities lead to the spread in T
c
displayed in
Fig. 14.14.
Replacing Bi with Sb leads to a less favorable sit-
uation for superconductivity [53].Maximum critical
temperatures are again reached for x ∼0.25 but they
barely exceed 3 K. It is not quite clear why T
c
is so
low in this case.
More successful in this respect were experiments
with material that was prepared by partly replacing
the divalent Ba by the monovalent alkaline metal K in
BaBiO
3
. This type of substitution and the concomi-
tant reduction of the electron concentration induces
the already mentioned structural phase transition
and the symmetry of the crystal lattice changes from
monoclinic to cubic. Likewise, the chemical substi-
tution leads from an insulating to a metallic state.
K-doped BaBiO
3
is electronically very similar to Bi-
doped BaPbO
3
. Optimal conditions are reached at
the composition Ba
0.6
K
0.4
BiO
3
for which T
c
is ap-
proximately 30 K [54,55].
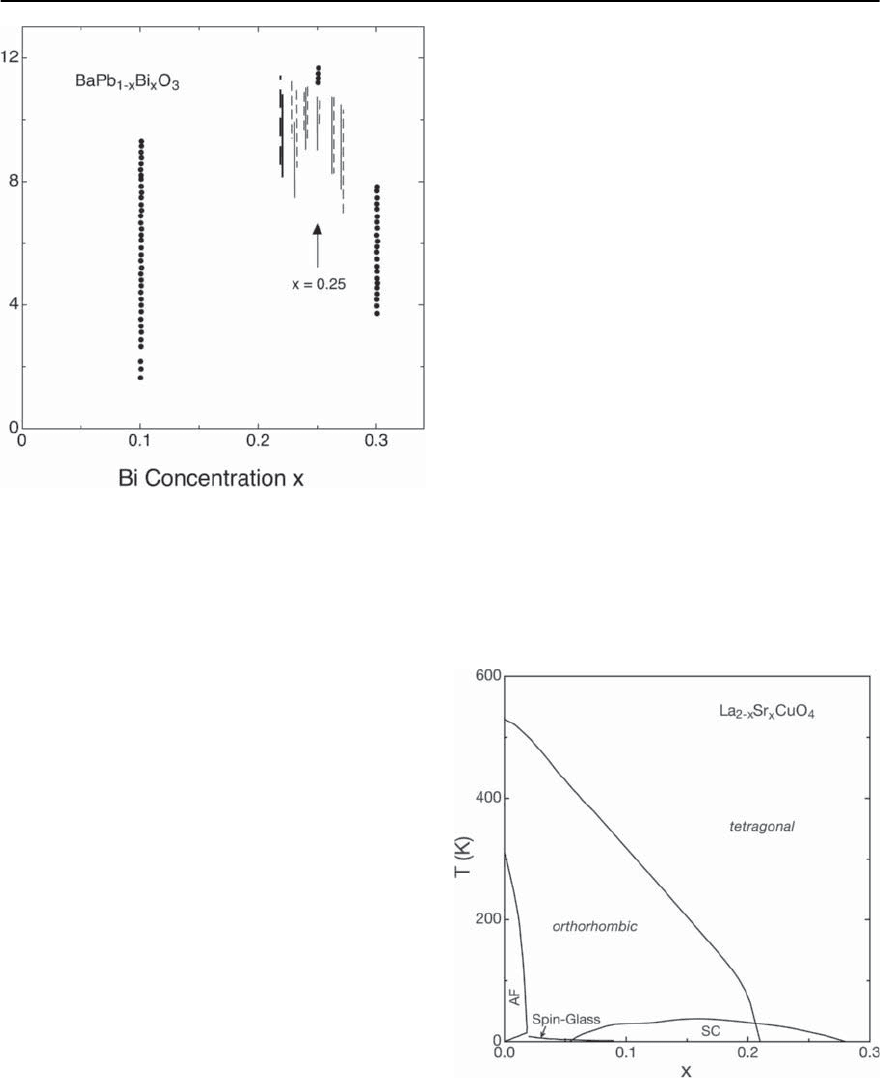
14 High-T
c
Superconductivity 775
Fig. 14.14. Onset and widths of the superconducting tran-
sition of BaPb
1−x
Bi
x
O
3
(see [52])
14.3.2 Copper Oxides
Very shortly after the discovery of superconductivity
inCuoxidesitwasrealized thatinthesematerials,the
occurrence of superconductivity is intimately related
with the number of itinerant charge carriers which,
in most cases, can be varied rather easily by simply
altering the chemical compositions of the materials
in specific ways. For some types of compounds, the
result of this procedure in the form of a transition
from an insulating to a metallic behavior and super-
conductivity, or vice versa, may be illustrated very
well, for others it proves to be less obvious. In what
follows, four rather instructive cases, representative
for the cuprate superconductors, are presented.
