Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

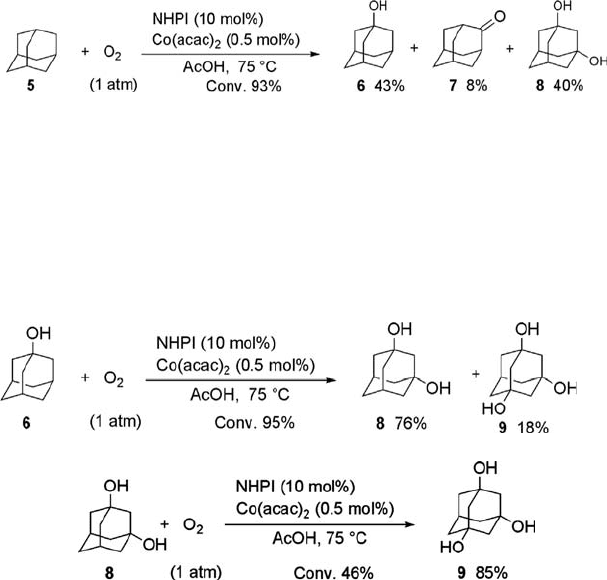
There have been a few reports on the catalytic hydroxylation of adamantane with
dioxygen in the presence of aldehydes [12]. Mizuno et al. reported that the aerobic
oxidation of adamantane by the PW
9
-Fe
2
Ni heteropolyanion without any reducing
agents gives 1-adamantanol and 2-adamantanone at 29% conversion [19a]. The
NHPI-catalyzed aerobic oxidation of adamantane is considerably accelerated by
adding a small amount of a Co salt [28, 40, 41]. Thus, the oxidation of adamantane
(5) in the presence of NHPI (10 mol%) and Co(acac)
2
(0.5 mol%) in acetic acid under
dioxygen (1 atm) for 6 h produced 1-adamantanol (6) (43%), 1,3-adamantanediol (8)
(40%), and 2-adamantanone (7) (8%) (Eq. (6.4)). The reactivity of the tertiary CH
bond relative to the secondary CH bond in the oxidation by NHPI/Co(II) was 31.1.
This value is considerably higher than that attained by the conventional autoxidation
(3.8–5.4). The preferential oxidation of the tertiary CH bond over the secondary
bond may be attributed to the electron-deficient character of PINO which is a key
radical species in the NHPI-catalyzed oxidation (see below).
ð6:4Þ
It is important that the oxidation led to diol 8 in high selectivity, because 8 is rarely
produced by conventional oxidation. Hirobe obtained 8 in 25% yield by the oxidation
of 5 using a Ru complex with 2,6-dichloropyridine N-oxide as the oxidant [42]. In the
stepwise hydroxylation of 5 by the NHPI/Co(acac)
2
system, the diol 8 and the triol 9
were obtained in high selectivity (Eqs. (6.5) and (6.6)). These alcohols are now
manufactured as important components of photoresistant polymer materials on an
industrial scale by Daicel Chemical Industry Ltd..
ð6:5Þ
ð6:6Þ
6.2.2
Oxidation of Alkylarenes
Aerobic oxidation of alkylbenzenes is a promising subject in industrial chemistry.
Many bulk chemicals such as terephthalic acid, phenol, benzoic acid, and so on are
6.2 NHPI-Catalyzed Aerobic Oxidation
j
193

manufactured by homogeneous liqu id-phase oxidations with O
2
[2, 43]. The largest-
scale liquid-phase oxidation is the conversion of p-xylene to terephthalic acid, which
is chiefl y used as polyethylene te rephthalate polymer raw material [2a]. m-Xylene is
also commercially oxidized to isophthalic acid. Benzoic acid derived from the
oxidation of toluene is an important raw material in the production of various
pharmaceuticals and pestic ides. Commercially important cumene hydroperoxide
and ethylbenzene hydroperoxide are also manufactured by the aerobic oxidation of
isopropylbenzene and ethylbenzene, respectively [2a ,5a]. These oxidation process-
es are usually operated at higher temperatures and pressures of air. A great deal of
effort has been made t o develop the homogeneous oxidations of alkylbenzenes with
better selectivity u nder milder conditions. The fi rst successfu l oxidation of a v ariety
of alkylbenzenes with O
2
by the use of NHPI as the catalyst under very mild
conditions is achieved.
