ASM Metals HandBook Vol. 8 - Mechanical Testing and Evaluation
Подождите немного. Документ загружается.

Potential Drop Method. The most common and sensitive in situ crack monitoring technique is reversing dc
potential drop, which typically applies a constant current to a specimen and measures the changes in potential
across the specimen as the crack grows. High-quality implementations of dc potential drop are consistently able
to achieve a crack length resolution on 1T compact-type specimens of about 1 mm (0.04 in.), and an overall
accuracy of <5% on the overall increment in crack advance. Current and potential leads can be insulated using
Teflon tubing for test temperatures up to 300 °C (570 °F); above 300 °C, zirconia is generally used.
During environmental testing, there are several special considerations. Solution conductivity can be a major
issue; an extreme example is the inability to use potential drop in liquid metal environments. Some deviations
in crack length versus measured potential response can also occur in highly conducting environments (e.g.,
aqueous solutions), and it must be recognized that the crack chemistry can be substantially more conductive and
at different pH than the bulk solution. However, despite the small distance between the upper and lower crack
flanks, the role of ionic (e.g., aqueous) conductivity is not large compared to that of metal conductivity, because
aqueous conductivities are typically measured in 10
-1
to 10
-6
S/cm (S, or Siemen, is W
-1
), whereas metal
conductivities are typically between 10
5
and 10
6
S/cm. Thus, errors associated with aqueous environments are
relatively small, although not always ignorable.
Another concern relates to inaccuracies in indicated crack length because of a nonuniform crack front or
because of metal contact along the crack flank during the fatigue cycle. In both cases, an abnormal fraction of
the dc current “shorts” through the uncracked metal ligament in the wake of the nominal crack front, and the
measured potential and indicated crack length is strongly affected. For example, if the crack front moves
forward in a 25 mm (1 in.) compact-type specimen by 3 mm (0.12 in.) in all locations except along one narrow,
rectangular ligament that is only 1 mm (0.04 in.) wide, the indicated crack advance by potential drop can be
very small (i.e., dramatically less than the area average of crack advance). Nonuniform crack fronts are much
more common when the environmental contribution to crack advance is high, and static loading (stress-
corrosion cracking) is generally much worse than dynamic loading (e.g., corrosion fatigue). Certain
microstructures, such as weld metal, can be quite susceptible to accelerated or retarded crack advance in
localized regions (i.e., along certain weld dendrites). The “unzipping” of the final metal ligament can lead to
anomalously high “apparent” crack growth rates over certain testing periods.
Other concerns for dc potential drop include electrochemical effects, particularly polarization. If a well-
designed, ground isolated power supply is used, then all of the dc current that leaves the “+” terminal must
return on the “-” terminal, and direct polarization of the specimen is not possible. In most cases, there is little
basis for concern for the electrochemical effects of using dc potential drop, although, for example, the small
potential difference between the crack flanks could have some influence in tight cracks in conductive solutions.
This potential difference is very small near the crack tip, so it is more likely to influence, for example,
dissolution of MnS inclusions at some distance toward the crack mouth, where the potential difference across
the crack flanks is higher. While the potential difference between the upper and lower surfaces of the crack is
small (typically 100 mV in many potential drop implementations), the gradient can be relatively large because
of the small separation of the crack faces. The importance of this issue can be quantified by establishing a
steady-state crack growth rate and disconnecting the potential drop system for a period of time, then
reconnecting it to evaluate its effect (or by comparing a duplicate experiment using an extensometer to monitor
the crack growth rate).
Electrochemical effects can also result from improperly insulated dc current leads. Because significant current
is passed through leads that are often relatively small, the potential drop in the current leads can be large (e.g.,
>1 V). If the current leads are not continuously insulated through the entire solution right up to the location
where they are spot welded onto the specimen, there is an opportunity for crosstalk with closely adjacent
potential leads (where the signal is typically 100 mV). Additionally, biasing of the specimen can occur if the
current leads are not continuously insulated through the system seals. Any ionic communication in the tight-
fitting seal area permits leakage to the metal (e.g., autoclave), and a circuit is established. The current leads act
like a 1 V battery that is shared across two resistors, one representing the water resistivity in the seal and one
representing the water resistivity between the specimen and the autoclave. This can cause some polarization of
the specimen in conductive solutions, or voltage (iR) drop in low-conductivity solutions. In the latter case, even
though no substantial polarization occurs, reference electrodes that are located between the specimen and the
autoclave “see” the voltage drop, and the apparent (measured) corrosion potential can be observed to fluctuate
as the direction of the dc current is reversed. This represents a good check of the integrity of the dc potential
drop system and wire insulation.

