Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

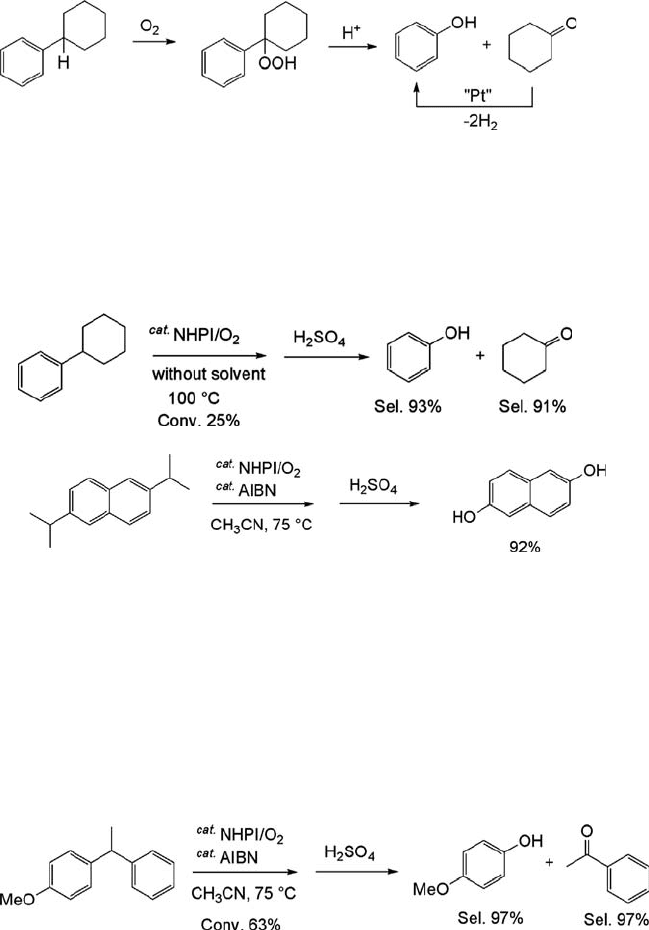
resulted in phenol (93% selectivity) and cyclohexanone (91% selectivity) at 25%
conversion (Eq. (6.15)) [73]. Similarly, the oxidation of 2,6-diisopropylnaphthalene
with air (20 atm) in the presence of NHPI/AIBN led to 2,6-naphthalenediol
(Eq. (6.16)) [74].
ð6:15Þ
ð6:16Þ
Various substituted phenols were selectively synthesized by a one-pot reaction
through the NHPI-catalyzed aerobic oxidation of 1,1
0
-diarylethanes to hydroperox-
ides followed by treatment with dilute sulfuric acid [75]. For example, the oxidation of
1-(4-methoxyphenyl)-1-phenylethane was performed under O
2
(1 atm) in the pres-
ence of AIBN (3 mol%) and NHPI (10 mol%) in MeCN (3 mL) at 75
C for 15 h, and
treatment with sulfuric acid afforded 4-methoxyphenol and acetophenone in 61%
yield (97% selectivity) at 63% conversion (Eq. (6.17)). In this reaction, the degradation
of hydroperoxides was selectively induced to give more electron-rich phenols in high
selectivity (Scheme 6.6).
ð6:17Þ
6.2.3
Preparation of Acetylenic Ketones by Direct Oxidation of Alkynes
a,b-Acetylenic carbonyl compounds (ynones) are important intermediates in organic
synthesis, since further elaboration of ynones can lead to highly valuable compounds
Scheme 6.5 A new route to phenol synthesis suggested by Sheldon et al.
