ASM Metals HandBook Vol. 8 - Mechanical Testing and Evaluation
Подождите немного. Документ загружается.


226. G. Venkataraman, T.S. Sriram, M.E. Fine, and Y.W. Chung, STM and Surface Analytical Study
of the Effect of Environment on Fatigue Crack Initiation in Silver Single Crystals I, Surface Chemical
Effects, Scr. Metall., Vol 24, 1990, p 273–278
227. P.G. Marsh, S.E. Harvey, M.D. Kriese, and W.W. Gerberich, Environmental Effects on Fatigue
Crack Development in HSLA Steel and Fine Grain αα-Titanium, Scr. Metall., Vol 33, 1995, p 825–829
228. M. Goken, H. Vehoff, and P. Neumann, J. Vac. Sci. Technol. B, Vol 14, 1996, p 1157
Fracture Resistance Testing of Plastics
Kevin M. Kit and Paul J. Phillips, University of Tennessee, Knoxville
Introduction
POLYMERIC MATERIALS are many and varied, ranging from pure glasses to blends to semicrystalline
solids. Their mechanical properties range from pure elasticity with very high strains to fracture (rubbers or
elastomers) to almost pure Hookian elasticity with low strains to fracture (glasses); the majority of polymers
have properties somewhere between these two extremes. Virtually all polymeric materials show some form of
inelastic behavior (Ref 1, 2). The elastomers show hysteresis, and the glasses show some form of yielding. The
inelastic behavior is not restricted to the tip of a crack, but is present in some form or another throughout the
material. The inelasticity is a direct result of the time dependence of the motions of the polymer chains. With
the exceptions of certain untoughened epoxy resins and related thermosets, inelasticity is the norm. Hence, the
expectation of many theories of fracture mechanics that Hookian behavior can be assumed is not to be realized.
Even theories that assume elastic-plastic criteria are inadequate because they assume plastic behavior at the
crack tip and elastic behavior throughout the remainder of the specimen, whereas in the real materials, there is
viscoelastic deformation of some form or other occurring in the bulk of the specimen.
The presence of inelasticity in the entire specimen, as well as at the crack tip, results in additional energy being
required for crack propagation. Hence, in any mechanical test the energy measured to propagate a crack
consists of the surface energy of the crack, energy of plastic deformation at the crack tip, and energy of inelastic
deformation of the entire specimen (Ref 3). Because the latter two forms of energy absorption are a direct result
of the time-dependent behavior of the polymer chains, the energy absorbed displays a strong dependence on the
rate at which stress is applied. The crack opening displacements in polymeric materials can be quite large and,
hence, the microstrain at a crack tip will be similarly large. In polymeric materials displaying minimal levels of
plasticity and/or inelasticity, such as untoughened epoxies, the crack opening displacement is quite small. At
the other extreme is the elastomer, or rubber, where the crack opening displacement is so large that the process
is usually referred to as tearing. The crack opening displacement can reflect two extremes in deformation
behavior: shear yielding or crazing (Ref 3). Both reflect large amounts of plastic deformation at the crack tip. In
the case of some polymers, for example, polycarbonate, a large yield zone is observed. In others, the
phenomenon is referred to as crazing, where the apparent crack is really a zone of fibrous material produced by
the stress field ahead of the crack. This phenomenon can be present in glassy materials as well as
semicrystalline materials, and it corresponds to microyielding to levels of several hundred percent strain. A
similar phenomenon can also be observed in unnotched specimens where regions in the bulk of the specimen
display what is usually described as stress whitening.
In addition to the behavior described above, polymers are also sensitive to the environment, both gaseous and
liquid (Ref 3). An example of the effects of gaseous environments is the effect of atmospheric ozone on crack
propagation rates in natural rubber (Ref 4). In the case of a liquid the behavior can be caused by several
different effects (Ref 5, 6). First, there is always the possibility that the liquid may be a solvent and be absorbed
by the polymer; the absorption process may occur more rapidly at the tip of a crack. In this case the liquid will

plasticize the polymer, lowering its glass transition temperature and thereby altering all of its fundamental
properties. Second, the liquid may react chemically with the polymer, changing its fundamental structure and
properties on a microscopic or macroscopic scale. Third, the liquid may simply wet the polymer, lowering the
surface energy and making crack or craze propagation much easier. A well-known example of such behavior is
the effect of carbon tetrachloride on polycarbonate.