La
2−x
Sr
x
CuO
4+ı
The ternary compound La
2
CuO
4
has long been
known to be an electrical insulator. Early measure-
ments revealed peak features in the temperature
dependence of the magnetic susceptibilitywhich in-
dicatedsometypeofmagneticorderbelowapprox-
imately 200 K [56]. It was only after the discovery
of superconductivity in exactly this class of cuprates,
when the physical properties of these materials were
studied in great detail. The results of these investi-
gations are being reviewed in some of the follow-
ing sections but we summarize part of them in the
form of low temperature phase diagrams, display-
ing the variation of the physical behavior as a func-
tion of the concentration of itinerant charge carriers.
The charge carrier doping may be accomplished in
two ways, either by enhancing the oxygen content
to above four atoms per formula unit or by replac-
ing part of the trivalent La ions by divalent ions of
the alkaline earth elements Sr and Ba. No successful
attempts to replace La by Ca have been reported.
Figure 14.15 captures the low temperature phases
that are identified if La is replaced by Sr [57].The di-
agram is somewhat more complicated if Ba is chosen
as the substitute. In the region of x ∼ 0.12 where a
slight indentation in T
c
(x) is apparent for the Sr dop-
ing (see Fig.14.15),T
c
is suppressed completely if the
dopant is Ba (see also below). As mentioned above,
the ternary compound La
2
CuO
4
is an insulator with
an antiferromagnetically ordered ground state.Upon
doping with Sr
2+
,theN´eel temperature T
N
of 325 K
for x = 0 is efficiently reduced, giving way to a spin-
glass type ground state for 0.02 < x < 0.05. Once
Fig. 14.15. Low temperature phase diagram of
La
2−x
Sr
x
CuO
4
(see [57])
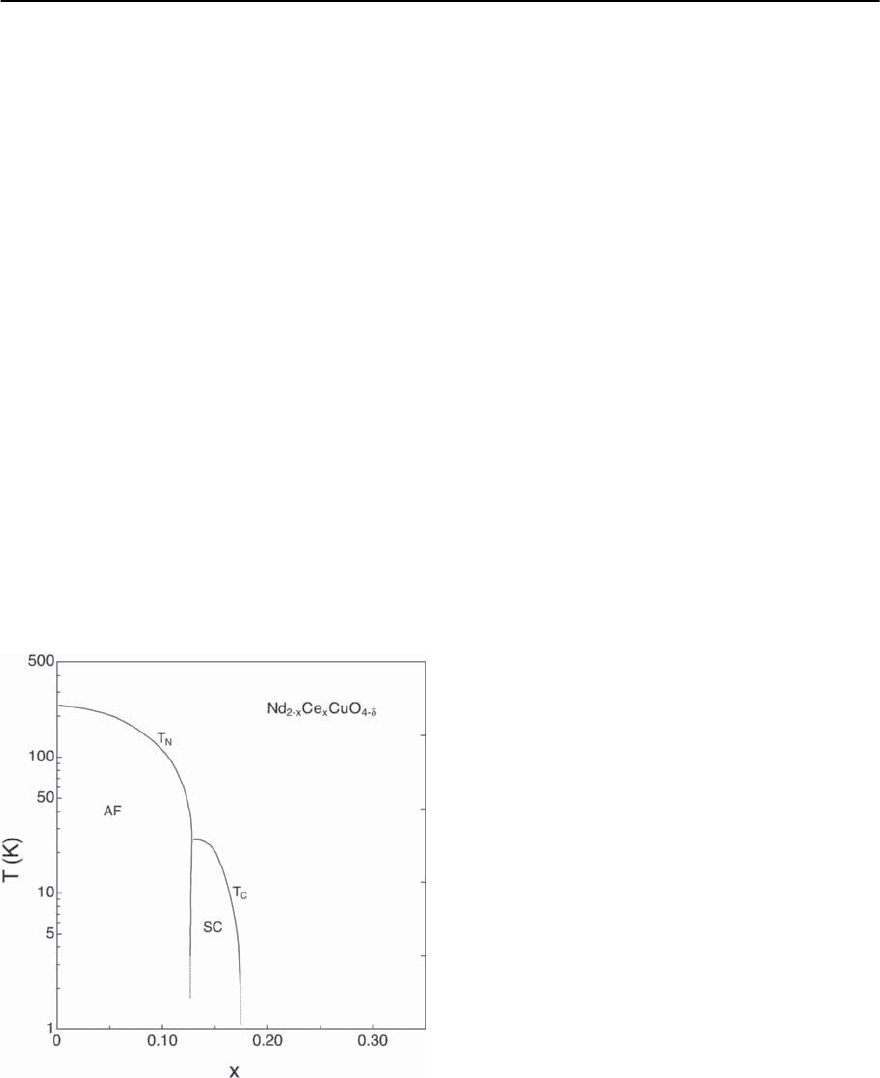
776 H.R.Ott
the Sr concentration exceeds 5%, superconductivity
sets in at low temperatures. The optimal doping, i.e.,
the highest transition temperature T
c
of about 40 K
is reached at x ∼ 0.15. Further increasing x reduces
T
c
and superconductivity is lost for x > 0.25. Since,
as mentioned above, the structural transition from
a tetragonal to an orthorhombic structure has been
reported to disappear at x ∼ 0.21, bulk supercon-
ductivity seems to persist into the tetragonal struc-
ture regime. This observation is important in view
of discussing relations between the crystal structure
symmetry and the occurrence of superconductiv-
ity, as illustrated in the following case. A tiny dip in
the T
c
(x)curveatx ∼ 0.125, displayed in Fig. 14.15,