6.2 NHPI-Catalyzed Aerobic Oxidation
j
203

such as heterocyclic compounds [76], a,b-unsaturated ketones [77], cyclopente-
nones [78], nucleosides [79], chiral pheromones [80], and so on. Several methods
have been reported for the synthesis of conjugated acetylenic ketones based on a
coupling reaction of acetylenides with activated acylating reagents such as acid
chloride or anhydrides [81]. Alternatively, the selective oxidation of alkynes to ynones
can be carried out by the use of CrO
3
/TBHP [82], CrO
3
(pyridine)
2
[83], Na
2
CrO
4
/
acetic anhydride [83], and SeO
2
/TBHP systems [84], but these oxidations are not
wholly successful [85]. An alternative approach for preparing ynones is the oxygen-
ation of the propargylic CH bonds of alkynes with dioxygen, since the bond
dissociation energy of these bonds (87 2 kcal/mol for 2-pentyne) is approximately
equal to that of the benzylic CH bonds of alkylbenzenes (88 1 kcal/mol for
toluene) [86]. However, the conventional oxidation of alkynes with dioxygen at higher
temperatures (around 150
C) results in undesired over-oxidation products like
carboxylic acids. Since the aerobic oxidation of alkylbenzenes by the NHPI catalyst
could be effected even at room temperature, the NHPI-catalyzed oxidation of alkynes
at lower temperature is expected to suppress undesired side reactions.
Treatmentof4-octynewithdioxygen(1 atm)under theinfluence ofNHPI(10 mol%)
and Cu(acac)
2
(0.5 mol%) in acetonitrile at room temperature for 30 h produces
4-octyn-3-one (77%) and 4-octyn-3-ol (22%) at 70% conversion (Eq. (6.18)) [87]. The
same reaction at 50
C for 6 h gives the ynone in 70% yield based on 83% conversion.
This oxidation would offer a facile catalytic method for the preparation of conjugated
ynones from alkynes, since 1-decyne, upon treatment with TBHP in the presence
of SeO
2
, leads to the acetylenic alcohol, 1-decyn-3-ol, rather than the ynone, 1-decyn-
3-one [84]. The NHPI/Cu(II) system can also promote the oxidation of acetylenic
alcoholsto ketones.Thereactionof1-octyn-3-ol underdioxygen(1 atm)in thepresence
of NHPI and Cu(acac)
2
affords 1-octyn-3-one in 95% yield based on 57% conversion.
ð6:18Þ
Scheme 6.6 A strategy of the selective phenol synthesis.
204
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
6.2.4
Oxidation of Alcohols
The oxidation of alcohols to the corresponding carbonyl compounds is a trans-
formation freq uently used in organic synthe sis [88]. There have been many
catalytic methods f or the aerobic oxidation of alcohols to the corresponding
carbonyl compounds [ 89, 90], but some of these oxidations are carried out in
the presence of a reducing agent such as an aldehyde which is e ventually
converted into carboxylic acid, or they are severely limite d to some reactive
alcohols such as be nzylic and allylic alcohols. Recently, a few aerobic oxidations
involving n onactivated alcohols have appeared, although expensive metal c atalysts
such as Ru and Pd must be employed to effect the oxidation [91]. In 1996, Markó
and coworkers developed an efficient aerobic oxidation system of aliphatic
alcohols using an inexpensive CuCl
2
/phenanthroline cat alyst comb ined wit h
azodicarboxylate [92]. Reusable heterogeneous catalysts consisting of Ru or Pd
have be en reported by Kaneda [93] and Uemura [94], respectively. Sheldon and
coworkers have succeeded in the aerobic oxidation of alcohols b y a water-soluble
Pd catalyst [95].
As described in the preceding sections, alkanes are oxidized by the NHPI/
Co(II) system w ith dioxygen under mild conditions. This catalytic system is
expected to promote the aerobic oxidation of the hydroxyl functions of alcohols to
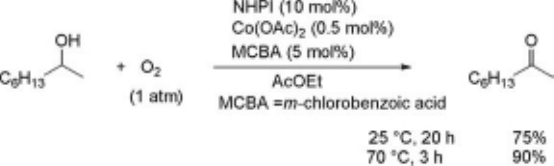
carbonyl functions [96, 97]. The oxidation of 2-octanol in ethyl acetate at 70
Cin
thepresenceofNHPI(10mol%)andCo(OAc)
2
(0.5 mol%) under dioxygen (1 atm)
gives rise to 2-octanone in quantitative yield. Benzoic acids such as m-chlor-
obenzoic acid (MCBA) enhance the oxidation of alcohols to carbonyl compounds.