References cited in this section
1. N.G. McCrum, B.E. Read, and G. Williams, Anelastic and Dielectric Effects in Polymeric Solids,
Wiley, 1967
2. I.M. Ward, Mechanical Properties of Solid Polymers, Wiley, 1983, p 15
3. E.H. Andrews, Cracking and Crazing in Polymeric Glasses, The Physics of Glassy Polymers, R.N.
Haward, Ed., Wiley, 1973, p 394
4. R. Natarajan and P.E. Reed, J. Polym. Sci. A, Polym. Chem., Vol 2 (No. 10), 1972, p 585
5. G.A. Bernier and R.P. Kambour, Macromolecules, Vol 1, 1968, p 393
6. E.H. Andrews, G.M. Levy and J. Willis, J. Mater. Sci., Vol 8, 1973, p 1000
Fracture Resistance Testing of Plastics
Kevin M. Kit and Paul J. Phillips, University of Tennessee, Knoxville
Historical Development
Fracture in polymers was first studied intensively for rubber, and tests were developed logistically in the early
1900s (Ref 7, 8). Standard test methods included tensile testing with “dog-bone” specimens where the breaking
strength was obtained. By the 1920s, standard tests for tear strength, using “trouser-type” specimens, were in
use. Such methods are still in common use, the tensile test to failure using a dog-bone specimen being one of
the most popular for the characterization of all kinds of polymers (Ref 8). As new polymers are developed and
testing is needed, tensile testing on sheets or thin films as a method of characterization still tends to be preferred
over the standardized ASTM tests for fracture strength. This may occur sometimes due to the amount of
specimen available and at other times due to the simplicity of specimen preparation and characterization.
Fracture testing using standardized linear fracture mechanics approaches, such as K
Ic
/G
Ic
methods, has been
used for decades as a means of carrying out fracture testing (Ref 3, 9). However, because of the previously
mentioned inelasticity problems, polymers have stress distributions at the tip of a crack that cannot be
calculated or described adequately by the assumptions of classical elasticity theory. Such approaches clearly
cannot describe adequately the behavior of even the most well-behaved systems. Early attempts at describing
the fracture phenomenon in a more realistic manner recognized that the most important parameter describing
the phenomenon was the energy absorbed by the fracture process (Ref 10). The energy balance approach was
suggested very early by Griffith (Ref 11) but was used for rubber by Rivlin and Thomas (Ref 12) who used a
to describe the total work needed to create a unit area of surface (or the tearing energy in the case of rubber).
Attempts at applying this approach were made successfully by Andrews and coworkers (Ref 3), Kambour (Ref
13), and Berry (Ref 14). The beginning of a generalized theory of fracture mechanics, not requiring linear
fracture assumptions, was developed by Andrews (Ref 15). Study of fracture then concentrated for several
years on the development and understanding of the mechanisms of craze formation because, clearly, the
formation of crazes ahead of the crack is the major contributor to the energy absorbed in fracture in most
polymers (Ref 15). Indeed, because crazing is the precursor to fracture itself, it justifies attention on that ground
alone.
A concurrent development, which now is used in the testing of polymers, is the J-integral method; it is
essentially the equivalent of G
I
for a nonlinear system. Discovered by Rice (Ref 16, 17), and developed
independently by Begley and Landes (Ref 18, 19), the J-integral method has been applied successfully to
polymers by the Williams group and others (Ref 20, 21, and 22). The disadvantage of the method is that it
requires multiple specimens in its strict form, discouraging widespread use. A single specimen method was
developed and used successfully on polypropylene by Ouederni and Phillips (Ref 23), but it has not yet been
converted into a standard ASTM method.
References cited in this section
3. E.H. Andrews, Cracking and Crazing in Polymeric Glasses, The Physics of Glassy Polymers, R.N.
Haward, Ed., Wiley, 1973, p 394
7. L.E. Weber, The Chemistry of Rubber Manufacture, Griffin, London, 1926, p 336
8. K. Memmler, The Science of Rubber, R.F. Dunbrook and V.N. Morris, Ed., Reinhold, 1934, p 523
9. G.R. Irwin, in Encyclopaedia of Physics, Vol 6, Springer Verlag, 1958
10. N.G. McCrum, C.P. Buckley, and C.B. Bucknall, Principles of Polymer Engineering, Oxford University
Press, 1997, p 201
11. A.A. Griffith, Phil. Trans. R. Soc. (London) A, Vol 221, 1921, p 163
12. R.S. Rivlin and A.G. Thomas, J. Polym. Sci., Vol 10, 1953, p 291
13. R.P. Kambour, Appl. Polym. Symp., Vol 7, John Wiley & Sons, 1968, p 215
14. J.P. Berry, J. Polym. Sci. A, Polym. Chem., Vol 2, 1964, p 4069
15. E.H. Andrews, J. Mater. Sci., Vol 9, 1974, p 887
16. J.R. Rice, J. Appl. Mech. (Trans. ASME), Vol 35, 1968, p 379
17. J.R. Rice, Fracture, Vol 2, 1968, p 191
18. J.A. Begley and J.D. Landes, in Fracture Toughness, ASTM STP 514, 1972, p 1
19. J.D. Landes and J.E. Begley, in Fracture Toughness, ASTM STP 514, 1972, p 24
20. J.M. Hodgkinson and J.G. Williams, J. Mater. Sci., Vol 16, 1981, p 50
21. S. Hashemi and J.D. Williams, Polym. Eng. Sci., Vol 26, 1986, p 760
22. Y.W. Mai and P. Powell, J. Polym. Sci. B, Polym. Phys., Vol 29, 1991, p 785
23. M. Ouederni and P.J. Phillips, J. Polym. Sci. B., Polym. Phys., Vol 33, 1995, p 1313

Fracture Resistance Testing of Plastics
Kevin M. Kit and Paul J. Phillips, University of Tennessee, Knoxville
Fracture Test Methods for Polymers
Several methods have been developed specifically for determining the fracture toughness of polymeric
materials. ASTM D 5045 (Ref 24) describes a method for determining the linear elastic fracture toughness (K
Ic
and G
Ic
) of polymers. This methodology is appropriate for highly crosslinked thermosets (e.g., epoxy) or glassy
thermoplastics incapable of significant plastic deformation (e.g., polystyrene). ASTM D 6068 (Ref 25)
describes a method for measuring J-R curves (a measure of elastic-plastic fracture toughness) for polymer
specimens that are not large enough to experience conditions of plane strain during loading. However, methods
originally developed to characterize the elastic-plastic fracture of ductile metallic materials are most commonly
used (with slight modifications) to characterize ductile polymers. These methods are based on the concept of
the J-integral to determine plane strain fracture toughness values. To date, the most commonly used method is
that of ASTM E 813 (Ref 26). This method was discontinued in 1989 and replaced by ASTM E 1737 (Ref 27).
