BD Diagnostic Systems (publ.). Difco Manual (Manual of Microbiological Culture)
Подождите немного. Документ загружается.

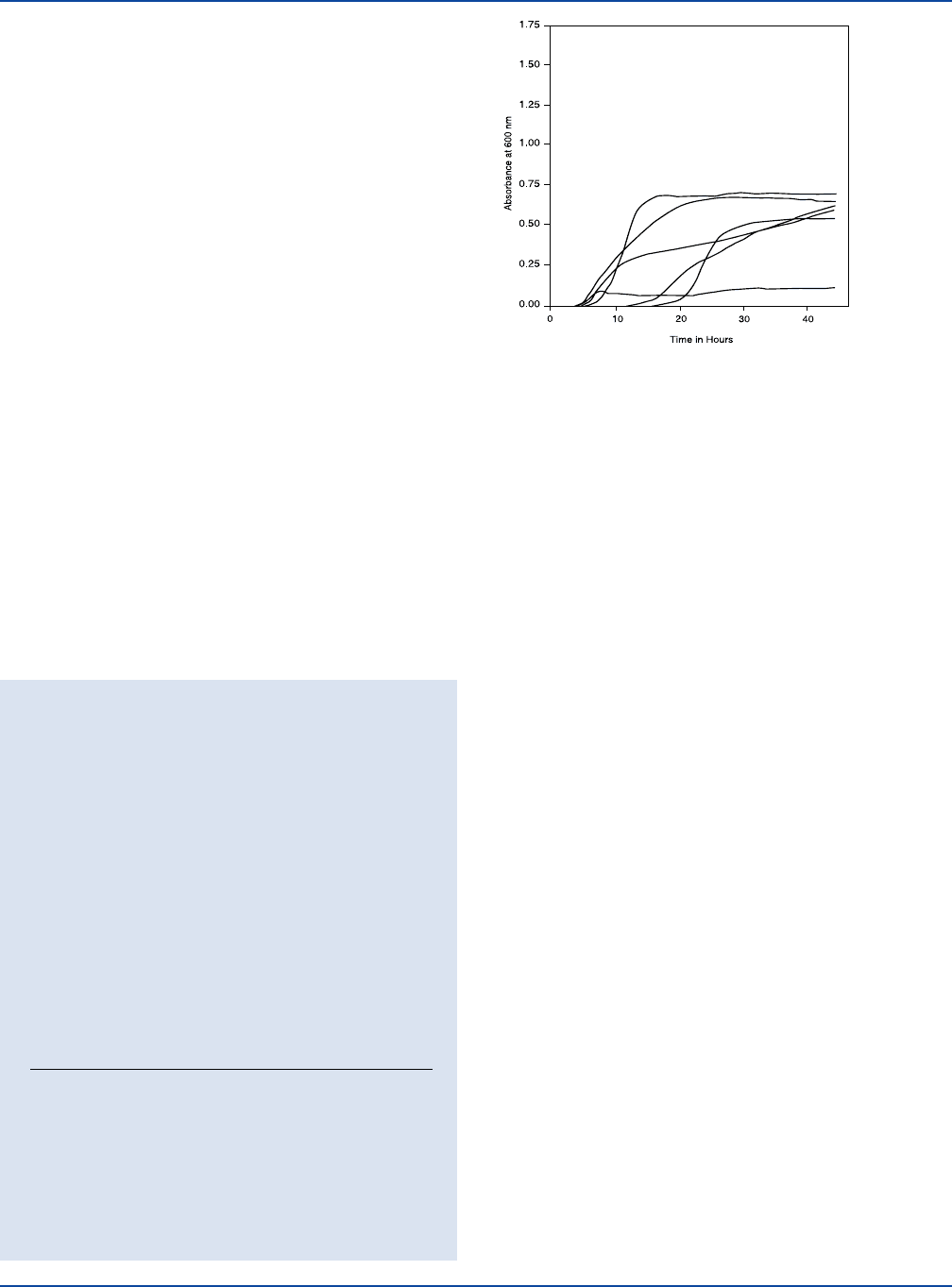
196 The Difco Manual
P. aeruginosa ATCC
®
27853
E. coli ATCC
®
25922
Y. ruckeri ATCC
®
29908
S. aureus ATCC
®
25923
S. cerevisiae ATCC
®
9763
B. subtilis ATCC
®
6633
Fish Peptone No. 1 Section II
User Quality Control
Identity Specifications
Dehydrated Appearance: Beige, free-flowing, homogeneous.
Solution: 1% solution is light to medium amber,
clear to very slightly opalescent, may
have a slight precipitate.
Reaction of 1%
Solution at 25°C: pH 6.7 ± 0.2
Cultural Response
Prepare a 1% concentration of Fish Peptone No. 1 with the
addition of 0.5% sodium chloride. Inoculate test organisms and
incubate for 18-48 hours at 35 ± 2°C. Incubate Vibrio tubiashii
for 18-48 hours at 25 ± 2°C. Saccharomyces cerevisiae is
tested with the addition of 0.5% dextrose. Vibrio tubiashii is
tested with the addition of 1.5% sodium chloride.
INOCULUM
ORGANISM ATCC
®
CFU GROWTH
Bacillus subtilis† 6633 100-1,000 fair to good
Escherichia coli 25922* 100-1,000 good
Saccharomyces cerevisiae 9763 100-1,000 good
Staphylococcus aureus 25923* 100-1,000 good
Vibrio tubiashii 19105 100-1,000 good
*These cultures are available as Bactrol
TM
Disks and should be
used as directed in Bactrol Disks Technical Information.
†Bacillus subtilis is available as Bacto Subtilis Spore Suspension.
Amino Acids (%)
Alanine 3.48 Lysine 2.51
Arginine 2.19 Methionine 0.83
Aspartic Acid 3.21 Phenylalanine 0.95
Cystine 0.24 Proline 2.19
Glutamic Acid 5.27 Serine 1.27
Glycine 5.29 Threonine 1.17
Histidine 1.54 Tryptophan 0.15
Isoleucine 0.92 Tyrosine 0.45
Leucine 2.16 Valine 1.43
Inorganics (%)
Calcium 0.020 Phosphate 3.848
Chloride 9.326 Potassium 4.183
Cobalt <0.001 Sodium 9.351
Copper 0.001 Sulfate 1.004
Iron 0.003 Sulfur 1.629
Lead <0.001 Tin <0.001
Magnesium 0.017 Zinc 0.002
Manganese <0.001
Vitamins (µg/g)
Biotin 0.3 PABA 95.0
Choline
(as Choline Chloride)
4170.7 Pantothenic Acid 63.2
Cyanocobalamin 0.3 Pyridoxine 7.2
Folic Acid 1.5 Riboflavin 26.8
Inositol 2820.0 Thiamine NA
Nicotinic Acid 603.0 Thymidine 55.0
Biological Testing (CFU/g)
Coliform negative Standard Plate Count 18
Salmonella negative Thermophile Count 379
Precautions
1. For Laboratory Use.
2. Follow proper established laboratory procedures in handling and
disposing of infectious materials.