2-Octanol can be converted into 2-octanone with O
2
even at room temperature by
adding a catalytic amount of MCBA to the NHPI/Co(OAc)
2
system (Eq. (6.19)).
The aerobic oxidation of aliphatic alcohols at room temperature has been
reported only by Ley et al., who used [Bu
4
N]
þ
[RuO
4
]
assisted by 4-A
molecular
sieves [91b].
ð6:19Þ
Figure 6.5 shows the oxidation of secondary and primary alcohols under ambient
conditions by the NHPI/Co(OAc)
2
/MCBA system. Aromatic and cyclic alcohols
afford the corresponding ketones in good to quantitative yields. Primary alcohols are
also oxidized to carboxylic acids in good yields, although MCPBA (m-chloroperben-
zoic acid) is added instead of MCBA. Lauryl alcohol leads to lauric acid (66% yield),
6.2 NHPI-Catalyzed Aerobic Oxidation
j
205
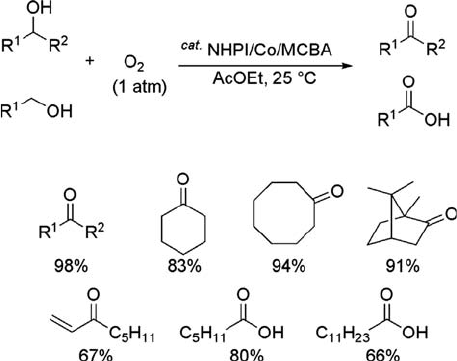
which is used as a surf actant raw material. In this oxidation, which proceeds
through a free radical process, primary alcohols are rapidly converted into
carboxylic acids without isol ation of aldehydes, because th e hydrogen atom
abstraction from aldehydes to af ford acyl radic als takes pl ace more easily than
that from alcoh ols to furnish a-hydroxyalkyl radicals [5a]. The oxidation of allylic
alcohols is easily achieved.
In contrast to oxidations of diols with stoichiometric oxidants such as NaIO
4
,
Pb(OAc)
4
[98], or hydrogen peroxide [99], which are often used in organic synthesis,
little work has been done so far for the oxidation of diols with dioxygen [100]. Recently,
Uemura and coworkers have reported the Pd(OAc)
2
-catalyzed lactonization of a,v-
primary diols with dioxygen in the presence of pyridine and 3-A
molecular
sieves [101]. Oxidative cleavage of aliphatic and cyclic 1,2-diols with O
2
furnishes
aldehydes and dialdehydes, respectively, using Ru(PPh
3
)
3
Cl
2
on active carbon [102].
The oxidation of 1,2-octanediol with dioxygen catalyzed by NHPI combined with
Co(acac)
3
afforded heptanoic acid in 70% yield at 80% conversion (Table 6.2) [92].
A precursor to heptanoic acid is an a-ketol, since 1-hydroxy-2-octanone is obtained as
a principal product at the limited stage of the reaction (Scheme 6.7). An independent
oxidation of the a-ketol leads to the carboxylic acid in good yield. Woodward and
coworkers have applied the NHPI-catalyzed oxidation to the carbon-carbon bond
cleavage of diols to carboxylic acids [103].
Unlike 1,2-diols, internal vicinal diols such as 2,3-octanediol are selectively
oxidized to diketones such as 2,3-octanedione rather than cleaved to carboxylic
acids. The conversion of vicinal diols to diketones is usually performed by oxidation
with metal oxidants such as AgCO
3
[104] and permanganate [105], by a TEMPO/
NaOCl system under electrochemical conditions [106], or by a catalytic method
using heteropolyoxometalates and H
2
O
2
[107]. Interestingly, 1,3- and 1,4-diols are
Figure 6.5 Aerobic oxidation of alcohols by NHPI/Co/MCBA system.
