Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

2.6.6
Solid Support
The i mmobilization of catalysts or catalyst precursors on s olid supports in order to
simplify reaction procedures and to increase the stability of the catalyst is a
common technique to render homogeneous systems heterogeneous. The MTO
catalyst can be transf err ed onto polyme ri c material in a number of different ways.
When an aqueous solution of MT O is heated for several hours (abou t 70
C), a
golden-colored polymeric material is formed. The composition of this organome-
tallic polymer is [H
0.5
(CH
3
)
0.92
ReO
3
] [61]. T his polymeric form of MTO is non-
volatile, stable to air and moisture, and insoluble in all non-coordinating solvents.
It can be used as a c atalyst precursor for ep oxidation of alkenes, since it is soluble
in hydrogen peroxide, where it reacts to form the peroxo-rhenium species. Of
course, the heterogeneous property of this material is lost upon usage, but from a
storage perspective, the polymeric MTO offers some advantages. MTO can,
however, easily be immobilized by t he add ition of a polymeric material containing
Lewis basic groups with the abilit y t o coordinate to the rhenium center. A nu mber
of different approaches have been reported. Herrmann and coworkers described
the use of polyvinylpyridines as the organic support, but the resulting MTO-
polymer complex showed low catalytic activity [61]. A serious drawback with this
supported catalyst was the oxidation of the polymeric backbone, leading to loss of
the rhenium catalyst.
In a recent improvement to this approach, poly(4-vinylpyridine) and poly(4-
vinylpyridine) N-oxides were used as the catalyst carrier [91]. The MTO catalyst
obtained from 25% cross-linked poly(4-vinylpyridine) proved to efficiently catalyze
the formation of even hydrolytically sensitive epoxides in the presence of aqueous
hydrogen peroxide (30%). This catalyst could be recycled up to 5 times without any
significant loss of activity. Attempts have been made to immobilize MTO with the use
of either microencapsulation techniques, including sol-gel techniques, to form silica-
bound rhenium compounds, or by the attachment of MTO to silica tethered with
polyethers. These approaches have provided catalysts with good activity using
aqueous hydrogen peroxide as the terminal oxidant [91–93]. In the latter case, high
selectivity for epoxide formation was also obtained for very sensitive substrates (e.g.,
indene).
An alternative approach to immobilization of the catalyst on a solid support is to
perform the MTO-catalyzed epoxidation reactions in the presence of NaY zeolites.
This technique has been employed by Malek and Ozin, and later by Bein et al., who
used highly activated zeolites for the preparation of NaY/MTO using vacuum
sublimation [94, 95]. More recently, Adam and coworkers found a significantly
simpler approach toward this catalyst. The active catalyst was formed by mixing
unactivated NaY zeolite with hydrogen peroxide (85%) in the presence of MTO and
the substrate alkene [96]. Using this catalytic mixture, various alkenes were trans-
formed into their corresponding epoxides without the formation of diols (typical diol
formation was <5%). The MTO catalyst is positioned inside the 12 A
supercages of
the NaY zeolite; hence, the role of the zeolite is to act as an absorbent for the catalyst
2.6 Rhenium-Catalyzed Epoxidations
j
63
and to provide heterogeneous microscopic reaction vessels for the reaction. The
supernatant liquid was demonstrated to be catalytically inactive, even if Lewis bases
(pyridine) were present. The high selectivity for epoxide formation was attributed to
inhibition of the Lewis acid-mediated hydrolysis of the product by means of steric
hindrance.
Recently, Omar Bouh and Espenson reported that MTO supported on niobia
catalyzed the epoxidation of various fatty oils using UHP as the terminal oxidant [97].
Oleic acid, elaidic acid, linoleic acid, and linolenic acid were all epoxidized in high
yields (80–100%) in less than two hours. Furthermore, it was demonstrated that the
catalyst could be recovered and reused without loss of activity.
2.6.7
Asymmetric Epoxidations Using MTO
The MTO-based epoxidation system offers a particularly effective and practical
route for the formation of racemic epoxides. Very few attempts to prepare chiral
MTO complexes and to employ them in catalytic asymmetric epoxidation have so
far been made, and the few existing reports are unfortunately quite discouraging.