Currently, the oxidation of toluene is commercially practiced in the presence of
a catalytic amount of cobalt(II) 2-ethylhexanoate under a pressure of 10 atm of air at
140–190
C [44]. The oxidation of toluene under normal pressure of dioxygen at
room temperatu re is achieved by the use of a combined catalyst of NHPI and a
Co(II) sp ecies. The fact that the toluene was oxidized with dioxyge n through the
catalytic process in high yield under ambient conditions is very important from
ecological and technical viewpoints as a promising strategy in oxidation chemistry.
As a typical example, the oxidation of toluene in the presence of NHPI (10 mol%)
and Co(OAc)
2
(0.5 mol%) in acetic acid under an atmosphere of O
2
at 25
C for 20 h
afforded benzoic acid and benzaldehyde in 81 and 3% yields, respectively
(Eq. (6.7)) [45]. This finding suggests that a n efficient cleavage of a CHbond,
having the bond dissociation energy (BDE) of 88 kcal mol
1
(corresponding to the
BDE of toluene), is possible at room temperature by the use of NH PI catalyst.
However, when Co(III) was employed in place of Co(II), n o reaction took place at a ll
at room temperature.
ð6:7Þ
Representative results for the NHPI-catalyzed aerobic oxidation of various alkyl-
benzenes in the presence of Co(OAc)
2
in acetic acid under ambient conditions are
listed in Table 6.1. Both p- and o-xylenes are selectively oxidized to p- and o-toluic acids
without the formation of dicarboxylic acids. o-Ethyltoluene undergoes selective
oxidation to form a mixture of the corresponding alcohol and ketone in which
the ethyl moiety was selectively functionalized. It is of interest to examine the
effect of substituents on the aromatic ring in the oxidation of substituted
toluenes. p-Methoxytoluene is more rapidly oxidized than the toluene itself, while
194
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
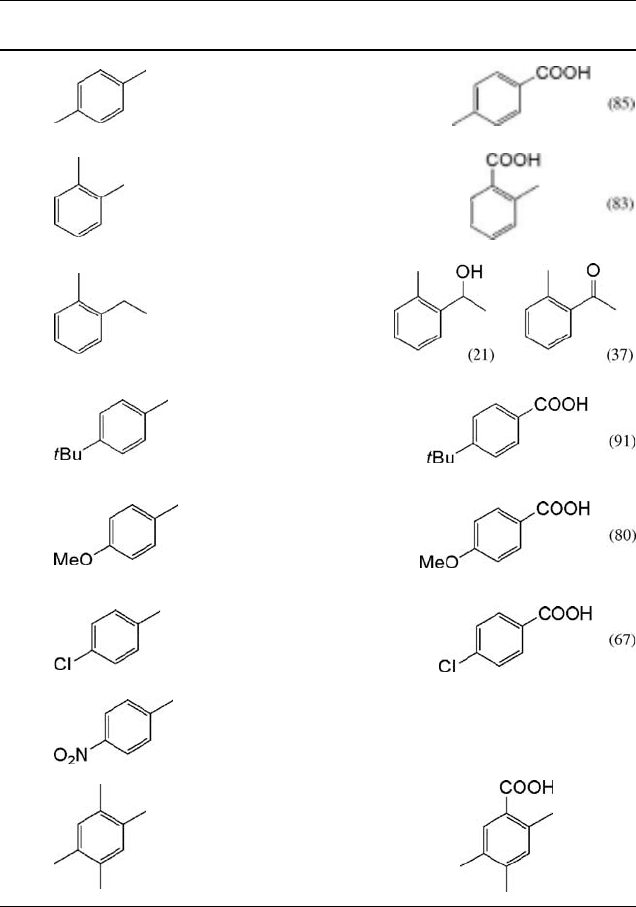
Table 6.1 Aerobic oxidation of various alkylbenzenes at room temperature
a)
.
Run Substrate Time (h) Conv. (%) Products (Yield (%))
1
20 95
2 20 93
3
b)
20 82
4 20 95
5
b)
689
6 20 71
7 20 No reaction
8 12 >99 (93)
a) Substrates (3 mmol) were allowed to react in the presence of NHPI (10 mol%) and Co(OAc)
2
(0.5 mol%) in AcOH (5 mL ) under dioxygen (1 atm) at 25
C.
b) CH
3
CN was used as the solvent.