The differences between the two are minor, but the methods for data analysis and reporting described in ASTM
E 1737 should now be followed.
J-Integral Testing
ASTM E 1737 is more general than ASTM E 813 and describes the method for determining either J
Ic
or J
c
under plane stress conditions. J
Ic
is the critical value of the J-integral at which onset of stable crack growth
occurs. If stable crack growth is not observed, then J
c
is defined as the value of the J-integral at which unstable
crack growth (i.e., failure) occurs. The J-integral is a measure of the amount of energy absorbed (due to both
elastic and plastic responses) during the growth of a crack through the material of interest.
Experimentally, J is determined as a function of crack extension, Δa, in a notched specimen loaded in tension. J
is calculated according to (Ref 28):
(Eq 1)
where U is the area under the load-displacement curve and B and b are the dimensions of the specimen in the
plane of the crack. Testing is most commonly performed on single-edge notched bend or on compact tension
specimens containing machined notches (see Fig. 2 in the article “Fracture Toughness Testing” in this Volume).
ASTM E 1737 specifies that the specimen be fatigued so that a sharp “precrack” is formed at the base of the
notch. However, this is not a viable technique for most thermoplastic polymers. The accepted method for
creating a precrack in polymer samples is to tap a fresh, unused razor blade into the notch immediately
preceding the test, as specified in ASTM D 6068 and D 5045.
To ensure the existence of plane strain conditions at the crack tip, specimen thickness, B, and the original
uncracked ligament, b
o
(i.e., the distance the crack would have to extend to separate the specimen into two
pieces), must be greater than 25J
Ic
/σ
y
where J
Ic
is the elastic-plastic fracture toughness and σ
y
is the yield
strength. Because J
Ic
is generally not known a priori, specimen dimensions must be based on an estimated value
of J
Ic
and then verified after testing. It has been shown (Ref 29) that the specimen size requirements specified
by ASTM E 1737 can be relaxed for some polymers, such as low-density polyethylene and a polypropylene
copolymer, to B, b
o
> 17 J
Ic
/σ
y
.
In order to arrive at a value of J
Ic
, J-integral values are plotted as a function of crack extension, Δa, to form a
so-called R-curve. This data may be collected using single specimen or multiple specimen techniques. The
multiple specimen technique is widely accepted as a valid measure of the elastic-plastic fracture toughness of
polymers and is commonly employed. However, results from the much simpler single specimen technique have
also been shown to be valid, and the implementation of this technique is increasing. These techniques differ
only in the determination of the R-curve; specimen requirements and data analysis to determine J
Ic
are identical.
Both are summarized in the following sections.

Multiple Specimen Technique. In both techniques, it is desirable to determine J at a minimum of ten equally
spaced Δa points. In the multiple specimen technique, each J-Δa point on the R-curve is generated with a
different specimen. Each specimen is loaded to a level judged to produce a desired, stable crack growth
extension, Δa, and is then unloaded. Polymer specimens are then removed from the test frame and fractured in
liquid nitrogen. (This last step deviates from ASTM E 1737, which specifies that the specimens be fatigued
first.) The precrack, stable crack growth and freeze-fracture regions of the fracture surface are usually easily
identifiable (Ref 25), and an optical microscope is used to measure Δa (the length of the stable crack growth
region) at nine points equally spaced across the thickness of the specimen. These nine values are averaged as
described by ASTM E 1737. J is then calculated according to:
J = J
cl
+ J
pl
(Eq 2)
where J
el
and J
pl
are the elastic and plastic components of J, calculated as:
(Eq 3)
(Eq 4)
K is a function of maximum load and specimen geometry, ν is Poisson's ratio, E is Young's modulus, A
pl
is the
area under the load-displacement curve for the entire loading-unloading cycle, and B
N
is specimen thickness.
For single-edge notch and compact tension specimens, η = 2, while for the disk-shape compact tension
specimen, η is a function of geometry. Equations for K for each specimen type are given in Annex 4 of ASTM
E 1737.
Single Specimen Technique. The single specimen technique relies on the ability to determine the extent of
crack growth, Δa, while the specimen is loaded in the test frame. If this can be done, then many J-Δa data pairs
can be collected from one specimen. Crack growth is usually determined by an elastic compliance method or by
an electrical resistance method. In the elastic compliance method, the specimen is unloaded periodically during
the test. At each unloading point, Δa is calculated as a function of the slope of the unload line, Young's
modulus, and specimen geometry. However, due to the viscoelastic behavior of polymers, accurate
determination of crack lengths by this method is suspect (Ref 30, 31).