206
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
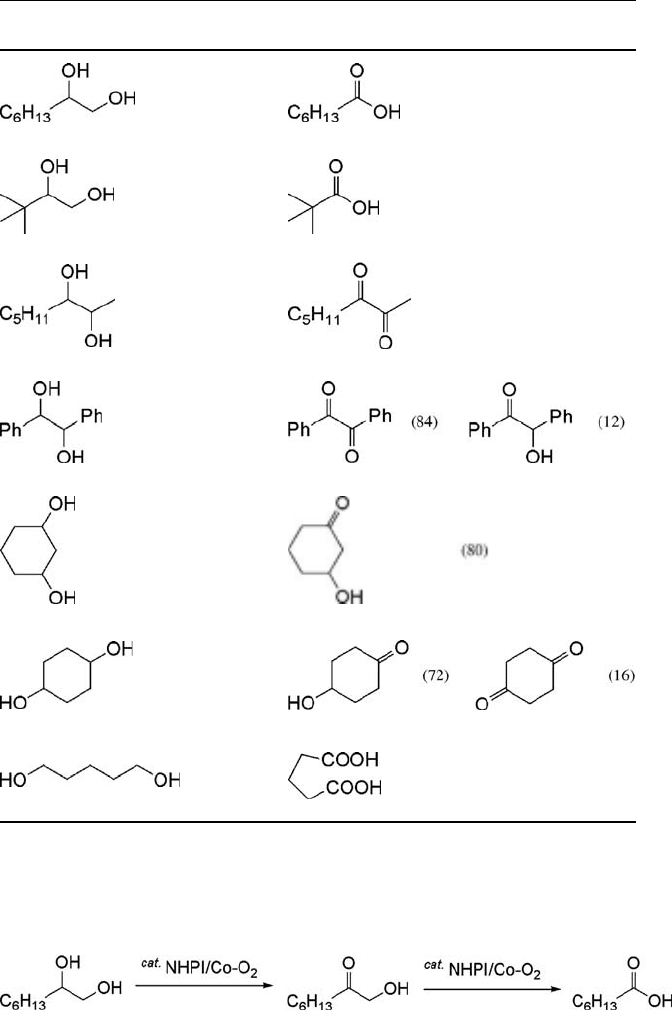
Table 6.2 Oxidation of various diols with dioxygen
a)
.
Diol Conv. (%) Products (Yield/%)
80 (70)
89 (71)
96 (86)
97
80
88
80 (66)
a) Diols (3 mmol) were allowed to react with molecular oxygen (1 atm) in the presence of NHPI
(10 mol%) and Co(acac)
3
(1 mol%) in CH
3
CN (5 mL).
Scheme 6.7 Oxidation of 1,2-octanediol to heptanoic acid.
6.2 NHPI-Catalyzed Aerobic Oxidation
j
207
selectively converted into the corresponding hydroxy ketones rather than to the
diketones. An a,v-diol such as 1,5-pentanediol gives rise to the dicarboxylic acid in
good yield. The present reaction provides an alternative and useful route to dicar-
boxylic acids from diols with dioxygen.
6.2.5
Epoxidation of Alkenes using Dioxygen as Terminal Oxidant
The epoxidation of alkenes using dioxygen via a catalytic process is a challenging
subject in the field of oxidation chemistry. Much effort has been devoted to the
epoxidation of alkenes with dioxygen using transition metals as catalysts
[5d,5e,6a,108–115]. For instance, b-diketonate complexes of Ni, V, and Fe are reported
to catalyze efficiently the epoxidation of alkenes with dioxygen in the presence of an
aldehyde, alcohol, or acetal as a reducing agent under mild conditions [109j]. On the
other hand, Ru-porphyrin complexes [113] and Ru-substituted polyoxometalates,
{[WZnRu
2
(OH)(H
2
O)](ZnW
9
O
34
)
2
}
11
[114], catalyze the epoxidation of alkenes
without any reducing agents.