Storage
Store the dehydrated product below 30°C. The dehydrated product is
very hygroscopic. Keep container tightly closed.
Expiration Date
The expiration date applies to the product in its intact container when
stored as directed. Do not use a product if it fails to meet specifications
for identity and performance.
Procedure
Materials Provided
Fish Peptone No. 1
Materials Required But Not Provided
Materials vary depending on the medium being prepared.
Method of Preparation
Refer to the final concentration of peptone in the formula of the
medium being prepared. Add Fish Peptone No. 1 as required.
Specimen Collection and Preparation
Obtain and process specimens according to the techniques and
procedures established by laboratory policy.
Test Procedure
See appropriate references for specific procedures on the medium
being prepared or the sample being analyzed.
Results
Refer to appropriate references and procedures for results.
Limitations of the Procedure
1. Since the nutritional requirements of organisms vary, some strains may
be encountered that fail to grow or grow poorly on prepared medium.
Packaging
Fish Peptone No. 1 500 g 0551-17
10 kg 0551-08

The Difco Manual 197
Section II Fletcher Medium Base
Bacto
®
Fletcher Medium Base
Intended Use
Bacto Fletcher Medium Base is used with sterile normal rabbit serum
for isolating and cultivating Leptospira.
Summary and Explanation
In 1816, Adolf Weil described the first recognized infections of
Leptospirosis in humans.
5
These cases were caused by Leptospira
interogans serovar icterohaemorrhagiae and the disease was subse-
quently named Weil’s Disease.
5
Leptospirosis is a zoonotic disease,
having its reservoir in wild, domestic, and peridomestic animals.
6
Infection usually results from direct or indirect exposure to the urine
of leptospiruric animals.
6
Indirect exposure through contaminated
water and soil accounts for most sporadic cases.
3
Direct exposure occurs
in pet owners, veterinarians and persons working with livestock.
3
Leptospirosis is typically a biphasic illness.
3,8
The infection is acute in
onset, with a flu- like syndrome persisting for 4 to 7 days.
4
Onset of a
second “immune” phase, in which meningitis, skin rash, and hepatic
and renal involvement may be present, occurs within a few days.
4
Fletcher Medium Base is prepared according to the formulation of
Fletcher.
1
Myers et al.
2
reported Fletcher Medium, with a basal agar
layer containing charcoal, to be superior to the standard medium for
the maintenance of leptospiral cultures. Fletcher Medium Base
prepared with sterile normal rabbit serum is specified for the isolation
of Leptospira.
3,4,7
Principles of the Procedure
Bacto Peptone and Beef Extract provide the nitrogen, vitamins, carbon
and amino acids in Fletcher Medium Base. Sodium chloride maintains
the osmotic balance of the medium. Bacto Agar is the solidifying agent.
Sterile normal rabbit serum is added to the formula to stimulate growth
of Leptospira.
Formula
Fletcher Medium Base
Formula Per Liter
Bacto Peptone . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.3 g
Bacto Beef Extract . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.2 g
Sodium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.5 g
Bacto Agar . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.5 g
Final pH 7.9 ± 0.1 at 25°C
Precautions
1. For Laboratory Use.
2. Follow proper established laboratory procedures in handling and
disposing of infectious materials.
3. Leptospira species are BioSafety Level 2 pathogens. Handling clini-
cal specimen material potentially infected with Leptospira species
should be performed in a Class II biological safety cabinet (BSC).
7
Storage
Store the dehydrated medium below 30°C. The dehydrated medium is
very hygroscopic. Keep container tightly closed.
Expiration Date
The expiration date applies to the product in its intact container when
stored as directed. Do not use a product if it fails to meet specifications
for identity and performance.
Procedure
Materials Provided
Fletcher Medium Base
Materials Required But Not Provided
Glassware
Autoclave
Incubator (35°C)
Waterbath (56°C)
Sterile normal rabbit serum
Method of Preparation
1. Suspend 2.5 grams in 920 ml distilled or deionized water.
2. Heat to boiling to dissolve completely.
3. Autoclave at 121°C for 15 minutes. Cool to 56°C.
4. Aseptically add 80 ml sterile normal rabbit serum at 56°C. Mix well.
5. Determine pH. If necessary, aseptically adjust to pH 7.9 ± 0.1 with
1 N HCl or 1 N NaOH.
Specimen Collection and Preparation
Obtain and process specimens according to the techniques and procedures
established by laboratory policy. Blood, cerebrospinal fluid (CSF) and
User Quality Control
Identity Specifications
Dehydrated Appearance: Beige, free-flowing, homogeneous.
Solution: 2.5 g in 920 ml distilled or deionized
water, soluble upon boiling. Solution
is very light amber, clear to very
slightly opalescent without
significant precipitate.
Prepared Medium: Very light amber, very slightly to
slightly opalescent without
significant precipitate.
Reaction of 2.5 g in
920 ml distilled water: pH 7.9 ± 0.1 at 25°C
Cultural Response
Prepare Fletcher Medium Base per label directions. Enrich
with sterile normal rabbit serum. Inoculate and incubate at
30 ± 2°C for up to 5 days.
ORGANISM ATCC
®
INOCULUM GROWTH
Leptospira interrogans 23605 2-3 drops good
serovar australis
Leptospira interrogans 23470 2-3 drops good
serovar canicola
Leptospira kirschneri 23604 2-3 drops good
serovar grippotyphosa
The cultures listed are the minimum that should be used for
performance testing.

198 The Difco Manual
2. Myers, D. M., V. M. Varela-Diaz, and A. A. Siniuk. 1973.
Long-term survival of Leptospira in a biphasic culture medium
containing charcoal. Am. Soc. Microbiol. 25:514-516.
3. Kaufmann, A. F., and R. S. Weyant. 1995. Leptospiraceae,
p. 621-625. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover
and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed.
American Society for Microbiology, Washington, D.C.