Another method determines the crack length by measuring the voltage drop across the uncracked ligament
through which a constant direct current is passed. This method is also not generally applicable to polymers
because most are poor conductors. However, Ouederni and Phillips (Ref 23) have developed a method that
involves measuring crack extension directly with a video camera. A thin copper grid deposited on the surface of
the specimen serves as a scale reference. Another J-integral technique that has been successfully applied to
polymers is the normalization method (Ref 31). This method does not require specimen unloading or in situ
measurements of crack growth. The crack length is calculated by separating total displacement into elastic and
plastic components, each of which is a function of crack length. After a fitting procedure is used to establish a
relationship between plastic displacement and crack length, the actual crack length can be calculated at any
point on the load-displacement curve. Zhou et al. (Ref 31) used this technique to determine J
Ic
for two rubber-
toughened nylons and found their results very close to values obtained by the standard multiple specimen
method.
Determination of J
Ic
. Before the data can be analyzed, it must be checked to verify that it spans a sufficiently
large range of Δa. This procedure to determine qualifying data is detailed in ASTM E 1737. Qualifying J data
must also be less than the smaller of b
o
σ
y
/20 and Bσ
y
/20 to ensure that all data points are measured under plane
strain conditions. Qualified data are fit by the method of least squares to the curve described by:
(Eq 5)
where C
1
and C
2
are fitting parameters and k = 1 mm (0.04 in.). A linear blunting line must also be constructed
along the line defined by:
J = 2σ
y
Δa
(Eq 6)
where σ
y
is the average of the 0.2% offset yield strength and the ultimate tensile strength. The blunting line
accounts for deflection that occurs due to plastic deformation near the crack tip prior to the onset of stable crack
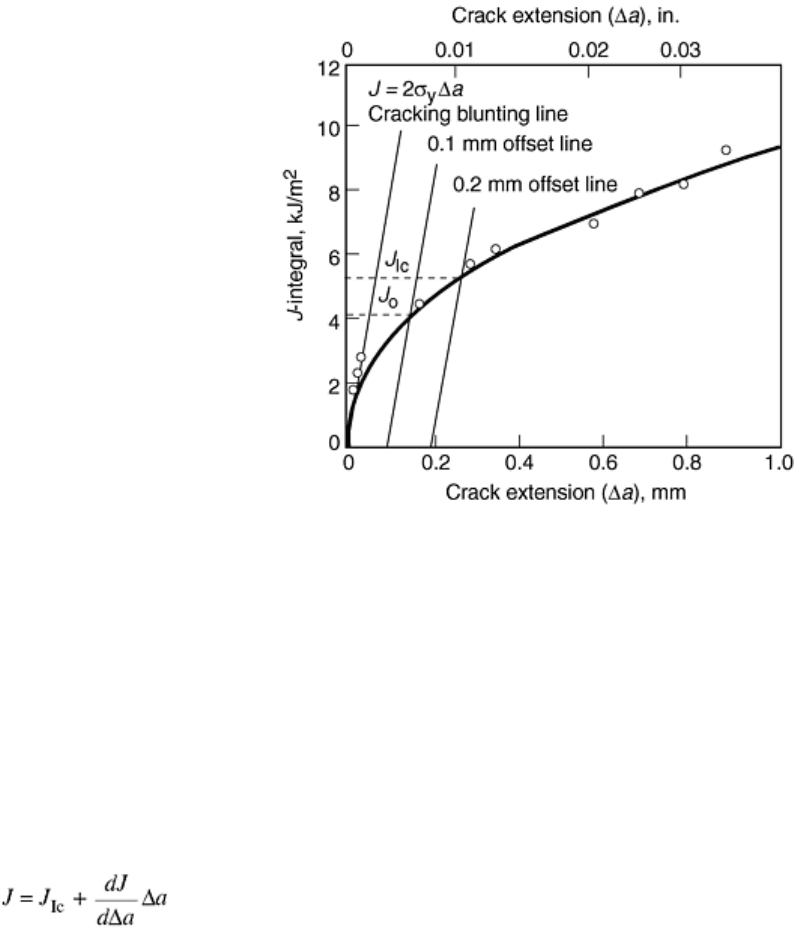
growth. ASTM E 1737 specifies that the J value at the intersection of the fit data and a line offset 0.2 mm
(0.008 in.) from the blunting line defines an interim value, J
Q
, which is used to verify the existence of plane
strain conditions. If both B and b
o
are indeed greater than 25J
Ic
/σ
y
, and some additional data qualifications are
met, then the value of J
Q
is taken to be equal to J
Ic
. Experimental and fit R-curves for an acrylonitrile-
butadiene-styrene (ABS) copolymer are shown in Fig. 1 along with the blunting and 0.2 mm offset lines. The
intersection of the fit R-curve and the 0.2 mm offset line indicates a J
Ic
of 5.31 kJ/m
2
.