6.2 NHPI-Catalyzed Aerobic Oxidation
j
195
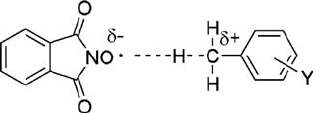
p-chlorotoluene is oxidized at a relatively slow rate. An electron-donating substituent
anchoring to toluene stabilizes the partial positive charge on the benzylic carbon
atom in the transition state for the abstraction of a benzylic hydrogen atom by PINO,
possessing an electrophilic character (Scheme 6.2) (see below) [46]. Therefore, the
oxidation of toluenes having electron-donating groups by the NHPI catalyst is
facilitated. Indeed, p-nitrotoluene substituted by a strongly electron-withdrawing
nitro group, is not oxidized at all under these conditions. Recently, various substi-
tuted NHPI derivatives were prepared and studied by Nolte et al. in the aerobic
oxidation of ethylbenzene [47]. It was found that NHPI, with an electron-withdrawing
fluorine substituent, increases the oxidation rate, while NHPI substituted by a
methoxy group decreases the rate.
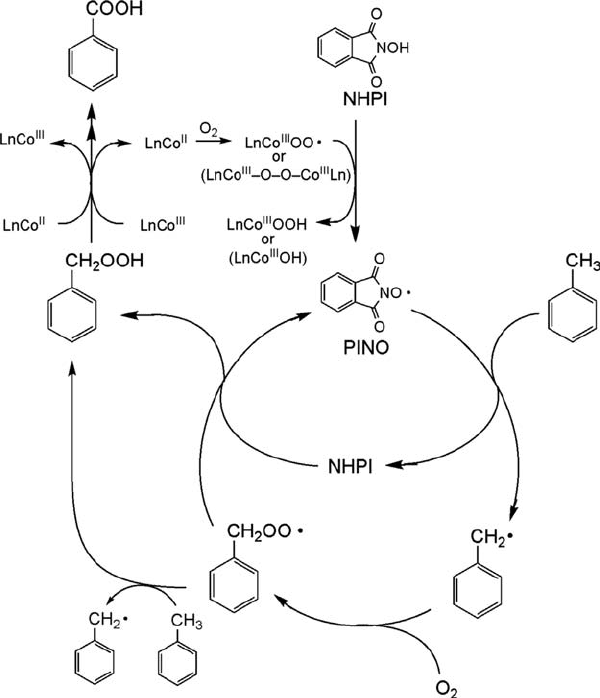
A plausible reaction pathway for the aerobic ox idation of alkanes cat alyzed by
NHPI and Co(II) is illu strated in Scheme 6.3. A labile dioxygen complex such as
superoxocobalt(III) or peroxocobalt(III) complexes is known to be formed by the
complexation of Co(II) with O
2
.Thein situ generation of PINO from NHPI by the
action of the cobalt(III)-oxygen complex formed is a key step in the present
oxidation. The next step involves the hydrogen atom abstraction from alkanes by
PINO to form alkyl radicals. Trapping the resulting alkyl radicals by dioxygen
provides peroxy radicals, which are eventually converted into oxygenated products
through alkyl hydroperoxides. In fact, on exposing NHPI in benzonitrile contain-
ing a small amount of Co(OAc)
2
to dioxygen at 80
C, an ESR signal attribu ted to
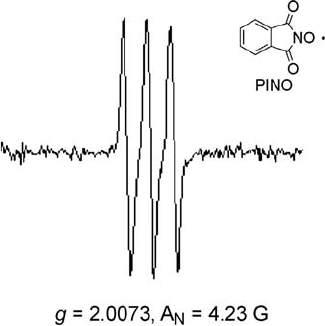
PINO as a triplet signal having hyperfine spl itting (hfs) by the nitrogen atom
(g ¼ 2.0074, A
N
¼ 4.3 G) is observed (Figure 6.3). T he g-value and hyperfine
splitting constants observed here are consistent with those (g ¼ 2.0073, A
N
¼ 4.23
G) of PINO report ed previously [48]. In addition, PINO is observed during the
oxidation of toluene by the N HPI/Co(II) system under ambient conditions [45].
Quite recently, Minisci, Pedulli, and coworkers found by means of ESR spectros-
copy that the BDE value of the OH bond for NHPI is >86 kcal mol
1
.This
suggests that PINO could abstract the benzylic hydrogen atom of t oluene, whose
BDE is 88 kcal mol
1
[49].