4. Koneman, E. W., S. D. Allen, V. R. Dowell, Jr., W. M. Janda,
H. M. Sommers, and W. C. Winn, Jr. 1988. Color atlas and
textbook of diagnostic microbiology, 3rd ed. J. B. Lippincott
Company, Philadelphia, PA.
5. Elliott, S. H. 1980. Discussion and clinical diagnosis of
Leptospirosis. J. Am. Med. Tech. 42:37-44.
6. Faine, S. (ed.). 1982. Guidelines for the control of Leptospirosis. W.
H. O. Offset publication no. 67. World Health Organization, Geneva.
7. Isenberg, H. D. (ed.). 1992. Clinical microbiology procedures
handbook. American Society for Microbiology, Washington, D.C.
8. Kelley, P. W. 1992. Leptospirosis, p. 1295-1301. In S. L. Gorbach,
J. G. Bartlett, and N. R. Blacklow (ed.), Infectious diseases. The
W. B. Saunders Co., Philadelphia, PA.
9. Weinman, D. 1981. Bartonellosis and anemias associated with
bartonella-like structures, p. 235-248. In A. Balows, and W. J.
Hausler, Jr. (ed.), Diagnostic procedures for bacterial, mycotic and
parasitic infections, 6th ed. American Public Health Association,
Washington, D.C.
Packaging
Fletcher Medium Base 500 g 0987-17
urine are the specimens of choice for the recovery of leptospires from
patients with leptospirosis.
3
Test Procedure
7
1. Aseptically dispense into sterile screw-cap tubes in 5-7 ml amounts.
Store at room temperature overnight.
2. Inactivate the whole medium the day following its preparation by
placing the tubes in a water bath at 56°C for 1 hour.
3. Allow the medium to cool before inoculation.
4. Growth is first seen in approximately 10 days at 35°C (2 to 4 weeks
at 25°C) as a cloud of minute granules that develop into
microcolonies just below the surface.
5. Gram stain is not satisfactory. The microcolonies can be fixed
with methanol and stained with Giemsa stain to show rod forms
at the edges.
9
Results
Refer to appropriate references and procedures for results.
Limitations of the Procedure
1. Since the nutritional requirements of organisms vary, some
strains may be encountered that fail to grow or grow poorly on
this medium.
References
1. Fletcher, W. 1928. Recent work on leptospirosis, tsutsugamushi
disease and tropical typhus in the federated Malay States. Trans.
Roy. Soc. Trop. Med. Hyg. 21: 265-287.
Folic AOAC Medium Section II
Bacto
®
Folic AOAC Medium
User Quality Control
Identity Specifications
Dehydrated Appearance: Off-white, free-flowing,
homogeneous.
Solution: 5.50% solution (single strength) and
11.0% solution (double strength),
soluble in distilled or deionized
water on boiling.
Solution is light amber, clear, may
have a slight precipitate.
Prepared Medium: Very light amber, clear.
Reaction of 5.50%
Solution at 25°C: pH 6.7 ± 0.1
Cultural Response
Prepare Folic AOAC Medium per label directions. This
medium should support the growth of E. hirae ATCC
®
8043
when prepared in single strength and supplemented with
folic acid.
Intended Use
Bacto Folic AOAC Medium is used for determining folic acid concen-
tration by the microbiological assay technique.
Also Known As
AOAC is an abbreviation for Association of Official Analytical Chemists.
Summary and Explanation
Vitamin Assay Media are prepared for use in the microbiological assay
of vitamins. Three types of media are used for this purpose:
1. Maintenance Media: For carrying the stock culture to preserve
the viability and sensitivity of the test organism for its intended
purpose.
2. Inoculum Media: To condition the test culture for immediate use.
3. Assay Media: to permit quantitation of the vitamin under test.
Folic AOAC Medium is prepared for use in the microbiological assay
of folic acid according to the procedures of the Folic Acid Assay in
AOAC.
1
Enterococcus hirae ATCC
®
8043 (Streptococcus faecium) is
the test organism in this assay.
Principles of the Procedure
Folic Acid AOAC Medium is a folic acid-free dehydrated medium
containing all other nutrients and vitamins essential for the cultivation
of E. hirae ATCC
®
8043. The addition of folic acid in specified

The Difco Manual 199
Section II Folic AOAC Medium
increasing concentrations gives a growth response that can be
measured turbidimetrically or titrimetrically.
Formula
Folic AOAC Medium
Formula Per Liter
Bacto Vitamin Assay Casamino Acids. . . . . . . . . . . . . . . . 10 g
L-Asparagine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.6 g
L-Tryptophane . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.2 g
L-Cysteine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . 0.76 g
Bacto Dextrose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 g
Adenine Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Guanine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Uracil. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Xanthine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
p-Aminobenzoic Acid. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 mg
Pyridoxine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . 4 mg
Thiamine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . 400 µg
Calcium Pantothenate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800 µg
Nicotinic Acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800 µg
Biotin. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 µg
Riboflavin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 mg
Glutathione . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2 mg
Sorbitan Monooleate Complex. . . . . . . . . . . . . . . . . . . . . . 0.1 g
Sodium Citrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52 g
Dipotassium Phosphate . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.4 g
Magnesium Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.4 g
Manganese Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Sodium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Ferrous Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Final pH 6.7 ± 0.1 at 25°C
Precautions
1. For Laboratory Use.
2. Follow proper established laboratory procedures in handling and
disposing of infectious materials.
3. Great care must be taken to avoid contamination of media or
glassware in microbiological assay procedures. Extremely small
amounts of foreign material may be sufficient to give erroneous
results. Scrupulously clean glassware free from detergents and
other chemicals must be used. Glassware is heated to 250°C for at
least 1 hour to burn off any organic residues that might be present.
4. Take precautions to keep sterilizing and cooling conditions uniform
throughout the assay.
Storage
Store the dehydrated medium at 2-8°C. The dehydrated medium is very
hygroscopic. Keep container tightly closed.
Expiration Date
The expiration date applies to the product in its intact container when
stored as directed. Do not use a product if it fails to meet specifications
for identity and performance.
Procedure
Materials Provided
Folic AOAC Medium
Materials Required But Not Provided
Glassware
Autoclave
Stock culture of Enterococcus hirae ATCC
®
8043
Sterile tubes
Distilled or deionized water
Folic Acid
0.01 N NaOH
Dilute HCl
Spectrophotometer or Nephelometer
Method of Preparation
1. Suspend 11 grams in 100 ml distilled or deionized water.
2. Heat to boiling for 2-3 minutes.
3. Distribute 5 ml amounts into tubes, evenly dispersing the precipitate.
4. Add standard or test samples.
5. Adjust tube volume to 10 ml with distilled or deionized water.
6. Autoclave at 121°C for 5 minutes.
Specimen Collection and Preparation
Assay samples are prepared according to references given in the
specific assay procedures. For assays, the samples should be diluted to
approximately the same concentration as the standard solution.