Fig. 1 Experimental R-curve for an ABS copolymer showing power-law fit, blunting line, and 0.2 mm
offset line. Source: Ref 32
Modifications for Polymeric Materials. Due to the unique properties of polymers, several modifications to the
J-integral method have been proposed and used. Some of these modifications that affect the collection of J-Δa
data have already been mentioned, and these are quite widely accepted as standard.
In some cases, crack tip blunting may not occur before or during stable crack growth in polymers. Crack tip
blunting can be verified by direct microscopic observation or if J data follows the blunting line (J = 2σ
y
Δa) for
small amounts of crack growth. Some of the data in Fig. 1 lie on the blunting line, indicating that blunting does
occur (Ref 32). If blunting is not known to occur, J
Ic
should be determined by extrapolating a linear fit to the J-
Δa data to zero crack growth (Δa = 0). It has been argued in Ref 33 that J-Δa data should, under conditions of
plane strain, follow:
(Eq 7)
For small crack growth, J should vary linearly with Δa, and the value of J
Ic
should be determined as previously
explained. Optical microscopy (Ref 34, 35) has shown that crack blunting does not occur in certain grades of
high-density polyethylene, toughened nylon 6/6, ABS, and toughened polycarbonate. As further evidence, the J
data collected from the high-density polyethylene (Ref 35) does not follow the blunting line for small Δa, as
shown in Fig. 2.
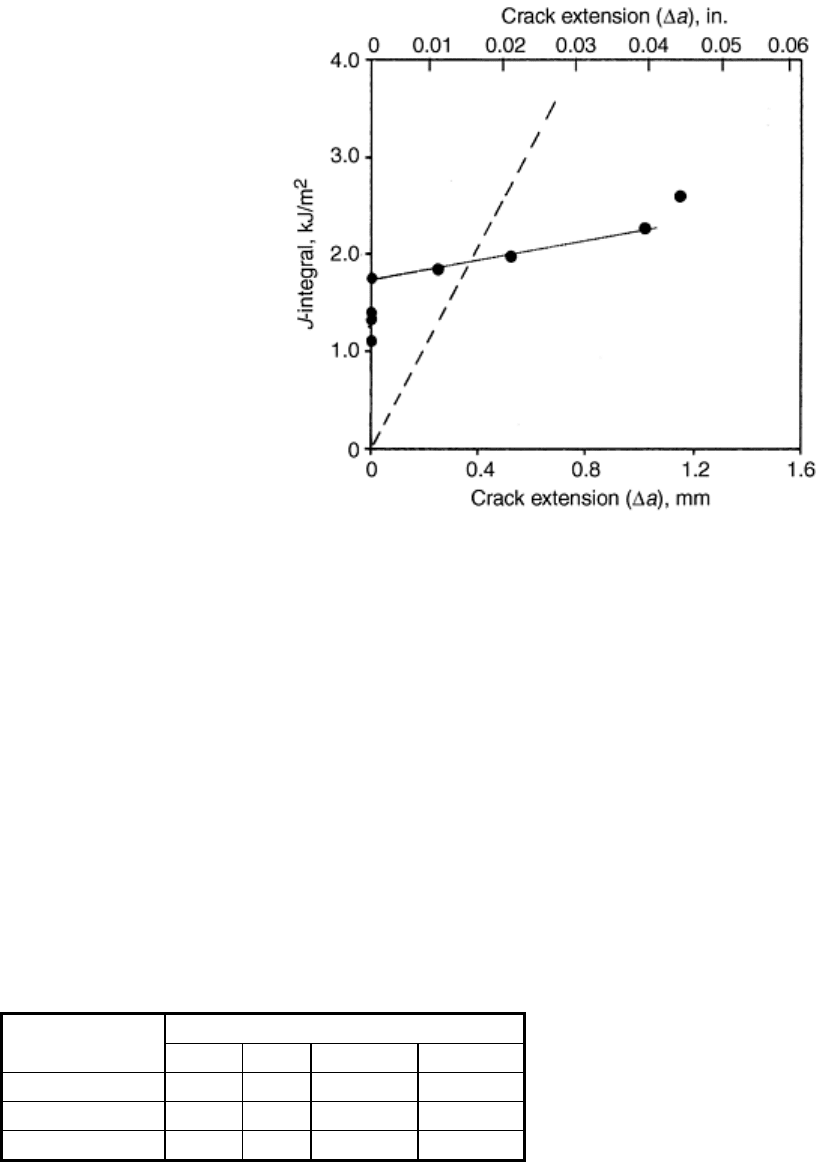
Fig. 2 Experimental R-curve for a high-density polyethylene showing the dashed blunting line and the
absence of blunting behavior. Source: Ref 35
If crack tip blunting does occur, the procedure described will yield conservative values of J
Ic
. If blunting is
known to occur, then J
Ic
should be determined by the methods of ASTM E 1737 or ASTM E 813. The
determination of J
Ic
by ASTM E 813 differs in that J
Ic
is taken at the intersection of a linearly fit R-curve and
the blunting line. This construction is shown in Fig. 3 for the same data used in Fig. 1. The intersection of the
linear R fit and the blunting line indicates a J
Ic
of 3.95 kJ/m
2
(compare to the ASTM E 1737 value of 5.31
kJ/m
2
). The method in ASTM E 813 usually gives more conservative values than that in ASTM E 1737. Chang
et al. (Ref 29, 31, 35, and 36) have analyzed J data of high-impact polystyrene (HIPS), ABS, a polycarbonate
(PC)/ABS blend, and a polycarbonate/polybutylene terephthalate (PBT) blend by three methods (ASTM E
1737, ASTM E 813, and the no-blunting method described previously). As can be seen in Table 1, the no-
blunting method is the most conservative, while ASTM E 1737 is the least conservative. If no direct evidence
of crack tip blunting exists, the most conservative method for calculating J
Ic
should be used.