6.2.2.1 Synthesis of Terephthalic Acid
Terephthalic acid (TPA) as well as dimethyl terephthalate (DMT) have recently
become very important as raw material for polyethylene terephthalate [50]. In
1999, about 17 10
6
tonnes of TPA were manufactured worldwide, and its
Scheme 6.2 Transition state for the reaction of PINO with substituted benzenes.
196
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
production has been estimated to have been increasing at a minimum growth rate
of 10% annually by the year 2002. Until the 1980s, the following fou r-step process
developed by Witten and modified by Hercules and Dynamit-Nobel (Witten-
Hercules process) h ad been mainly operated to produc e DMT [2a, 50]. The first
step is the conversion of p-xylene (PX) to p-toluic acid (PTA). It then passes to an
esterification step to form methyl p-toluate, which is subjected to further oxidation
to monometh yl terephthalate, followed by esterification to DMT. From the 1990s,
these processes we re changed to the aerobic one-stage oxidation of PX to TPA by the
combineduseofcobaltandmanganesesaltsinthepresenceofbromideasa
promoter in acetic acid at 175–225
Cunder15– 30 atm of air, followed b y hydro-
genation of the crude TPA to remove 4-carboxybenzaldehyde (4-CBA) by a Pd
Scheme 6.3 A plausible reaction path for the aerobic oxidation of toluene catalyzed by NHPI
combined with Co(II).
6.2 NHPI-Catalyzed Aerobic Oxidation
j
197
catalyst [50–52]. This process was developed by Scientific Design and Amoco Ltd.
(Amoco process). Currently, about 70% of TPA produced worldwide is based on the
Amoco process, and almost all of the new plants adopt this method. However, there
are several disadvantages in th e Amoco process: (i) significant combustion of acetic
acid used as the s olvent to form CO and CO
2
, (ii) use of the highly corrosive bromid e
ion, which calls for the use of vessels lined with expensive metals like t itanium, and
(iii) contamination of 4-CBA in crude TP A, wh ich necessitates elaborate hydro-
genation and recrystallization procedures in manufacturing the purified TPA
required for PET. Therefore, a new oxidation system for the production of TPA
is desired to ove rcome t hese disadvanta ges. Partenheimer recen tly published a
review devoted to the aerobic oxidation of alkylbenzenes, espe cially PX, using the
Co/Mn/Br syste m [52].
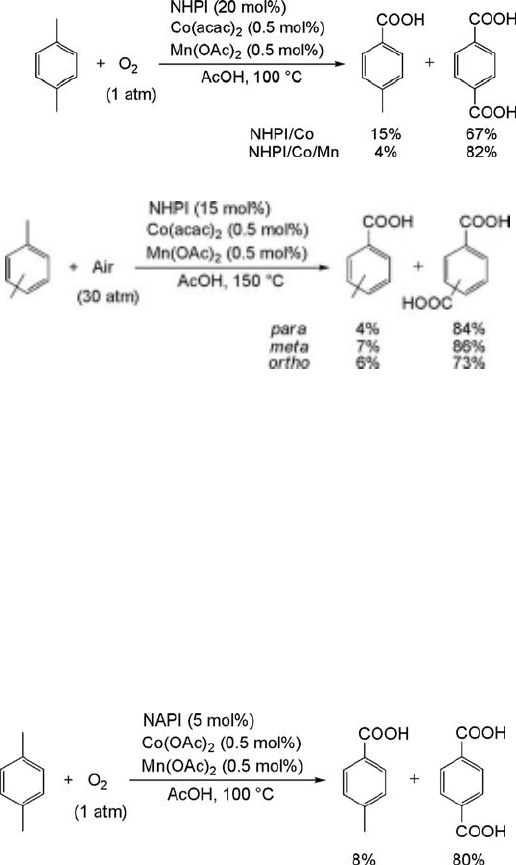
The aerobic oxidation of PX to TPA was examined by the NHPI catalyst to develop
a halogen-free catalytic system [53]. The oxidation of PX with dioxygen (1 atm) in the
presence of catalytic amounts of NHPI (20 mol%) and Co(OAc)
2
(0.5 mol%) in
acetic acid at 100
C for 14 h produced TPA in 67% yield and PTA (15%) together
with small amounts of 4-CBA, 4-carboxybenzyl alcohol, 1,4-diacetoxymethylben-
zene, and 4-acetoxymethylbenzoic acid, as well as several unidentified compounds
in 1–2% yields, respectively, at over 99% conversion (Eq. (6.8)). The yield of TPA is
improved to 82% when Mn(OAc)
2
(0.5 mol%) is added to the NHPI/Co(OAc)
2
system. The synergistic effect of Co and Mn salts in the aerobic oxidation of
alkylbenzenes has been well documented [52, 54, 55]. From a practical point of view,
it is important that the aerobic oxidation of PX under air (30 kg cm
2
) by the
NHPI/Co/Mn system is completed within 3 h at 150
C to form TPA in 84% yield
(Eq. (6.9)). Both o- and m-xylenes were also successfully converted into the
corresponding dicarboxylic acids, isophthalic acid and phthalic acid, respectively,
in high yields.