Test Procedure
Follow assay procedures as outlined in AOAC.
1
It is essential that a
standard curve be set up for each separate assay. Autoclaving and
incubation conditions that can influence the standard curve readings
cannot always be duplicated. The standard curve is obtained by using
folic acid at levels of 0.0, 1, 2, 4, 6, 8 and 10 ng per assay tube (10 ml).
Folic AOAC Medium may be used for both turbidimetric and titrimetric
analysis. Turbidimetric readings should be taken after incubation at
35-37°C for 16-18 hours. Titrimetric determinations are best made
following incubation at 35-37°C for 72 hours.
The folic acid required for the preparation of the standard curve may
be prepared as follows:
A. Dissolve 50 mg dried folic acid in about 30 ml 0.01N NaOH
and 300 ml distilled water.
B. Adjust the pH reaction to 7.5 ± 0.5 with diluted HCl solution.
Dilute to 500 ml with distilled water.
C. Add 2 ml of the solution to 50 ml distilled water. Adjust the
pH reaction to 7.5 ± 0.5. Dilute to 100 ml with distilled water.
This yields a stock solution containing 2 mcg folic acid per ml.
D. Prepare the stock solution fresh daily.
The standard solution for the assay is made by diluting 1 ml of this
stock solution to 1 liter with distilled water. This solution contains 2 ng
folic acid per ml. Use 0.0, 0.5, 1, 2, 3, 4, and 5 ml per assay tube.
Some laboratories may wish to alter the concentration of folic acid
recommended above for the standard curve. This is permissible if the
concentration used is within the limits specified by AOAC.
1
Results
1. Prepare a standard concentration response curve by plotting the
response readings against the amount of standard in each tube,
disk or cup.

200 The Difco Manual
2. Determine the amount of vitamin at each level of assay solution by
interpolation from the standard curve.
3. Calculate the concentration of vitamin in the sample from the
average of these volumes. Use only those values that do not vary
more than ±10% from the average. Use the results only if two thirds
of the values do not vary more than ±10%.
Limitations of the Procedure
1. The test organism used for inoculating an assay medium must
be cultured and maintained on media recommended for this
purpose.
2. Aseptic technique should be used throughout the assay procedure.
Bacto
®
Folic Acid Assay Medium
User Quality Control
Identity Specifications
Dehydrated Appearance: Off white to very light beige,
free-flowing, homogeneous.
Solution: 3.75% (single strength) or 7.5%
(double strength) solution, soluble
in distilled or deionized water upon
boiling for 2-3 minutes. Solution is
light amber, clear, may have a slight
precipitate.
Prepared Medium: Very light amber, clear, may have a
very slight precipitate.
Reaction of 3.75%
Solution at 25°C: pH 6.8 ± 0.2
Cultural Response
Prepare single-strength Folic Acid Assay Medium per label
directions. The medium should support the growth of E. hirae
ATCC
®
8043. The most effective range is 2-10 ng folic acid
per 10 ml tube.
Intended Use
Bacto Folic Acid Assay Medium is used for determining folic acid
concentration by the microbiological assay technique.
Summary and Explanation
Vitamin Assay Media are prepared for use in the microbiological assay
of vitamins. Three types of medium are used for this purpose:
1. Maintenance Medium: For carrying the stock culture to preserve the
viability and sensitivity of the test organism for its intended purpose.
2. Inoculum Medium: To condition the test culture for immediate use.
3. Assay Medium: To permit quantitation of the vitamin under test.
Folic Acid Assay Medium is used in the microbiological assay of folic
acid with Enterococcus hirae ATCC
®
8043 as the test organism. Folic
Acid Assay Medium is prepared according to the formula described by
Capps, Hobbs and Fox,
1
modified with sodium citrate instead of
sodium acetate.
Principles of the Procedure
Folic Acid Assay Medium is a folic acid-free dehydrated medium
containing all other nutrients and vitamins essential for the cultivation
of E. hirae ATCC
®
8043. The addition of folic acid in specified
increasing concentrations gives a growth response that can be measured
turbidimetrically.
Formula
Folic Acid Assay Medium
Formula Per Liter
Bacto Vitamin Assay Casamino Acids. . . . . . . . . . . . . . . . 12 g
Bacto Dextrose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 g
Sodium Citrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 g
L-Cystine. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.2 g
DL-Tryptophane . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.2 g
Adenine Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Guanine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Uracil. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Thiamine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 mg
Pyridoxine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . 4 mg
Riboflavin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 mg
Niacin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 mg
p-Aminobenzoic Acid. . . . . . . . . . . . . . . . . . . . . . . . . . . . 200 µg
Biotin. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.8 µg
Calcium Pantothenate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 400 µg
Dipotassium Phosphate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
Monopotassium Phosphate. . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
Magnesium Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.4 g
Sodium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Ferrous Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Manganese Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Final pH 6.8 ± 0.2 at 25°C
Precautions
1. For Laboratory Use.
2. Follow proper established laboratory procedures in handling and
disposing of infectious materials.
3. Great care must be taken to avoid contamination of media or glass-
ware in microbiological assay procedures. Extremely small
Folic Acid Assay Medium Section II
3. The use of altered or deficient media may cause mutants
having different nutritional requirements that will not give a
satisfactory response.
4. For successful results of these procedures, all conditions of the
assay must be followed precisely.
References
1. Association of Official Analytical Chemists. 1995. Official
methods of analysis of AOAC international, 16th ed. AOAC
International, Arlington, VA.
Packaging
Folic AOAC Medium 100 g 0967-15*
*Store at 2-8°C

The Difco Manual 201
amounts of foreign material may be sufficient to give erroneous
results. Scrupulously clean glassware free from detergents and
other chemicals must be used. Glassware must be heated to 250°C
for at least 1 hour to burn off any organic residues that might be
present.
4. Take precautions to keep sterilization and cooling conditions
uniform throughout the assay.
Storage
Store the dehydrated medium at 2-8°C. The dehydrated medium is very
hygroscopic. Keep container tightly closed.
Expiration Date
The expiration date applies to the product in its intact container when
stored as directed. Do not use a product if it fails to meet specifications
for identity and performance.