signals an effect that is much more pronounced in
La
1−x
Ba
x
CuO
4
. Again around a concentration of
x ∼ 0.125, T
c
oftheBa-dopedseriesissuppressed
to zero and static magnetic order is observed in-
stead [58,59]. Detailed studies have traced this back
to the occurrence of a low temperature tetragonal
structure [23,59,60].
As already mentioned in a previous section en-
hancing the oxygen content leads to phase separation
phenomena in La
2
CuO
4+ı
. The oxygen rich metallic
Fig. 14.16. Low temperature phase diagram of
Nd
2−x
Ce
x
CuO
4−ı
-type compounds (see [63])
phase is superconducting at low temperatures and
again, T
c
values exceeding 30 K are achieved. An in-
structive discussion of the ground state properties of
La
2
CuO
4+ı
is offered in [57].
Ln
2−x
Ce
x
CuO
4−ı
(Ln = Pr, Nd, Sm)
This variety of compounds is, with respect to elec-
tronic properties, distinctly different from all the
other Cu-oxide compounds. Here, the doping is not
withholesbutwithelectrons[61].Thisdifference
is also reflected in the phase diagram capturing the
occurrence ofsuperconductivity [62,63].Theantifer-
romagnetic phase is again rapidly suppressed upon
doping and gives way to a superconducting ground
state rather abruptly, as may be seen in Fig. 14.16.
The maximum value of T
c
is found next to the phase
boundary and a further enhancement of doping
leads to a reduction of the critical temperature and,
again, to a rather abrupt disappearance of supercon-
ductivity. The range of doping where superconduc-
tivity has been observed is much more narrow than
in the hole doped cuprates.The discovery of electron
doped superconductors was initiated by the preced-
ing observation of a superconductingground statein
the chemically similar but somewhat more compli-
cated series adopting the T
∗
structure [64] (see also
Sect. 14.2.2).
YBa
2
Cu
3
O
6+ı
In this series of compounds, tetragonal YBa
2
Cu
3
O
6
represents the electrically insulating and antiferro-
magnetically ordering parent compound [65]. The
doping of this material is accomplishedby enhancing
the oxygen content. Superconductivity sets in at oxy-
gen concentrations close to those where the struc-
tural instability leads to an orthorhombic crystal lat-
tice,i.e.,ı ∼ 0.45.The critical temperature increases
roughly in two steps, with two plateaus at approxi-
mately 60 and 90 K [66]. Optimal values for T
c
,ofthe
order91to92Kareobtainedforı between 0.9 and
0.95. Further enhancing the oxygen content leads to
aslightdecreaseofT
c
,by1or2K.Theschematic
T
c
(x) variation is displayed in Fig. 14.17.