Figure 6.3 ESR spectrum of PINO.
198
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
ð6:8Þ
ð6:9Þ
As shown in Eq. (6.8), about 20 mol% of NHPI must be used to obtain TPA in
satisfactory yield (over 80%), because NHPI gradually decomposes to inert phtha-
limide and phthalic anhydride during the oxidation. If the NHPI used could be
reduced by a simple modification, the present oxidation would be more desirable.
Efforts to reduce the amount of the NHPI led to the discovery of an efficient catalyst,
N-acetoxyphthalimide (NAPI), which can be easily prepared by the reaction of NHPI
with acetic anhydride. Surprisingly, PX was oxidized to TPA in high yield (80%) even
by the use of 5 mol% of NAPI, Co(OAc)
2
(0.5 mol%) and Mn(OAc)
2
(0.5 mol%)
(Eq. (6.10)). The effect of NAPI is considered to be to resist the rapid decomposition
to phthalimide or phthalic anhydride at the early stage of the reaction where violent
chain reactions take place, since NAPI is gradually hydrolyzed to NHPI by water
present in acetic acid as well as by the water resulting during the oxidation.
ð6:10Þ
6.2.2.2 Oxidation of Methylpyridines and Methylquinolines
Pyridinecarboxylic acids are useful and important intermediates in pharmaceutical
syntheses. Although the synthesis of these carboxylic acids by the aerobic oxidation
of alkylpyridines is straightforward, the oxidation is usually difficult to carry out
selectively owing to their low reactivities [52, 56]. Pyridinecarboxylic acids are readily
prepared by the oxidation of alkylpyridines with nitric acid or by the hydrolysis of
pyridinecarboxamides derived from pyridinecarbonitrile [57]. According to the
6.2 NHPI-Catalyzed Aerobic Oxidation
j
199
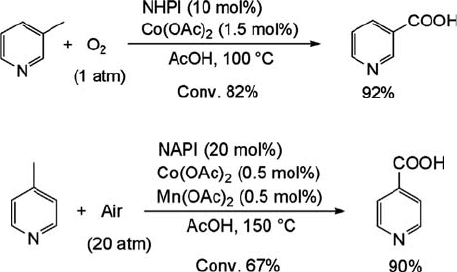
recent literature, nicotinic acid is obtained in about 50% yield at 52% conversion by
the oxidation of b-picoline in the presence of Co(OAc)
2
and Mn(OAc)
2
using LiCl as
a promoter under air (16 atm) at 170
C [58]. The aerobic oxidation of b-picoline to
nicotinic acid catalyzed by NHPI has been examined [59]. Nicotinic acid is used as a
precursor of vitamin B
3
and is commercially manufactured on a large scale by nitric
acid oxidation of 5-ethyl-2-methylpyridine [58a]. The oxidation of b-picoline in the
presence of NHPI (10 mol%) and Co(OAc)
2
(1.5 mol%) under dioxygen (1 atm) at
100
C for 15 h in acetic acid affords nicotinic acid in 76% yield at 82% conversion
(Eq. (6.11a)) [59]. This is the first successful oxidation of picolines with O
2
under mild
conditions. In contrast to the oxidation of b-picoline by the NHPI/Co/Mn system
where nicotinic acid was formed in good yield, c-picoline is oxidized with some
difficulty under these conditions to form 4-pyridinecarboxylic acid in low yield (22%).
After optimization of the reaction conditions, 4-pyridinecarboxylic acid was obtained
by the use of NHPI (20 mol%), Co(OAc)
2
(1 mol%) and Mn(OAc)
2
(1 mol%) in 60%
yield at 67% conversion (Eq. (6.11b)) [59].