In the epoxidation of cis-b-methylstyrene with MTO and hydrogen peroxide in
the presence of the additive (S)-(N,N-dimethyl)-1-phenylethylamine, a product with
an enantiomeric excess of 86% has been claimed [98]. The epoxides from
other substrates like styrene and 1-octene were obtained in significantly lower
enantioselectivity (13% ee). Furthermore, the MTO-catalyzed epoxidation of 1-
methylcyclohexene with
L-prolineamide, ( þ )-2-aminomethylpyrrolidine or (R)-1-
phenylethylamine as additives was reported to yield the product in low yield and
enantioselectivity (up to 20% ee) [99]. A significant amount of the diol was formed in
these reactions. More recently, Herrmann and coworkers reported on the use of
chiral pyrazole derivatives as ligands in the MTO-catalyzed epoxidation of cis-
b-methylstyrene [100]. Conversion and enantiomeric excess were unfortunately
rather poor, with a maximum of 27% ee. They also reported on the use of chiral diols
as ligands for the epoxidation of the same substrate (cis-b-methylstyrene), the best
enantioselectivity obtained being 41% ee, although in this particular case the
reaction was only completed to 5% conversion. Hence, a general protocol for the
enantioselective formation of epoxides using rhenium catalysts is still lacking.
There would certainly be a breakthrough if such a system could be developed,
considering the efficiency of the MTO-catalyzed epoxidation reactions using
hydrogen peroxide as the terminal oxidant.
2.7
Iron-Catalyzed Epoxidations
The use of iron salts and complexes for alkene epoxidation is in many respects similar
to that of manganese catalysts. Thus, iron porphyrins can be used as epoxidation
catalysts, but often conversion and selectivity is inferior to that obtained with its
64
j
2 Transition Metal-Catalyzed Epoxidation of Alkenes
manganese counterpart. The possibilities to efficiently use hydrogen peroxide as the
terminal oxidant are limited because of its rapid decomposition, catalyzed by iron.
However, a recent breakthrough by Beller and coworkers [101] has resulted in a series
of catalytic systems which use only 2–3 equivalents of aqueous hydrogen peroxide for
the epoxidation of predominantly aromatic alkenes (see below). The traditional iron
catalysts used for epoxidation of alkenes are either of the porphyrin type or based on
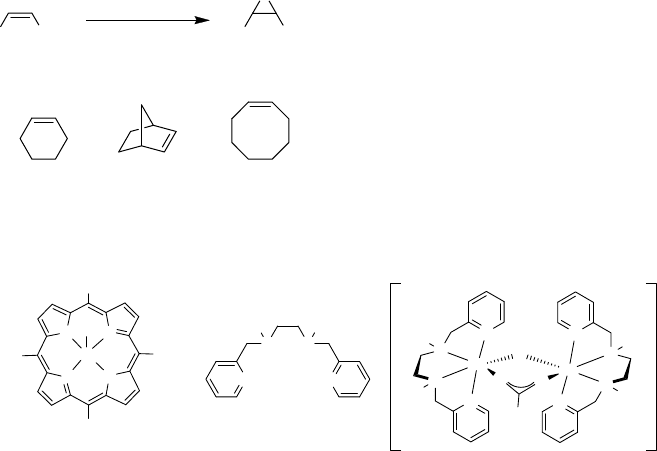
various non-heme biomimetics. Traylor and coworkers found conditions where a
polyfluorinated Fe(TPP)-catalyst (17) was employed in the epoxidation of cyclooctene
to yield the corresponding epoxide in high yield (Scheme 2.17) [102]. High catalyst
loading (5 mol%) and slow addition of the oxidant was required, which certainly
limits the usefulness of this procedure.