Procedure
Materials Provided
Folic Acid Assay Medium
Materials Required But Not Provided
Glassware
Autoclave
Stock culture of Enterococcus hirae ATCC
®
8043
Sterile tubes
Sterile 0.85% saline
Distilled or deionized water
0.01 N NaOH
Dilute HCl
Folic Acid USP
Lactobacilli Agar AOAC
Lactobacilli Broth AOAC
Centrifuge
Spectrophotometer
Method of Preparation
1. Suspend 7.5 grams in 100 ml distilled or deionized water.
2. Boil 2-3 minutes to dissolve completely.
3. Dispense 5 ml amounts into tubes, evenly dispersing the precipitate.
4. Add standard or test samples.
5. Adjust tube volume to 10 ml with distilled or deionized water.
6. Autoclave at 121°C for 10 minutes.
Specimen Collection and Preparation
Prepare assay samples according to references given in the specific
assay procedures. Dilute the samples to approximately the same
concentration as the standard solution.
Test Procedure
Prepare stock cultures of E. hirae ATCC
®
8043 by stab inoculation of
Lactobacilli Agar AOAC. Incubate at 35-37°C for 24-48 hours. Store
tubes in the refrigerator. Make transfers at monthly intervals. Prepare
the inoculum for assay by subculturing a stock culture of E. hirae
ATCC
®
8043 into a tube containing 10 ml of Lactobacilli Broth AOAC.
After incubation at 35-37°C for 18-24 hours, centrifuge the cells under
aseptic conditions and decant the supernatant. Wash the cells three
times with 10 ml of sterile 0.85% saline. After the third wash, dilute
the cell suspension 1:100 with sterile 0.85% saline. Use one drop of
this latter suspension to inoculate each of the assay tubes.
It is essential that a standard curve be set up for each separate assay.
Autoclaving and incubation conditions that influence the standard
curve readings cannot always be duplicated. The standard curve is
obtained by using folic acid at levels of 0.0, 2, 4, 6, 8 and 10 ng per
10 ml assay tube. Turbidimetric readings should be made after
incubation at 35-37°C for 18-24 hours. Refrigerate tubes for 15-30
minutes to stop growth before reading.
Prepare the folic acid stock solution required for the standard curve as
follows:
1. Dissolve 50 mg dried Folic Acid USP Reference Standard or
equivalent in about 30 ml of 0.01 N NaOH and 300 ml distilled
water.
2. Adjust to pH 7.5 ± 0.5 with diluted HCl solution. Add distilled
water to give a volume of 500 ml.
3. Add 2 ml of the solution from step 2 to 50 ml distilled water.
Adjust the pH to 7.5 ± 0.5 with HCl solution. Dilute to 100 ml with
distilled water to give a stock solution containing 2 mcg folic acid
per ml. Prepare the stock solution fresh daily.
Prepare the standard solution for the assay by diluting 1 ml of this
stock solution in 1 liter with distilled water. This solution contains 2 ng
folic acid per ml. Use 0.0, 0.5, 1, 2, 3, 4 and 5 ml per assay tube.
Following incubation, place the tubes in the refrigerator for 15-30
minutes to stop growth. The growth can be measured by a turbidimetric
method and the curve constructed from the values obtained. The most
effective assay range is between the levels of 2 and 10 ng folic acid per
10 ml tube.
Results
1. Prepare a standard concentration response curve by plotting the
response readings against the amount of standard in each tube, disk
or cup.
2. Determine the amount of vitamin at each level of assay solution by
interpolation from the standard curve.
3. Calculate the concentration of vitamin in the sample from the
average of these volumes. Use only those values that do not vary
more than ±10% from the average. Use the results only if two thirds
of the values do not vary more than ±10%.
Limitations of the Procedure
1. The test organism used for inoculating an assay medium must be
cultured and maintained on media recommended for this purpose.
2. Aseptic technique should be used throughout the assay procedure.
3. The use of altered or deficient media may cause mutants having
different nutritional requirements that will not give a satisfactory
response.
4. For successful results of these procedures, all conditions of the
assay must be followed precisely.
References
1. Capps, Hobbs, and Fox. 1948. J. Bacteriol. 55:869.
Packaging
Folic Acid Assay Medium 100 g 0318-15
Section II Folic Acid Assay Medium

202 The Difco Manual
Folic Acid Casei Medium & Folic Buffer A, Dried Section II
Bacto
®
Folic Acid Casei Medium
Bacto Folic Buffer A, Dried
User Quality Control
Identity Specifications
Folic Acid Casei Medium
Dehydrated Appearance: Off-white, homogeneous, with a
tendency to clump.
Solution: 4.7% (single strength) and 9.4%
(double strength) solution, soluble
in distilled or deionized water upon
boiling 1-2 minutes. Single-strength
solution is light amber, clear, may
have a slight precipitate.
Prepared Medium: Single-strength solution is very light
amber, clear, may have a very slight
precipitate.
Reaction of 4.7%
Solution at 25°C: 6.7 ± 0.1
Folic Buffer A, Dried
Dehydrated Appearance: White to off-white, free-flowing,
homogeneous.
Solution: 1.54% solution, soluble in distilled
or deionized water.
Solution Appearance: Colorless to very light amber, clear.
Reaction of 1.54%
Solution at 25°C: pH 6.1 ± 0.05
Cultural Response
Prepare Folic Acid Casei Medium per label directions. The
medium is tested by creating a standard curve using Folic Acid
at concentrations of 0 to 1.0 ng per 10 ml. This medium should
support the growth of L. casei subsp. rhamnosus ATCC
®
7469
when prepared in single strength and supplemented with
ascorbic acid and Folic Acid.
Intended Use
Bacto Folic Acid Casei Medium is used for determining folic acid
concentration by the microbiological assay technique.
Bacto Folic Buffer A, Dried is prepared for use with Folic Acid Casei
Medium in the microbiological assay of serum folic acid.
Summary and Explanation
Vitamin Assay Media are prepared for use in the microbiological assay
of vitamins. Three types of media are used for this purpose:
1. Maintenance Media: For carrying the stock culture to preserve the
viability and sensitivity of the test organism for its intended purpose;
2. Inoculum Media: To condition the test culture for immediate use;
3. Assay Media: To permit quantitation of the vitamin under test.
Folic Acid Casei Medium is prepared for the microbiological assay of
folic acid, particularly folic acid in serum. Lactobacillus casei subsp.
rhamnosus ATCC
®
7469 is used as the test organism in this assay.
Folic Acid Casei Medium is prepared according to the formulation
described by Flynn, Williams, O’Dell and Hogan
1
and modified by
Baker et al.