ð6:11aÞ
ð6:11bÞ
Quinolines and their derivatives are common in natural products, and have
attractive applications as pharmaceuticals and agrochemicals [60]. For example,
3-quinolinecarboxylic acid derivatives are reported to be potent inhibitors of bacterial
DNA gyrase. So far, there have been only limited methods for the preparation
of quinolinecarboxylic acids despite their potential importance [61]. The synthesis of
quinolinecarboxylic acids from the corresponding methylquinolines by direct oxi-
dation seems to be the simplest method, but the reaction has been difficult to carry
out selectively because of low reactivity of the methyl group bearing the quinoline
ring. Classically, the oxidation was examined by the use of a stoichiometric amount of
a metal oxidant like KMnO
4
[62], CrO
3
[62], or nickel peroxide [63], or by Pd-catalyzed
oxidation with H
2
O
2
[64].
Treatment of 3-methylquinoline (10) by the NHPI-Co-Mn system under the
reaction condition used for b-picoline, however, results in the recovery of the
starting 10. This is believed to be because the activation of O
2
by the Co(II) becomes
difficult, probably because of coordination of 10 to Co(OAc)
2
. As described below, the
nitration of alkanes with NO
2
is enhanced in the presence of NHPI catalyst, in which
200
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
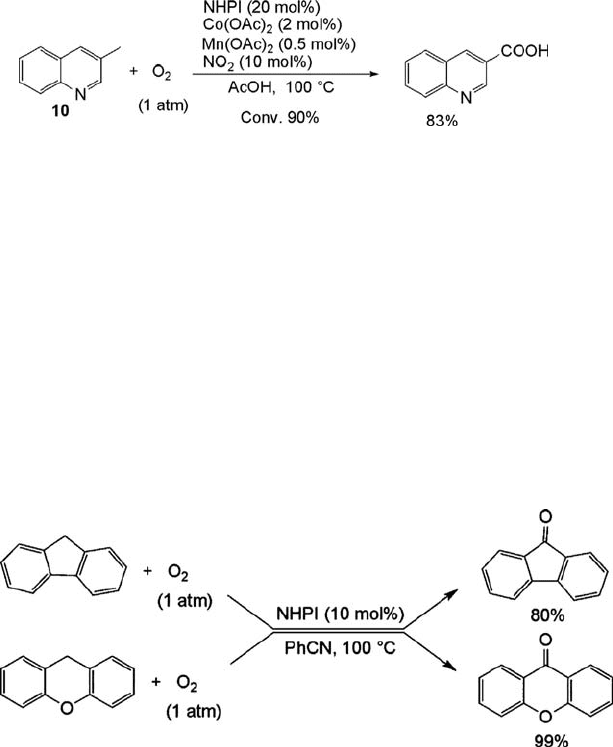
the generation of PINO from NHPI is easily achieved by NO
2
without any transition
metal. Hence, the oxidation of 10 by adding a small amount of NO
2
produced
3-quinolinecarboxylic acid. For instance, the oxidation of 10 with O
2
(1 atm) catalyzed
by NHPI (20 mol%), Co(OAc)
2
(2 mol%) and Mn(OAc)
2
(0.1 mol%) in the presence of
NO
2
(10 mol%) gave 3-quinolinecarboxylic acid in 75% yield at 90% conversion
(Eq. (6.12)). Other methylquinolines are also successfully oxidized under reaction
conditions similar to those used for 10 [65].
ð6:12Þ
6.2.2.3 Oxidation of Hydroaromatic and Benzylic Compounds
Various hydroaromatic and benzylic compounds can be oxidized under a normal
pressure of dioxygen catalyzed by NHPI even in the absence of a transition metal
species, giving the corresponding oxygenated compounds in good yields. For
example, treatment of fluorene with dioxygen in the presence of a catalytic amount
of NHPI in benzonitrile at 100
C for 20 h affords fluorenone in 80% yield. Similarly,
xanthene produced xanthone in excellent yield (Eq. (6.13)) [8, 66]. After our finding
of NHPI catalysis in the aerobic oxidation, Einhorn et al. reported the oxidation of
these substrates with O
2
at room temperature in the presence of NHPI and
acetaldehyde, and they concluded that the active species is the PINO formed by the
reaction of NHPI with an acetylperoxy radical (Scheme 6.4) [67]. They prepared chiral
N-hydroxyimides and used them as the catalyst in the asymmetric oxidation of
indanes to give indanones in 8% ee (Figure 6.4) [68].