Table 1 Comparison of J
Ic
data for several polymers determined by different methods
J
Ic
, kJ/m
2
Method
HIPS
ABS
PC/ABS
PC/PBT
No blunting 3.24 3.57 3.00 5.47
ASTM E 813 3.60 3.95 3.55 7.17
ASTM E 1737
4.30 5.31 7.85 13.41
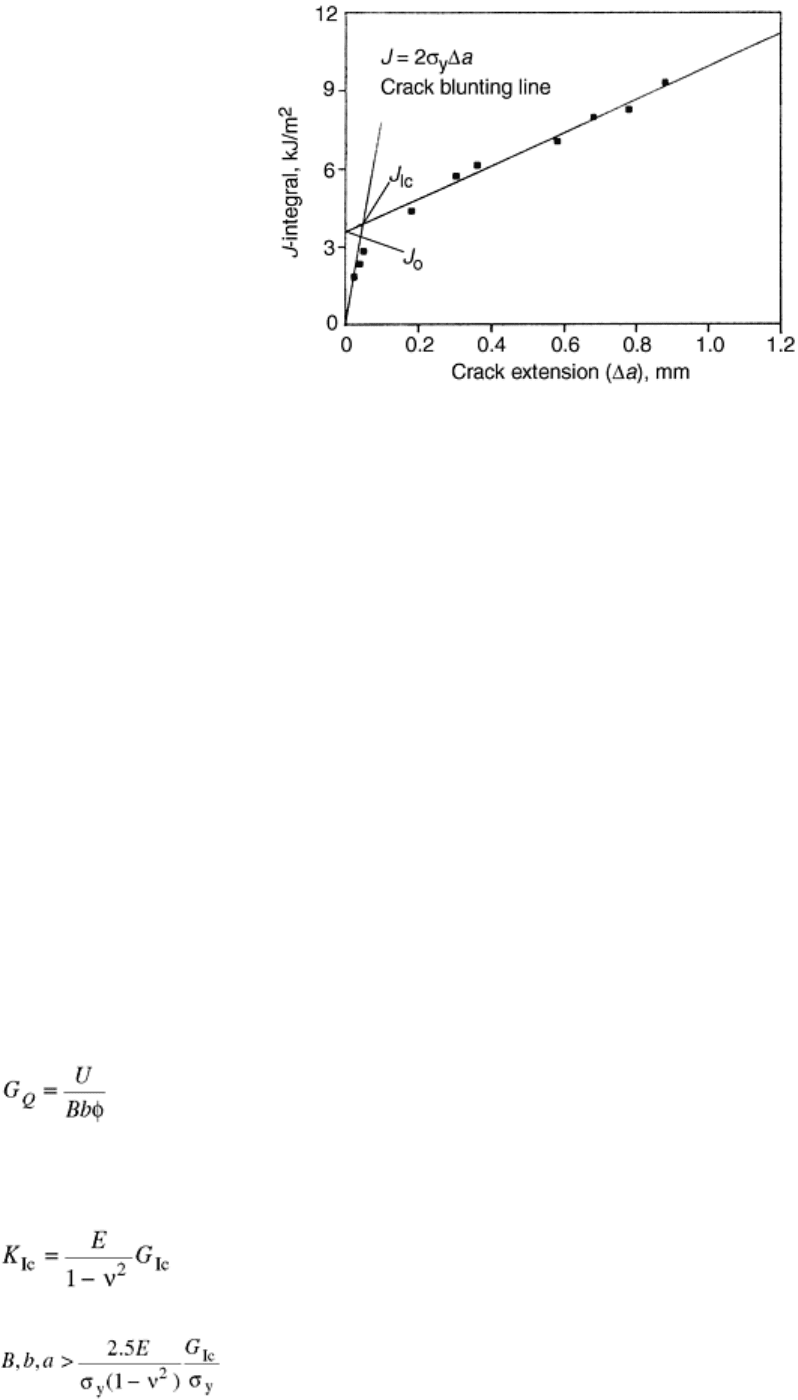
Fig. 3 Experimental R-curve for an ABS copolymer showing linear fit and blunting line. Source: Ref 32
Several workers have shown that the plane strain thickness requirements specified by ASTM E 813 and ASTM
E 1737 are too conservative in certain cases, while not conservative enough in others. Rimnac et al. (Ref 38)
and Huang (Ref 39) have shown that the requirement is too conservative for tough thermoplastics, ultrahigh-
molecular-weight polyethylene (J
Ic
= 95 kJ/m
2
), and rubber-toughened nylon 6/6 (J
Ic
= 30 kJ/m
2
). Both studies
found that size-independent values of J
Ic
were obtained for specimen thicknesses greater than 6J
Ic
/σ
y
, which is
approximately 25% of the recommended minimum thickness. Conversely, Lu et al. (Ref 40) found that size-
independent values of J
Ic
for a relatively brittle PC/ABS blend (J
Ic
= 4 kJ/m
2
) were not obtained until the
thickness was greater than 64J
Ic
/σ
y
, which is more than twice the recommended minimum thickness. In light of
these results, it is recommended that J
Ic
be determined for various thicknesses to ensure that the true plane
strain value is obtained.