2
and Waters and Mollin.
3
Total serum folic acid activity can vary depending on the disease state.
It has been reported that normal subjects have a mean serum folic acid
level of 9.9 ng per ml. Patients with uncomplicated pernicious anemia
have a mean serum folic acid level of 16.6 ng per ml while patients
with megaloblastic anemia have levels less than 4.0 ng per ml.
Folic Buffer A, Dried is used for preparing both the standard and the
serum specimen in the microbiological assay of folic acid.
Principles of the Procedure
Folic Acid Casei Medium is a folic acid-free dehydrated medium
containing all other nutrients and vitamins essential for the cultivation
of L. casei subsp. rhamnosus ATCC
®
7469. The addition of folic acid
in specified increasing concentrations gives a growth response that can
be measured turbidimetrically.
Formula
Folic Acid Casei Medium
Formula Per Liter
Charcoal Treated Casitone . . . . . . . . . . . . . . . . . . . . . . . . . 10 g
Bacto Dextrose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 g
Sodium Acetate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 g
Dipotassium Phosphate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
Monopotassium Phosphate. . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
DL-Tryptophane . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.2 g
L-Asparagine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.6 g
L-Cysteine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . 0.5 g
Adenine Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Guanine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Uracil. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
Xanthine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Sorbitan Monooleate Complex. . . . . . . . . . . . . . . . . . . . . . 0.1 g
Glutathione (reduced) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 mg
Magnesium Sulfate, Anhydrous . . . . . . . . . . . . . . . . . . . . . 0.2 g
Sodium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Ferrous Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Manganese Sulfate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15 mg
Riboflavin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 mg
p-Aminobenzoic Acid. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 mg
Pyridoxine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . . 4 mg
Thiamine Hydrochloride . . . . . . . . . . . . . . . . . . . . . . . . . 400 µg
Calcium Pantothenate . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800 µg
Nicotinic Acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800 µg
Biotin. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 µg
Final pH 6.7 ± 0.1
Folic Buffer A, Dried
Formula Per Liter
Monopotassium Phosphate. . . . . . . . . . . . . . . . . . . . . . 10.656 g
Dipotassium Phosphate . . . . . . . . . . . . . . . . . . . . . . . . . 3.744 g
Ascorbic Acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
Final pH 6.1 ± 0.05

The Difco Manual 203
Section II Folic Acid Casei Medium & Folic Buffer A, Dried
Precautions
1. For Laboratory Use.
2. Great care must be taken to avoid contamination of media or glass-
ware in microbiological assay procedures. Extremely small
amounts of foreign material may be sufficient to give erroneous
results. Scrupulously clean glassware free from detergents and
other chemicals must be used. Glassware must be heated to 250°C
for at least 1 hour to burn off any organic residues that might be present.
3. Take precautions to keep sterilization and cooling conditions
uniform throughout the assay.
4. Follow proper established laboratory procedures in handling and
disposing of infectious materials.
Storage
Store Folic Acid Casei Medium and Folic Buffer A, Dried at 2-8°C.
The dehydrated medium is very hygroscopic. Keep container tightly
closed.
Expiration Date
The expiration date applies to the product in its intact container when
stored as directed. Do not use a product if it fails to meet specifications
for identity and performance.
Procedure
Materials Provided
Folic Acid Casei Medium
Folic Buffer A, Dried
Materials Required But Not Provided
Glassware
Autoclave
Stock culture of Lactobacillus casei subsp. rhamnosus ATCC
®
7469
Ascorbic acid (25 mg)
Sterile 0.85% saline
Distilled and deionized water
0.01 N NaOH
0.05 N HCl
Lactobacilli Agar AOAC
Folic Acid
Incubator (35-37°C)
Micro Inoculum Broth
Centrifuge
Spectrophotometer
Method of Preparation
Folic Acid Casei Medium
1. Suspend 9.4 grams in 100 ml distilled or deionized water.
2. Add 50 mg ascorbic acid if standard and test samples are not prepared
in Folic Buffer A.
3. Boil for 1-2 minutes.
4. Dispense 5 ml amounts into tubes, evenly dispersing any precipitate.
5. Add standard or test samples.
6. Adjust tube volume to 10 ml with distilled or deionized water.
7. Autoclave at 121°C for 5 minutes.
Folic Buffer A Dried
1. Dissolve the contents of one vial (15.4 grams) in 1 liter distilled or
deionized water.
Specimen Collection and Preparation
Assay samples are prepared according to references given in the
specific assay procedures. For assays, the samples should be diluted to
approximately the same concentration as the standard solution.
Test Procedure
Preparation of Stock Cultures and Inoculum
Prepare stock cultures of the test organism, L. casei subsp. rhamnosus
ATCC
®
7469, by stab inoculation into prepared tubes of Lactobacilli Agar
AOAC. Incubate the cultures at 35-37°C for 18-24 hours. Store cultures
in the refrigerator at 2-8°C. Stock transfers are made at monthly intervals.
Prepare the inoculum for assay by subculturing from a stock culture of
L. casei subsp. rhamnosus into a tube containing 10 ml prepared
Micro Inoculum Broth. Incubate at 35-37°C for 16-18 hours. Under
aseptic conditions, centrifuge the tubes to sediment the cells and decant
the supernatant. Wash the cells in 10 ml sterile single-strength Folic
Acid Casei Medium. Resediment the cells by centrifuging aseptically
and decant the supernatant. Repeat washing two more times. After the
third washing, resuspend the cells in 10 ml sterile single-strength
medium and dilute 1 ml with 99 ml of the same medium. One drop of
this suspension is used to inoculate each of the assay tubes. Read the
growth response of the assay tubes turbidimetrically after 18-24 hours
incubation at 35-37°C. (Some laboratories use 0.85% saline instead of
the single-strength basal medium to wash and dilute the inoculum.)
Preparation of the Standard
It is essential that a standard curve be constructed for each separate
assay. Autoclave and incubation conditions can influence the standard
curve readings and cannot always be duplicated. The standard curve
may be obtained by using folic acid at levels of 0.0, 0.1, 0.2, 0.4, 0.6,
0.8 and 1 ng per assay tube (10 ml).
The folic acid required for preparation of the standard curve may be
prepared as follows:
Dissolve 50 mg dried folic acid in about 30 ml 0.01 N NaOH and 300
ml distilled water. Adjust to pH 7-8 with 0.05 N HCl and dilute to 500
ml with distilled water. Dilute 10 ml of this solution with 500 ml
distilled water. Further dilute 1 ml in 1 liter distilled water to make a
stock solution containing 2 ng per ml folic acid. Prepare the standard
solution containing 0.2 ng per ml folic acid by diluting 10 ml of stock
solution with 90 ml of Folic Buffer A, Dried solution. Use 0.0, 0.5, 1,
2, 3, 4 and 5 ml per assay tube.