N N
N N
H
3
C CH
3
N
N
N
N
C
6
F
5
C
6
F
5
C
6
F
5
Fe
III
Cl
C
6
F
5
1817
N
N
N
N
H
3
C
H
3
C
Fe
N
N
N
N
CH
3
CH
3
Fe
O
O
O
H
3
C
19
(SbF
6
)
2
Recently, a number of iron complexes with biomimetic non-heme ligands were
introduced as catalysts for alkane hydroxylation, alkene epoxidation and dihydrox-
ylation. These complexes were demonstrated to activate hydrogen peroxide without
the formation of free hydroxyl radicals, a feature commonly observed in iron
oxidation chemistry. A particularly ef ficient catalytic system for selective epoxidation
of alkenes was developed by Jacobsen and coworkers [103]. In this protocol, a
tetradentate ligand (BPMEN ¼ N,N
0
-dimethyl-N,N
0
-bis(2-pyridylmethyl)-diami-
noethane, 18) was combined with an iron(II) precursor and acetic acid to yield a
self-assembled m -oxo, carboxylate-bridged diiron(III) complex (19). This dimeric
iron-complex, resembling the active site found in the hydroxylase methane mono-
oxygenase (MMO), was demonstrated to efficiently epoxidize alkenes in the presence
R R
O
H30%
2
O
2
(aq)
17 mol%)(5
CH
2
Cl
2
(1:3)MeOH:
25
o
h1-24C,
R
R
yield100%yield64%yield80%
Scheme 2.17
2.7 Iron-Catalyzed Epoxidations
j
65
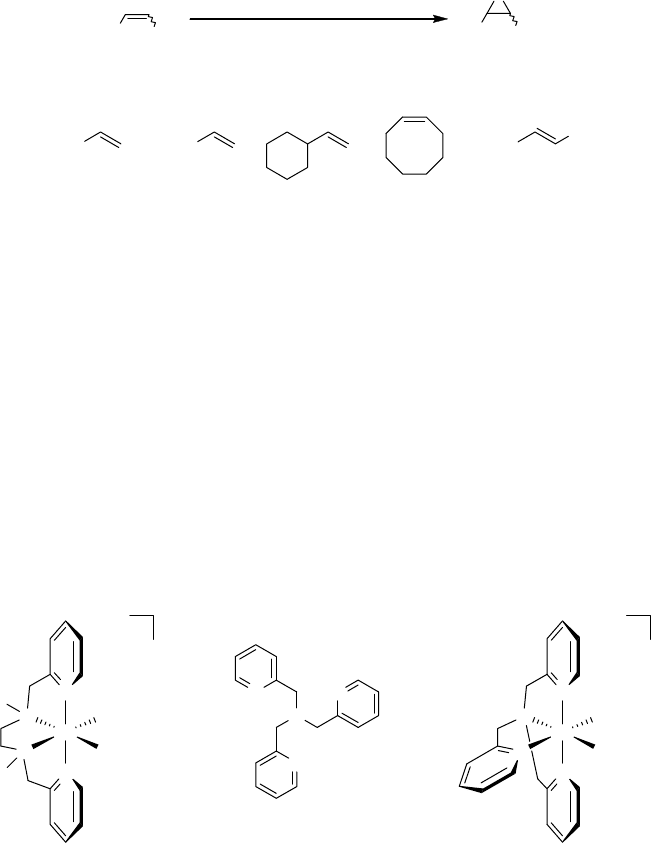
of aqueous hydrogen peroxide (50%). This catalyst turned out to be particularly active
for the epoxidation of terminal alkenes, which normally are the most difficult
substrates to oxidize. Thus, 1-dodecene was transformed into its corresponding
epoxide in 90% yield within 5 min using 3 mol% of the catalyst. This system was also
effective for the epoxidation of other simple non-terminal alkenes, like cyclooctene
and trans-5-decene (Scheme 2.18).
Que and coworkers reported on a similar monomeric iron complex, formed with
the BPMEN ligand but excluding acetic acid [104]. This complex was able to epoxidize
cyclooctene in reasonably good yield (75%), but at the same time a small amount of
the cis-diol (9%) was formed. The latter feature, observed with this class of complexes,
has been further studied, and more selective catalysts have been prepared. Even
though poor conversion is often obtained with the current catalysts, this method
represents an interesting alternative to other cis-dihydroxylation systems [105, 106].
Using similar chiral ligands based on 1,2-diaminocyclohexane resulted in complexes
which were able to catalyze the formation of epoxides in low yields and in low
enantioselectivity (0–12% ee). The simultaneous formation of cis-diols was occurring
with significantly better enantioselectivity (up to 82% ee), but these products were also
obtained in low yields.