The hexafluoroacetone (HFA)-catalyzed epoxidation of alkenes utilizing H
2
O
2
obtained in situ by the NHPI-catalyzed aerobic oxidation of alcohols was examined
(Scheme 6.8). A hydroperoxide derived from HFA and H
2
O
2
has been reported to
epoxidize various alkenes in fair to good yields [116, 117]. This epoxidation system
seems to be an interesting industrial strategy, for it does not require the storage and
transportation of explosive H
2
O
2
[118]. In addition, the resulting ketones can be easily
reduced to the original alcohols. 2-Octene was allowed to react under O
2
(1 atm) in the
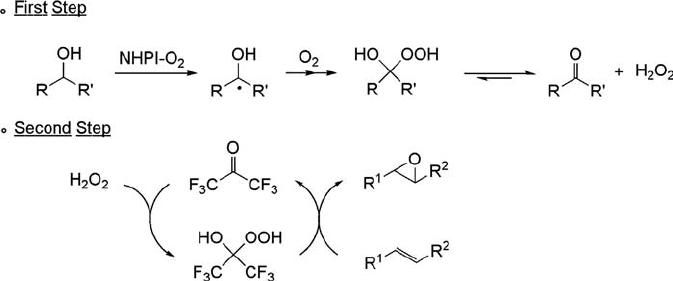
presence of 1-phenylethanol under the influence of catalytic amounts of NHPI
(10 mol%) and HFA (10 mol%) in benzonitrile at 80
C for 24 h, giving 2,3-epox-
yoctane in 93% selectivity based on 93% conversion (Eq. (6.20)) [119]. This is the first
successful epoxidation with H
2
O
2
generated in situ from alcohols and O
2
without
any metal catalysts. The important feature of this reaction is that the epoxidation of
Scheme 6.8 A new strategy for the epoxidation of alkenes.
208
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
cis- and trans-2-octenes proceeded in a stereospecific manner to form cis- and trans-
2,3-epoxyoctanes respectively, in high yields., although O
2
is used as a terminal
oxidant.
ð6:20Þ
6.2.6
Baeyer-Villiger Oxidation of KA Oil
KA oil, a mixture of cyclohexanone and cyclohexanol obtained by the aerobic
oxidation of cyclohexane, is an important intermediate in petroleum industrial
chemistry for the production of adipic acid and e-caprolactam, which are key
materials for manufacturing 6,6-nylon and 6-nylon, respectively [120]. Baeyer-Villiger
oxidation is a frequently used synthetic tool for conversion of cycloalkanones to
lactones. Usually, this transformation is carried out by the use of peracids like
peracetic acid and mCPBA [121], hydrogen peroxide [122], and bis(trimethylsilyl)
peroxide [123]. However, the catalytic Baeyer-Villiger oxidation using dioxygen is
limited to the in situ generation of peracids using excess aldehydes and O
2
[124].
In industry, e-caprolactone is manufactured by the reaction of cyclohexanone with
peracetic acid generated by the aerobic oxidation of acetaldehyde [120]. From both a
synthetic and an industrial point of view, it is very convenient that the KA oil can be
used as the starting material for the production of e-caprolactone with molecular
oxygen via a catalytic process.