Linear Elastic Fracture Toughness
Other methods also exist to determine the plane strain fracture toughness of polymers. ASTM D 5045 specifies
a procedure for determining the critical strain energy release rate, G
Ic
, of polymers. This parameter is equivalent
to J
Ic
for materials that exhibit linear (or nearly linear) elastic behavior (Ref 41). ASTM D 5045 specifies the
use of single-edge notch bend or compact tension specimens. Precracks are created by tapping a fresh, unused
razor blade into the machined notch immediately preceding the test. The samples are then loaded to a level that
causes a 2.5% apparent crack extension. However, significant deviation from linear elastic behavior must not
occur at this load level. The procedure for testing this requirement is detailed in ASTM D 5045. An interim
value of the critical strain energy release rate, G
Q
is determined by:
(Eq 8)
where φ is a function of b and the original crack length, a. This interim value can be qualified as the plane
strain critical strain energy release rate if plane strain conditions are verified. The standard specifies that B, b,
and a must be greater than 2.5 (K
Ic
/σ
y
)
2
where K
Ic
is the plane strain fracture toughness and is related to G
Ic
by:
(Eq 9)
Using this relation, the size requirement for plane strain conditions can be written as:
(Eq 10)
Using typical values for E (1 GPa, or 145 ksi), σ
y
(60 MPa, or 8.7 ksi), and ν (0.4), the size requirement is B, b,
a > 50G
Ic
/σ
y
, which is twice the size requirement for determining plane strain J
Ic
.
Due to the viscoelastic properties of polymers, test temperature and strain rate should be well controlled and
reported. The standard recommends 23 °C (73 °F) and a crosshead speed of 10 mm/min (0.4 in./min). The
orientation of the specimen with respect to processing direction (e.g., extrusion direction and mold flow
direction) should also be reported because of the strong dependence of mechanical properties on molecular
orientation that often develops during processing.
Testing of Thin Sheets and Films
In order to ensure the existence of plane strain state, the dimensions of the sample normal to the applied stress
are usually required to be greater than 25J
Ic
/σ
y
, where J
Ic
is the elastic-plastic fracture toughness and σ
y
is the
yield strength. Both J
Ic
and σ
y
are generally considerably lower than the corresponding values for metallic
materials, but the ratio J
Ic
/σ
y
is usually much larger for polymeric materials. Therefore, the plane strain size
requirements for polymeric fracture specimens are often unrealistic (on the order of 5 cm or 2 in.). In many
applications, the properties of polymeric materials are strongly dependent on the level of molecular orientation
and crystallinity. These levels, in turn, are strongly dependent on the thermal and mechanical histories
experienced during processing. Specimens that are produced to fulfill the plane strain condition are likely to
have quite different thermal and mechanical histories than polymer materials processed into sheet or film.
Therefore, the thicker test specimens do not reflect the actual properties of the polymer for the intended
application. For these reasons, ASTM D 6068 is often a more desirable method than the plane strain method of
ASTM E 813 or E 1737. This method was developed specifically for the determination of R-curves from thin
sheets or films. However, this is not a valid method for determining J
Ic
, and results should not be reported as
such. When using this method, specimen size and the values of C
1
and C
2
(which characterize the power-law fit
of the R-curve) should be reported.
Other Methods
Alternative methods for determining the fracture toughness of polymer materials have recently been proposed.
Most notable are the normalization and hysteresis methods, which are both single specimen techniques. The
normalization method does not require unloading cycles or online crack measurement and has been used
successfully for metallic materials (Ref 31). The method is based on the assumption that the load, P, on the
specimen can be represented by:
P = G(a)H(ν
pl
)
(Eq 11)
where G(a) is a known function of crack length and specimen geometry, and H(ν
pl
) is a function of plastic
displacement, ν
pl
. After the form of H(ν
pl
) is fit to experimental data, values of a (and hence J) can be
determined at any point on the load-displacement curve. J
Ic
can then determined from the R-curve using the
methods described above. Zhou et al. (Ref 31) found that the results of this method are slightly less
conservative than those determined by ASTM E 813 and more conservative than ASTM E 1737 for two rubber-
toughened nylons (nylon 6/6 and an amorphous nylon).
The hysteresis method requires the application of multiple load-unload cycles to successively larger
displacements (Ref 30, 32, and 37), as shown in Fig. 4. The area between the loading and unloading lines on the
load-displacement curve is defined as the hysteresis energy, and this is plotted against maximum displacement
for each loading cycle, as shown in Fig. 5. For small displacements, crack growth does not occur, and the
hysteresis energy varies linearly with displacement. This data is fit with a linear blunting line. After crack
growth commences, the hysteresis energy varies nonlinearly with displacement and can be fit with a power law.