N
N
N N
TPA
Fe
N NCCH
3
NCCH
3
N
N
N
2+
[(TPA)Fe
II
(CH
3
CN)
2
]
2+
Fe
N NCCH
3
NCCH
3
N
N
N
2+
[(BPMEN)Fe
II
(CH
3
CN)
2
]
2+
R
1
R
1
O
Hequiv1.5
2
O
2
(aq)
18 Fe(CH+
3
(SbFCN)
6
)
2
mol%)(3
mol%)(30acidAcetic
CH
3
CN
4
o
min5C,
C
10
H
21
C
8
H
17
yield76%yield90%yield85%
R
2
R
2
C
4
H
9
C
4
H
9
yield87%yield86%
Scheme 2.18
66
j
2 Transition Metal-Catalyzed Epoxidation of Alkenes
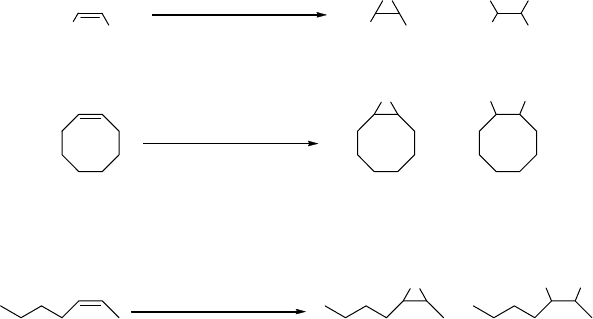
Upon closer inspection of the Fe(II)-complexes formed with either the BPMEN
or the tetradentate trispicolylamine (TPA) ligands, respectively, Que and cow-
orkers found that the presence of acetic acid in the catalytic mixture had a strong
influence on the selectivity of the oxidation process [107, 108]. Hence, the use of
either [(BPMEN)Fe(CH
3
CN)
2
](OTf)
2
or [(TPA)Fe(CH
3
CN)
2
](OTf)
2
as catalysts for
the oxidation of cyclooctene or cis-2-heptene in a mixture of acetonitrile and
acetic acid (1 : 2), with hydrogen peroxide as terminal oxidant, led predominantly
to the formation of the epoxide (Scheme 2.19). It should be noted that slow
addition of hydrogen peroxide (60 min) was required for successful alkene
oxidation. When the corresponding reactions were performed without addition
of acetic acid, the oxidation of cyclooctene using [(BPMEN)Fe(CH
3
CN)
2
](OTf)
2
as
catalyst precursor resulted in the formation of 63% cyclooctene oxide and 6% of
the corresponding diol. Using the TPA-containing catalyst gave merely 32%
epoxide and 37% diol.
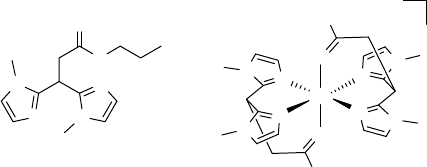
An additional contribution to the field of biomimetic non-heme iron complexes for
alkene oxidation was recently reported by Klein Gebbink and coworkers [109]. They
found that iron(II) complexes formed with the neutral ligand propyl 3,3-bis(1-
methylimidazol-1-yl)propionate (20) were active as catalysts for the oxidation of
various simple alkenes. When complex 21 was employed as the catalyst for the
oxidation of cyclooctene in the presence of 10 equivalents of hydrogen peroxide, a
mixture of epoxide and diol in a ratio of 2.5 : 1 was obtained. However, the conversion
was rather poor (39%).
R
1
R
2
R
1
R
2
R
1
R
2
O HO OH
Hequiv1.5
2
O
2
Fe
II
mol%)(0.5-catalyst
CH
3
CN:CH
3
1:2COOH
0
o
min.90C,
+
[(BPMEN)Fe
II
(CH
3
CN)
2
(OTf)]
2
:
+
HO
OH
O
0.3%99%
[(TPA)Fe
II
(CH
3
CN)
2
(OTf)]
2
1%98%:
[(BPMEN)Fe
II
(CH
3
CN)
2
(OTf)]
2
:
+
(50%0.3%77%
cis
)
[(TPA)Fe
II
(CH
3
CN)
2
(OTf)]
2
(66%1%44%:
cis
)
O
HO OH
Scheme 2.19
2.7 Iron-Catalyzed Epoxidations
j
67
O
O
N
N
N
N
Fe
O
N
N
N
N
OPr
O
N
N
N
N
PrO
2+
(OTf)
2
20
21
The major breakthrough in the field of iron-catalyzed epoxidation using aqueous
hydrogen peroxide as the terminal oxidant was recently made by Beller and co-
workers. The combination of iron(III) chloride hexahydrate with a pyridine carboxylic
acid ligand and an organic base allowed for alkene epoxidation under seemingly
neutral conditions. After screening several different catalytic systems containing a
variety of pyridine ligands and organic or inorganic bases, the combination of
pyridine-2,6-dicarboxylic acid (H
2
pydic) and pyrrolidine turned out to be the most
successful one [101, 110]. Using slow addition of hydrogen peroxide and the above-
mentioned catalyst combination allowed for the efficient epoxidation of a series
of aryl substituted alkenes (Table 2.8). Interestingly, this catalyst system seems to be
most efficient and selective for the epoxidation of mono-substituted or trans-
1,2-disubstituted aromatic alkenes. The epoxidation of cis-1,2-disubstituted or tri-
substituted substrates led in all cases to lower yields and poor chemoselectivity.