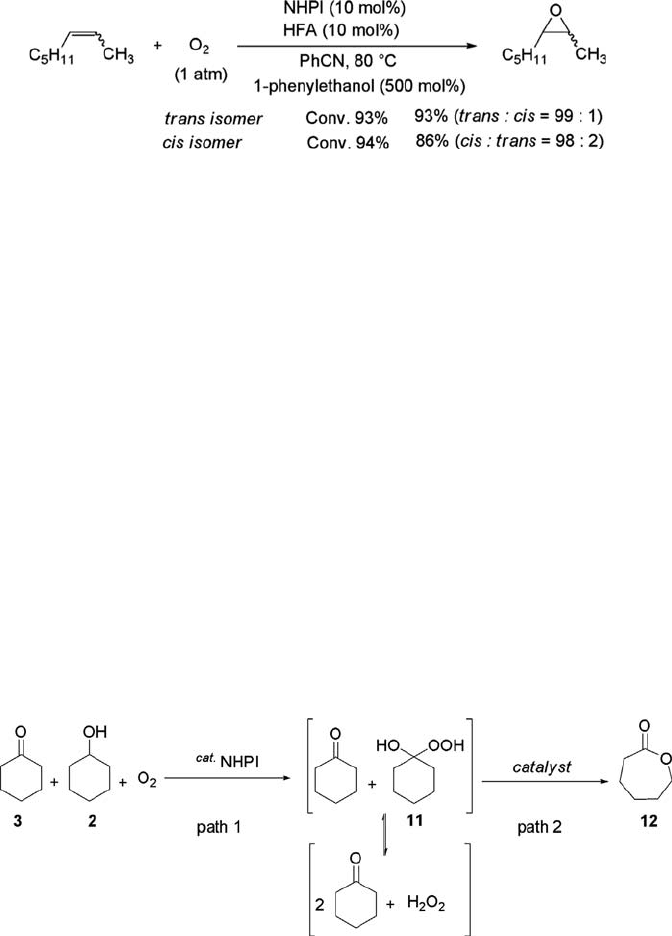
A new strategy for e-caprolactone synthesis is outlined in Scheme 6.9. The aerobic
oxidation of cyclohexanol (3) catalyzed by NHPI gives a mixture of cyclohexanone (2)
Scheme 6.9 A new strategy for the Baeyer-Villiger oxidation of KA-oil.
6.2 NHPI-Catalyzed Aerobic Oxidation
j
209

and hydrogen peroxide through the formation of 1-hydroxy-1-hydroperoxycyclohex-
ane (11) (path 1). Treatment of the resulting reaction mixture with an appropriate
catalyst would produce e-caprolactone (12) (path 2). A KA oil consisting of a 1 : 1
mixture of 3 and 2 was employed as a model starting material. If the aerobic oxidation
of the KA oil in the presence of NHPI is completed, 2 equiv. of 3 and 1 equiv. of H
2
O
2
are expected to be formed. Treatment of a 1 : 1 mixture of 3 (6 mmol) and 2 (6 mmol)
by catalytic amounts of NHPI (0.6 mmol) and 2,2
0
-azobisisobutyronitrile (AIBN)
(0.3 mmol) under an O
2
atmosphere in CH
3
CN at 75
C for 15 h, followed by InCl
3
(0.45 mmol) at 25
C for 6 h affords 12 in 57% selectivity based on the KA oil reacted,
and 77% of KA oil was recovered (Eq. (6.21)). Water-stable Lewis acids such as
Sc(OTf)
3
and Gd(OTf)
3
afford e -caprolactone in somewhat lower yields [125].
ð6:21Þ
6.2.7
Preparation of e-Caprolactam Precursor from KA Oil
e-Caprolactam (CL) is a very important monomer for the production of nylon-6, and
about 4.2 million tons of CL were manufactured worldwide in 1998 [126]. Most
current methods of CL production involve the conversion of cyclohexanone with
hydroxylamine sulfate into cyclohexanone oxime followed by Beckmann rearrange-
ment by the action of oleum and then treatment with ammonia, giving CL. A serious
drawback of this process is the co-production of a large amount of ammonium sulfate
waste [126, 127]. Raja and Thomas reported a method for one-step production of
cyclohexanone oxime and CL by the reaction of cyclohexanone with ammonia under
high-pressure air (34.5 atm) in the presence of a bifunctional molecular sieve
catalyst [128]. Hydrogen peroxide oxidation of cyclohexanone in the presence of
NH
3
catalyzed by titanium silicate is reported to produce CL [129]. In patent work, on
the other hand, the transformation of 1,1
0
-peroxydicyclohexylamine (PDHA) to a 1 : 1
mixture of CL and cyclohexanone by LiBr has been reported [130].