Prepare the stock solution fresh daily.
Preservation of Serum Specimens
1. Allow the blood specimen to clot and the serum to separate from
the clot.
2. Aspirate the serum into a clean dry tube and centrifuge to remove
any cells that may be present. Avoid hemolysis. Dispense 5 ml
of each serum sample into clean dry test tubes and add 25 mg
ascorbic acid to each tube.
3. If the test is not begun immediately, place tubes in a freezer and
hold below -20°C.

204 The Difco Manual
Preparation of Serum Specimen
1. Thaw the serum containing ascorbic acid.
2. Add 5 ml of the uniform sample to 45 ml rehydrated Folic Buffer
A, Dried.
3. Incubate the serum-buffer solution at 37°C for 90 minutes. Autoclave
the incubated mixture at 121°C for 2.5 minutes.
4. Remove the coagulated protein by centrifuging and transfer the
clear supernatant to a clean dry tube. The clear solution is the
sample to use in the folic acid assay.
Procedure for Total Folic Acid
1. Use 0.5, 1.0, 1.5 ml or other volumes of the prepared serum
extracts as described above.
2. Fill each assay tube with 5 ml of rehydrated Folic Acid Casei
Medium and sufficient distilled or deionized water to give a total
volume of 10 ml per tube.
3. Autoclave tubes at 121°C for 5 minutes.
4. Add 1 drop of inoculum described under Preparation of Stock
Culture and Inoculum to each assay.
5. Incubate at 35-37°C for 18-24 hours. Tubes are refrigerated for
15-30 minutes to stop growth before reading turbidimetrically.
Results
The amount of folic acid in the test samples can be determined by
Fraser Broth Section II
Fraser Broth
Bacto
®
Fraser Broth Base
.
Fraser Broth Supplement
Intended Use
Bacto Fraser Broth Base is used with Bacto Fraser Broth Supplement
in selectively enriching and detecting Listeria.
Summary and Explanation
First described in 1926 by Murray, Webb and Swann,
1
Listeria
monocytogenes is a widespread problem in public health and the food
industries. This organism has the ability to cause human illness
and death, particularly in immunocompromised individuals and
pregnant women.
2
The first reported food-borne outbreak of listeriosis
was in 1985,
3
and since then, microbiological and epidemiological
evidence from both sporadic and epidemic cases of listeriosis has
indicated that the principle route of transmission is via the consumption
of foodstuffs contaminated with Listeria monocytogenes.
4
Implicated vehicles of transmission include turkey frankfurters,
5
coleslaw, pasteurized milk, Mexican-style cheese, paté, and pickled pork
tongue. The organism has been isolated from commercial dairy and other
food processing plants, and is ubiquitous in nature, being present in a
wide range of unprocessed foods as well as in soil, sewage, silage
and river water.
6
Bacto Fraser Broth Base and Bacto Fraser Broth Supplement are based
on the formulation of Fraser and Sperber.
7
The medium is use in
the rapid detection of Listeria from food
8
and environmental samples.
Many common food contaminants such as streptococci, enterococci,
Bacillus species, Escherichia coli, Pseudomonas aeruginosa and Proteus
vulgaris interfere with the isolation of Listeria monocytogenes.
9
Listeria species grow over a pH range of 5.0-9.6, and survive in food
products with pH levels outside these parameters.
10
Listeria spp. are
microaerophilic, gram-positive, asporogenous, non-encapsulated, non-
branching, regular, short, motile rods. Motility is most pronounced at 20°C.
Identification of Listeria is based on successful isolation of the
organism, biochemical characterization and serological confirmation.
Principles of the Procedure
Bacto Tryptose, Bacto Beef Extract and Bacto Yeast Extract provide
nitrogen, vitamins and minerals. Sodium phosphate and potassium
phosphate are buffering agents. Differentiation is aided by including
ferric ammonium citrate in the final medium. Since all Listeria species
hydrolyze esculin, the addition of ferric ions to the medium will detect
the reaction. A blackening of the medium by cultures containing
esculin-hydrolyzing bacteria is the result of the formation of
6,7-dihydroxycoumarin that reacts with the ferric ions.
7
Selectivity is provided by the presence of lithium chloride, nalidixic
acid and acriflavine in the formula. The high salt tolerance of Listeria
is used as a means to inhibit growth of enterococci.
interpolating the results with the values obtained on the standard curve,
taking into consideration the dilutions of the samples.
Limitations of the Procedure
1. The test organism used for inoculating an assay medium must
be cultured and maintained on media recommended for this
purpose.
2. Aseptic technique should be used throughout the assay procedure.
3. The use of altered or deficient media may cause mutants having
different nutritional requirements that will not give a satisfactory
response.
4. For successful results of these procedures, all conditions of the
assay must be followed precisely.
References
1. Flynn, Williams, O’Dell, and Hogan. 1951. Anal. Chem. 23:180.
2. Baker, Herbert, Frank, Pasher, Hunter, Wasserman, and
Sobotka. 1959. Clin. Chem. 5:275.