Furthermore, aliphatic alkenes were less favorable substrates, leading in all cases to
lower conversions and selectivity.
In further optimizations, Beller and coworkers examined various benzyl amines as
replacement for pyrrolidine in the FeCl
3
-H
2
pydic catalyst system. They found that the
use of different benzyl amines resulted in higher yields and better selectivity for the
formation of epoxides, predominantly from aliphatic alkenes [111]. As seen in
Table 2.8, the epoxidation of trans-1,2-disubstituted alkenes such as trans-2-octene
and trans-5-decene resulted in high yields, whereas aliphatic terminal alkenes appear
to be problematic substrates. The epoxidation of aromatic alkenes using this catalytic
system gave similar results to those obtained using pyrrolidine as base.
In a recent study, the above-mentioned iron(III) chloride catalyst system was
further simplified [112, 113]. Beller and coworkers examined the use of a series of
simple imidazoles as ligands for iron, and the in-situ-formed complexes were used as
epoxidation catalysts in combination with aqueous hydrogen peroxide in tert-amyl
alcohol. Initially the best catalyst combination consisted of a mixture of FeCl
3
6H
2
O
(5 mol%) and 5-chloro-1-methylimidazole (15 mol%). Upon further optimization, it
was found that the use of 12 mol% of 2,6-diisopropyl-N-phenylimidazole (IPrPIm)
together with the iron(III) chloride salt resulted in a superior catalyst. The latter
catalyst system displays reactivity features similar to those of the H
2
pydic/pyrrolidine
system, in that mono-substituted or trans-1,2-disubstituted aromatic alkenes are
easily converted to their corresponding epoxides, whereas other substrates behave
more erratically.
68
j
2 Transition Metal-Catalyzed Epoxidation of Alkenes
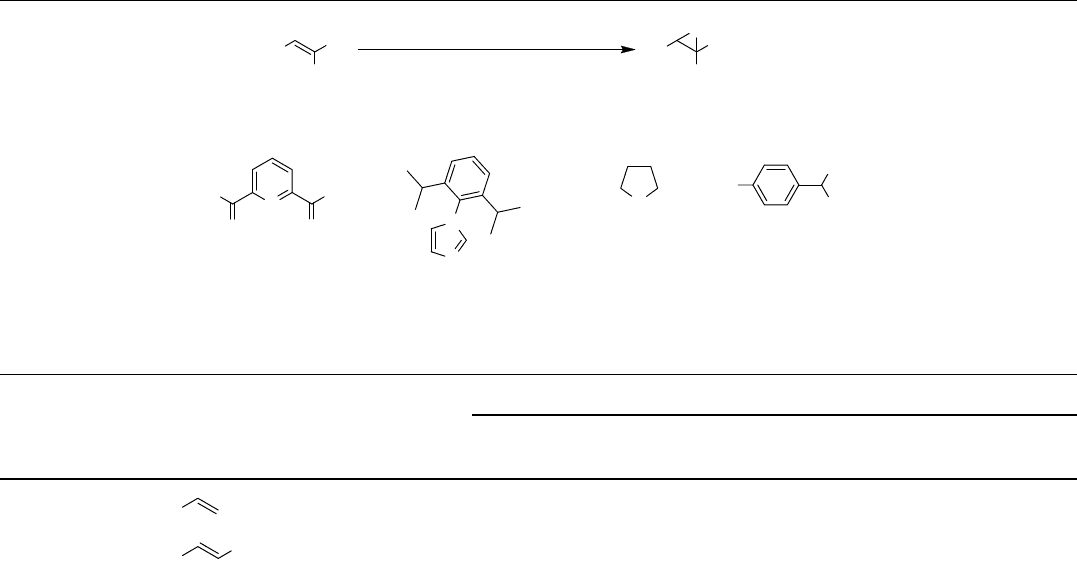
Table 2.8 Iron-catalyzed epoxidations.