The displacement at which the linear blunting line intersects with the power-law curve is taken as the critical
displacement to initiate crack growth, and the value of J at this displacement is taken as J
Ic
. It has been found
that the results of this method are slightly less conservative than those determined by ASTM E 813 and more
conservative than ASTM E 1737 for several polymers (ABS, PC/ABS, HIPS, and PC/PBT) (Ref 32, 36, 37, and
42).
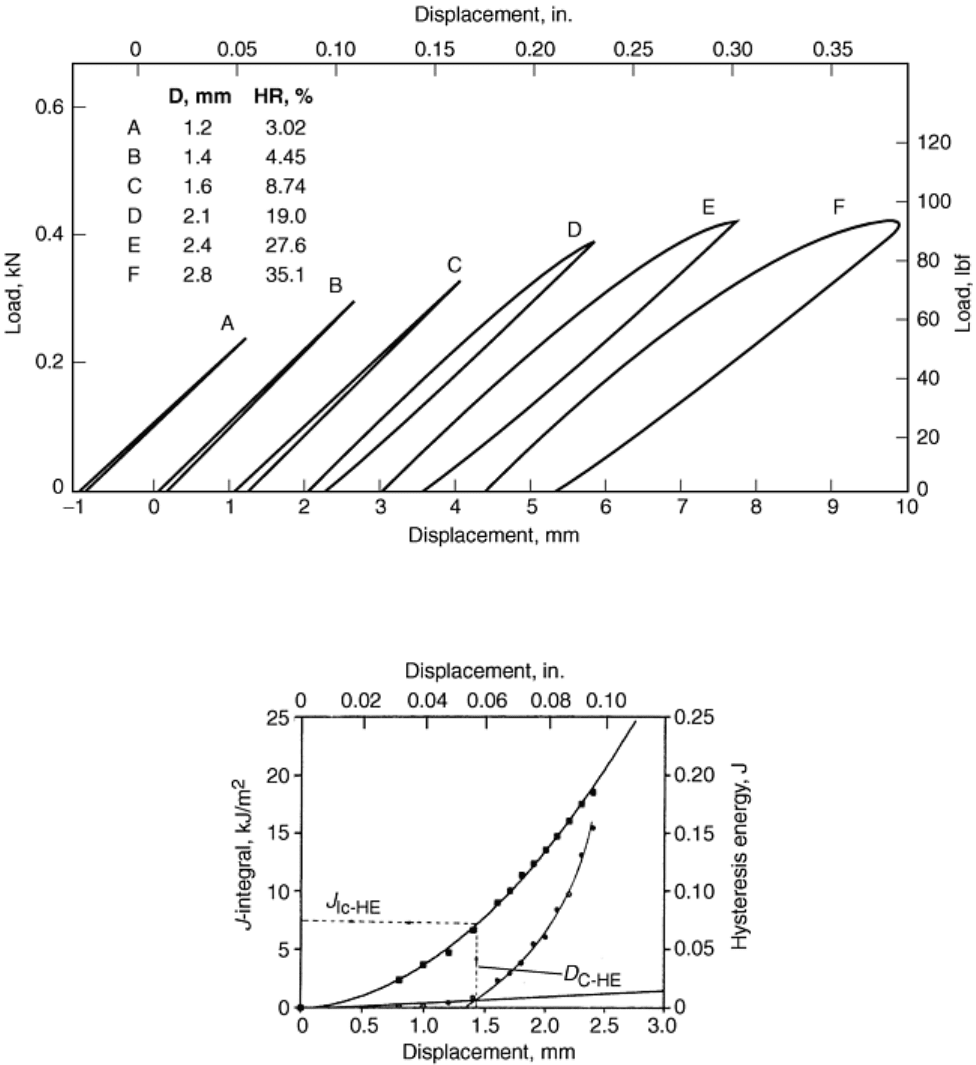
Fig. 4 Hysteresis loops for several loading-unloading cycles for a PC/PBT blend. D, specimen
displacement; HR, ratio of hysteresis energy to total strain energy. Source: Ref 37
Fig. 5 J-integral and hysteresis energy vs. displacement for a PC/PBT blend. Test rate, 2 mm/min (0.08
in./min). J
IC-HE
and D
C-HE
are critical values of J and D for initation of crack propagation. Source: Ref 37
References cited in this section
23. M. Ouederni and P.J. Phillips, J. Polym. Sci. B., Polym. Phys., Vol 33, 1995, p 1313
24. “Standard Test Methods for Plane Strain Fracture Toughness and Strain Energy Release Rate of Plastic
Materials,” ASTM D 5045, Annual Book of Standards, Vol 08.03, ASTM, 1996
25. “Standard Test Method for Determining J-R Curves of Plastic Materials,” ASTM D 6068, Annual Book
of Standards, Vol 08.03, ASTM, 1996
26. “Standard Test Method for J
Ic
, A Measure of Fracture Toughness,” ASTM E 813, Annual Book of
Standards, Vol 03.01, ASTM, 1989