It is interesting to develop a novel route to the CL precursor, PDHA, which was
hitherto prepared by hydrogen peroxide oxidation of cyclohexanone (3) followed by
treatment with ammonia [126, 130]. Because of the ease of transformation of PDHA
to a 1 : 1 mixture of CL and 3 under the influence of an appropriate catalyst such as
lithium halides, the CL production via PDHA is considered to be a superior candidate
for a next-generation waste-free process for CL. The NHPI-catalyzed aerobic oxida-
tion of KA oil was applied to the synthesis of PDHA without formation of
any ammonium sulfate waste. The strategy is outlined in Scheme 6.10. The
NHPI-catalyzed oxidation of KA oil (a mixture of 3 and 2) with O
2
produces 1,1
0
-
dihydroxydicyclohexyl peroxide, which seems to exist in equilibrium with cyclohex-
anone and H
2
O
2
(path 1). Subsequent treatment of the resulting reaction mixture
210
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
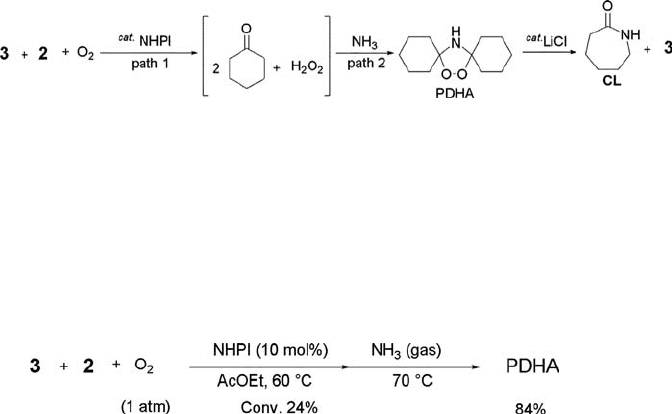
with NH
3
would afford PDHA (path 2). A 1 : 2 mixture of 3 and 2 was reacted under
dioxygen atmosphere (1 atm) in the presence of small amounts of NHPI and AIBN
in ethyl acetate at 60
C for 20 h, followed by the reaction with an ammonia at
atmospheric pressure at 70
C for 2 h to give 84% of PDHA at 24% conversion of
KA oil (Eq. (6.22)). This route provides a more economical and environmentally
friendly process than that by the current method using hydroxylamine sulfate.
ð6:22Þ
6.3
Functionalization of Alkanes Catalyzed by NHPI
6.3.1
Carboxylation of Alkanes with CO and O
2
Carbonylation and carboxylation of alkanes with carbon monoxide (CO) are chal-
lenging transformations in organic synthesis [131]. There have been several impor-
tant discoveries including the Rh-catalyzed photocarbonylation of alkanes by
Tanaka [132, 133] and the carboxylation of methane with CO/O
2
using Pd/Cu [134]
or RhCl
3
[135] catalysts by Fujiwara and Sen et al. The carbonylation of adamantanes
under the influence of Lewis acid and superacids has also been reported [136, 137].