ð6:13Þ
Hydroperoxides are used not only as oxidizing agents of alkenes but also as
important precursors for the synthesis of phenols. For instance, a-hydroperoxyethyl-
benzene, obtained by aerobic oxidation of ethylbenzene, is used as an active oxygen
6.2 NHPI-Catalyzed Aerobic Oxidation
j
201
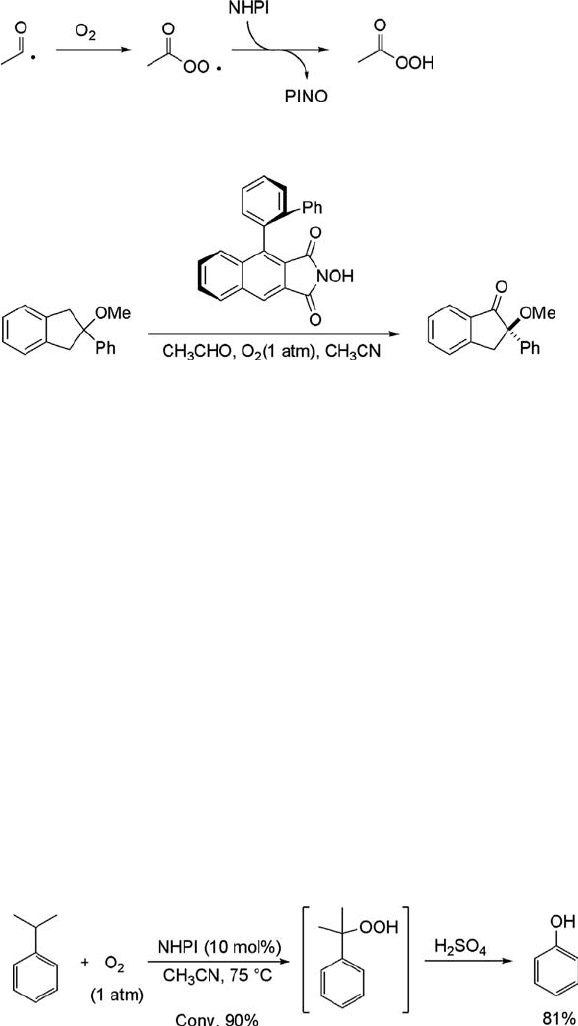
carrier in the epoxidation of propylene, which is known as the Halcon process [69].
The cumene-phenol process (Hock Process) based on the decomposition of cumene
hydroperoxide with sulfuric acid to phenol and acetone is the current method for
phenol synthesis used worldwide [70]. An efficient approach to phenols through the
formation of hydroperoxides from alkylbenzenes is successfully achieved by aerobic
oxidation using NHPI as a catalyst. The oxidation of several alkylbenzenes with
dioxygen by NHPI followed by treatment with an acid affords phenols in good yields.
For example, the aerobic oxidation of cumene in the presence of a catalytic amount
of NHPI at 75
C and subsequent treatment with H
2
SO
4
leads to phenol in 81%
selectivity at 90% conversion (Eq. (6.14)) [71]. Hydroquinone (61%) and 4-isopro-
pylphenol (33%) are obtained from 1,4-diisopropylbenzene. More recently, Sheldon
et al. have reported the highly selective oxidation of cyclohexylbenzene to cyclohex-
ylbenzene-1-hydroperoxide (CHBH). The aerobic oxidation of cyclohexylbenzene in
the presence of NHPI (0.5 mol%) and some CHBH (2 mol%) as an initiator without
solvent affords the desired CHBH (98% selectivity) at 32% conversion [72]. They
considered that this oxidation provides an overall co-product-free route to phenol
production. The acid-catalyzed decomposition of the CHBH would give a mixture of
phenol and cyclohexanone, which is subsequently dehydrogenated with an appro-
priate catalyst to form phenol (Scheme 6.5).
ð6:14Þ
A one-pot synthesis of phenol and cyclohexane from cyclohexylbenzene was later
achieved by using NHPI catalyst followed by treatment with sulfuric acid, which
Scheme 6.4 Formation of PINO by reaction of NHPI with acetylperoxy radical.
Figure 6.4 Oxidation with chiral N-hydroxyimides prepared by Einhorn et al.
202
j
6 Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