N
HO
O
OH
O
R
1
FeCl
3
6Hx
2
mol%)(5O
mol%)(5-10Ligand
mol%)(10-12Base
Hequiv.2-3
2
O
2
aq),(30%
t
-AmylOH
R
2
R
3
R
1
R
2
R
3
O
2,6-dicarboxylicpyridine
(Hacid
2
pydic)
Bases:
N
H
mol%)(10
X
pyrrolidine
NHR
5
R
4
22a
RCl,=X)
4
RMe,=
5
H=
22b)RH,=X
4
REt,=
5
H=
mol%)(12
Ligands:
mol%)(5
N
N
2,6-diisopropyl-
N
-phenyl
(IPrPIm)imidazole
mol%)(10
Catalyst combinations: FeCl
3
6H
2
O (5 mol%) and:
H
2
pydic/pyrrolidine
a)
H
2
pydic/benzylamine
b)
IPrPIm
c)
Entry Substrate Yield (%) Yield (%) Yield (%)
1
Ph
93 91
d)
87
2
Ph
CH
3
95 94
(Continued)
2.7 Iron-Catalyzed Epoxidations
j
69
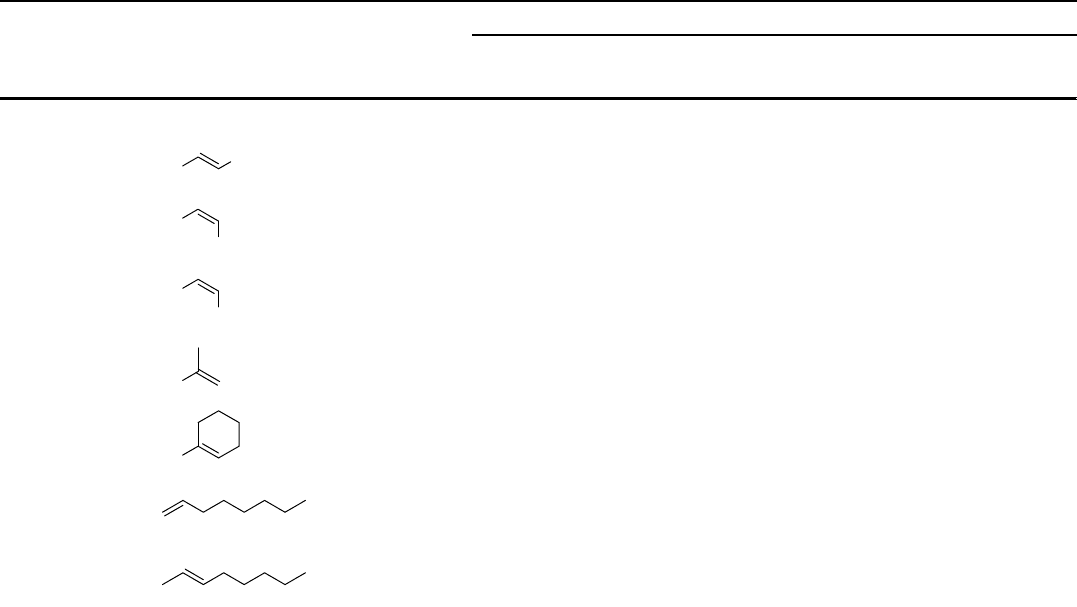
Table 2.8 (Continued)
Catalyst combinations: FeCl
3
6H
2
O (5 mol%) and:
H
2
pydic/pyrrolidine
a)
H
2
pydic/benzylamine
b)
IPrPIm
c)
Entry Substrate Yield (%) Yield (%) Yield (%)
3
Ph
Ph
56
4
Ph
CH
3
84 84
d)
66
5
Ph
Ph
824
d)
23
6
Ph
64
7
Ph
21
8
32
d)
18
9
36 87
d)
70
j
2 Transition Metal-Catalyzed Epoxidation of Alkenes

10 96
e)
37
11
32 58
e)
a) H
2
pydic (5 mol%) and pyrrolidine (10 mol%). Slow addition of 2 equiv. H
2
O
2
over 60 min at r.t.
b) H
2
pydic (5 mol%) and benzylamine (12 mol%). Slow addition of 2 equiv. H
2
O
2
over 60 min at r.t.
c) IPrPIm (10 mol%). Slow addition of 3 equiv. H
2
O
2
over 60 min at r.t.
d) Benzylamine 22a was employed.
e) Benzylamine 22b was employed.