Following the first report on the free-radical-mediated carbonylation by Coffmann
et al. in 1952 [138], this type of reaction was not investigated for a long time, probably
because it has to be conducted under extremely high CO pressure (200–300 atm)
[139]. In 1990, Ryu performed a successful free-radical carbonylation of alkyl halides
with CO mediated by tributyltin hydride [131e,140]. Sen et al. disclosed a free-radical
carboxylation of methane to acetic acid by the use of peroxydisulfate as a radical
source [141]. Benzophenone- [142] and polyoxotungstate-photocatalyzed [143] as
well as mercury-photosensitized [144] carbonylations of cyclohexane afford cyclo-
hexanecarbaldehyde. The trapping of alkyl radicals generated from alkanes under
the influence of NHPI catalyst by CO followed by O
2
leads to carboxylic acids
(Scheme 6.11) [145]. The carboxylation of adamantane under CO/air (15/1 atm) in the
presence of NHPI (10 mol%) in a mixed solvent of acetic acid and 1,2-dichloroethane
at 95
C for 4 h affords 1-adamantanecarboxylic acid, 1,3-adamantanedicarboxylic
Scheme 6.10 A new strategy for the synthesis of e-caprolactam precursor, PDHA.
6.3 Functionalization of Alkanes Catalyzed by NHPI
j
211
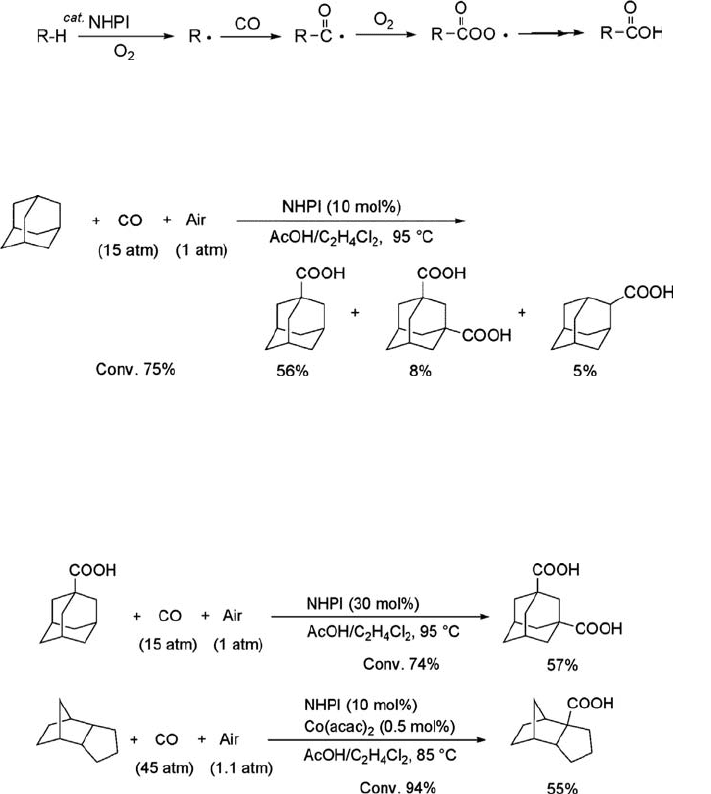
acid, 2-adamantanecarboxylic acid, and several oxygenated products such as
1-adamantanol and 2-adamantanone (Eq. (6.23)).
ð6:23Þ
The present strategy was successfully applied to the preparation of adamantane-
dicarboxylic acid, which is an interesting monomer in polymer chemistry, through a
stepwise procedure (Eq. (6.24)), although the dicarboxylic acid is difficult to obtain by
conventional methods. Similarly, 1,3-dimethyladamantane and endo-tricyclo[5.2.1.0]
decane were carboxylated to the respective mono- and dicarboxylic acids.
ð6:24Þ
6.3.2
First Catalytic Nitration of Alkanes using NO
2
Nitration of lower alkanes such as methane and ethane with nitric acid or nitrogen
dioxide is industrially practiced to produce nitroalkanes [146, 147]. However, a major
problem in current industrial nitration is that the reaction must be run at fairly
high temperature (250–400
C) because of the difficulty of obtaining CH bond
homolysis by NO
2
[146]. Under such high temperatures, higher alkanes undergo not
only homolysis of the CH bonds but also cleavage of the CC skeleton. Hence, the
Scheme 6.11 Carbonylation of alkanes with CO and O
2
catalyzed by NHPI.
212
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