3. Waters and Molin. 1961. J. Clin. Pathol. 14:335.
Packaging
Folic Acid Casei Medium 100 g 0822-15
Folic Buffer A, Dried 6 x 15.4 g 3246-33
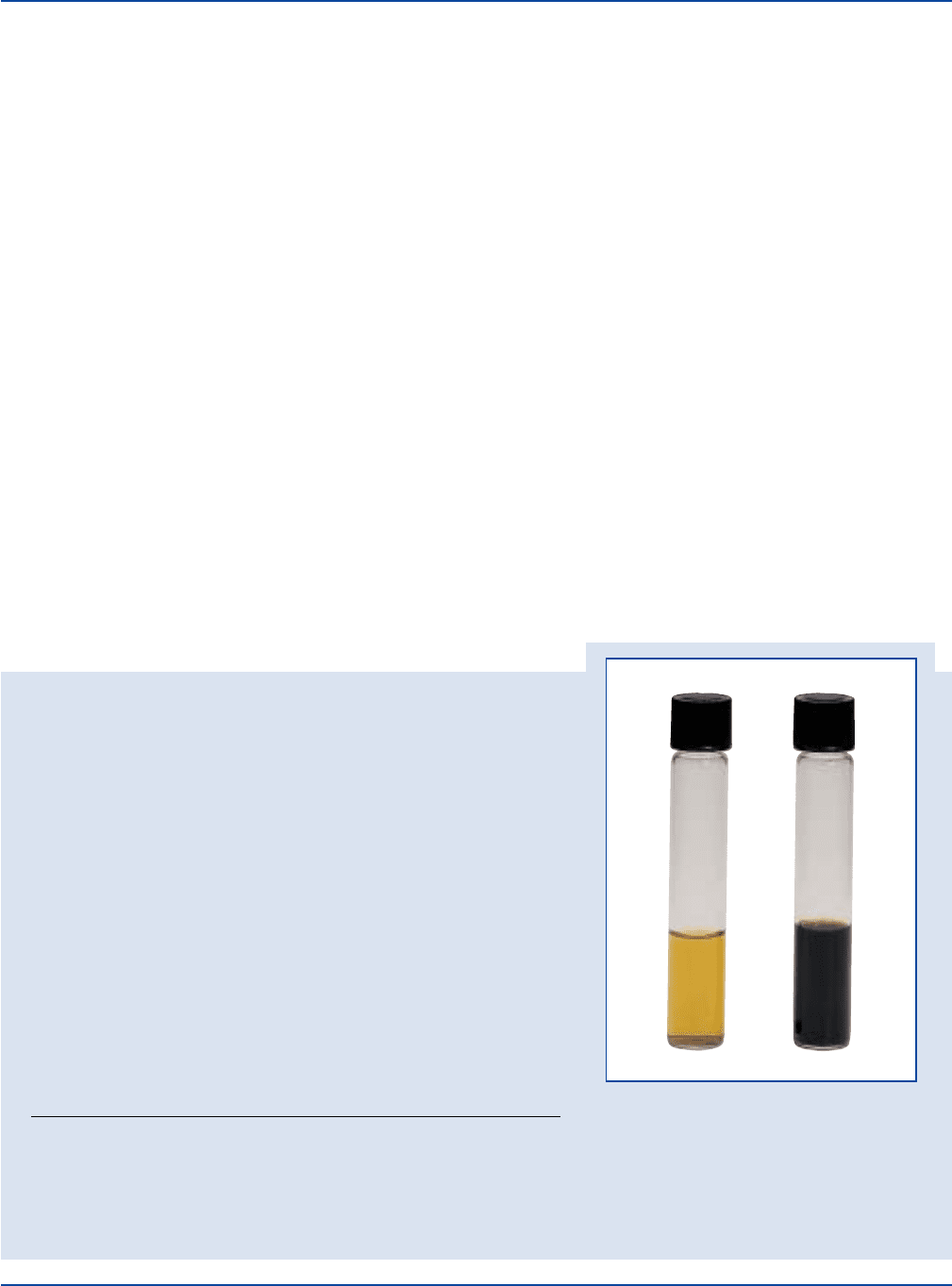
The Difco Manual 205
Section II Fraser Broth
Listeria monocytogenes
ATCC
®
19114
Uninoculated
tube
User Quality Control
Identity Specifications
Fraser Broth Base
Dehydrated Appearance: Tan, free-flowing, homogeneous.
Solution: 5.5% solution, soluble in distilled or deionized water on
boiling. Solution is medium amber, clear to slightly
opalescent with a fine precipitate.
Prepared Tubes: Medium amber, clear to slightly opalescent with
a fine precipitate.
Reaction of 5.5%
Solution at 25°C: pH 7.2 ± 0.2
Bacto Fraser Broth Supplement
Solution Appearance: Dark brown solution.
Cultural Response
Prepare Fraser Broth Base per label directions. Add Fraser Broth Supplement.
Inoculate and incubate at 35 ± 2°C for 24-48 hours.
INOCULUM ESCULINE
ORGANISM ATCC
®
CFU GROWTH REACTION
Escherichia faecalis 29212* 1,000-2,000 marked to complete inhibition –
Escherichia coli 25922* 1,000-2,000 marked to complete inhibition –
Listeria monocytogenes 19114 100-1,000 good +
The cultures listed are the minimum that should be used for performance testing.
*These cultures are available as Bactrol™ Disks and should be used as directed in Bactrol Disks Technical Information.
Formula
Fraser Broth Base
Formula Per Liter
Bacto Tryptose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 g
Bacto Beef Extract . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 g
Bacto Yeast Extract . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 g
Sodium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 g
Sodium Phosphate, Dibasic . . . . . . . . . . . . . . . . . . . . . . . . 9.6 g
Potassium Phosphate, Monobasic . . . . . . . . . . . . . . . . . . 1.35 g
Esculin. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 g
Nalidixic Acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.02 g
Acriflavine HCl. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.024 g
Lithium Chloride . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 g
Final pH 7.2 ± 0.2 at 25°C
Fraser Broth Supplement
Ingredients per 10 ml vial
Ferric Ammonium Citrate . . . . . . . . . . . . . . . . . . . . . . . . . 0.5 g
Precautions
1. For Laboratory Use.
2. Fraser Broth Base:
HARMFUL. IRRITATING TO EYES, RESPIRATORY SYSTEM
AND SKIN. MAY CAUSE HARM TO THE UNBORN CHILD.
Avoid contact with skin and eyes. Do not breathe dust. Wear
suitable protective clothing. Keep container tightly closed.
TARGET ORGAN(S): Blood, Kidneys, Nerves
FIRST AID: In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice. After contact with skin,
wash immediately with plenty of water. If inhaled, remove to fresh
air. If not breathing, give artificial respiration. If breathing is
difficult, give oxygen. Seek medical advice. If swallowed seek
medical advice immediately and show this container or label.
Fraser Broth Supplement:
IRRITANT. IRRITATING TO EYES, RESPIRATORY SYSTEM
AND SKIN. Avoid contact with skin and eyes. Do not breathe mist.
Wear suitable protective clothing. Keep container tightly closed.
FIRST AID: In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice. After contact with skin,
wash immediately with plenty of water. If inhaled, remove to fresh
air. If not breathing, give artificial respiration. If breathing is
difficult, give oxygen. Seek medical advice. If swallowed seek
medical advice immediately and show this container or label.
3. Follow proper, established laboratory procedures in handling and
disposing of infectious materials.
Storage
Store the dehydrated medium below 30°C. The dehydrated medium is
very hygroscopic. Keep container tightly closed.
Store Bacto Fraser Broth Supplement at 2-8°C.
Store the prepared medium at 2-8°C.