2.7 Iron-Catalyzed Epoxidations
j
71
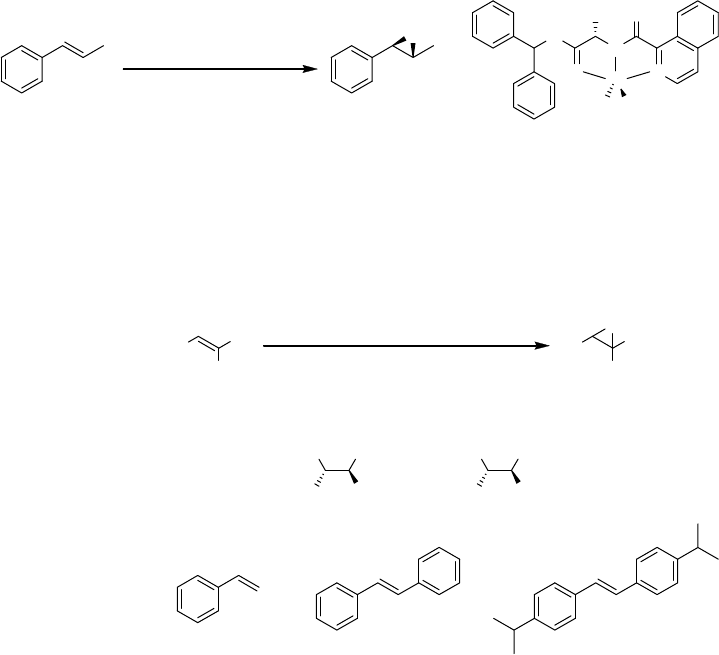
2.7.1
Iron-Catalyzed Asymmetric Epoxidations
The use of iron catalysts in combination with aqueous hydrogen peroxide as the
terminal oxidant for the asymmetric epoxidation of alkenes has been significantly
less studied. Using high-throughput screening techniques, Francis and Jacobsen
discovered a novel iron-based protocol for the preparation of enantiomerically
enriched epoxides [114]. In this system, chiral complexes prepared from polymer-
supported peptide-like ligands and iron(II) chloride were evaluated as catalysts for the
epoxidation of trans-b -methylstyrene employing aqueous hydrogen peroxide (30%)
as the terminal oxidant. The best polymer-supported catalysts yielded the correspond-
ing epoxide in up to 78% conversion with enantioselectivities ranging from 15 to 20%
ee. Employing a homogeneous catalyst derived from this combinatorial study, trans-
b-methylstyrene was epoxidized in 48% ee after 1 h (100% conversion, 5 mol%
catalyst, 1.25 equiv. 50% hydrogen peroxide in t-BuOH) (Scheme 2.20) [115].
Recently, Gelalcha, Beller, and coworkers reported that the combination of the
FeCl
3
-H
2
pydic system with chiral monosulfonated diamine ligands (e.g., TsDPEN)
resulted in complexes which catalyzed the formation of enantiomerically enriched
epoxides using aqueous hydrogen peroxide as the terminal oxidant (Scheme 2.21)
H
N
N
O
O N
Fe
ClCl
CH
3
23
O
Hequiv.1.25
2
O
2
(50%)
23 mol%),(5
t
1hBuOH,
conv.100%
ee48%
Scheme 2.20
R
1
FeCl
3
6Hx
2
mol%)(5O
H
2
mol%)(5pydic
mol%)(12Ligand
Hequiv.2
2
O
2
aq),(30%
t
-AmylOH
R
2
R
3
R
1
R
2
R
3
O
TsHN NH
2
Ph Ph
TsHN NHBn
Ph Ph
Ligands:
(
S
,
S
)-TsDPEN
(
S
,
S
)-TsDPEN:
(
S
,
S
)-
N
BnTsDPEN: ee)(8%84%
(-))ee(28%86%
ee)(71%90%(+))ee(42%87%
(
S
,
S
)-
N
BnTsDPEN
Scheme 2.21
72
j
2 Transition Metal-Catalyzed Epoxidation of Alkenes