Finally, there is a potential concern for self-heating of the specimen by the applied dc current. While this is not
a problem in aqueous environments or at common current densities, there have been cases where high current
densities coupled with air or vacuum exposure resulted in significant self-heating.
Compliance Method and Other Cracking Monitoring Methods. The next most common crack monitoring
technique is mechanical compliance, which relies on the relationship between crack mouth opening
displacement and load during an unload/reload cycle). Resolution is typically limited by the strain gage or
proximity sensors (e.g., eddy current or capacitance) that must monitor crack opening displacement in the
(high-temperature water) environment. See the article “Fatigue Crack Growth Testing” in this Volume.
Another method is the ac potential drop technique, which relies on the “skin” (surface) effect of high-frequency
current in metals. The advantage of the skin effect for detecting crack nucleation is generally more than offset
by the higher noise (poorer noise rejection) of the ac measurement, even with sophisticated lock-in amplifiers,
although improved instrumentation is closing the gap. Other crack-following techniques include burst detection
by monitoring pressurized tubes, periodic ultrasonic or eddy current scans to detect small cracks, and periodic
interruption and inspection.
In aqueous environments, electrochemical noise can be used as a semiquantitative crack monitoring technique.
This technique measures the small variations in corrosion potential and/or corrosion current as cracks (or other
corrosion phenomena, such as pitting) nucleate and grow. This technique is good at discriminating the early
stages of crack initiation. However, the correlation between crack depth (or number of cracks) and the
electrochemical noise signal is at best semiquantitative, because: (a) the noise signal intensity decreases with
increasing crack depth, increasing distance between sensors, and the location of cracking on the specimen
surface (especially in low-conductivity solutions); and (b) noise from multiple small cracks cannot be
distinguished from noise from longer cracks.
References cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
102. R. Gangloff, Corrosion Fatigue, Corrosion Tests and Standards: Application and Interpretation,
R. Baboian, Ed., ASTM, 1995
103. Electrochem. Soc., Vol 126, 1979, p 908
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Vacuum and Gases at Room Temperature (Ref 90)
One of the most critical considerations for fatigue tests in vacuum and gaseous environments is the maintenance
of the purity of (and the reduction and measurement of the impurity level in) the test environment. As
mentioned above, small amounts of contaminants (impurities) in the test environment can lead to fatigue crack
growth rates that are not representative of the resistance of the material to fatigue crack growth in that
environment.
A clean environmental test chamber that provides a very low background pressure and quantifiable impurity
levels (below 10
-7
to 10
-6
Pa, or 7.5 × 10
-10
to 7.5 × 10
-9
torr) is essential, even if the tests are to be carried out in
gaseous environments at relatively high pressures (i.e., above the background).
Environment Containment. An all-metal environmental test chamber with mechanical-force feedthroughs is
preferred for the study of environmentally assisted fatigue crack growth in vacuum and gaseous environments.
Stainless steels are suitable materials for the environmental test chamber, with copper used as the gasketing

material. The test chamber usually is equipped with a glass viewport that enables the operator to visually
monitor the progress of the experiment.
With adequate pumping, the background pressure in the clean test chamber is usually below 10
-6
Pa (7.5 × 10
-9
torr). Maintaining an ultraclean test system is important, because a small amount of impurities can either
significantly reduce or accelerate the fatigue crack growth rate, depending on the material and the types of
impurities.
To achieve a low background pressure, the test chamber frequently is baked out (with the test specimen in
place) at a temperature above ambient (60–400 °C, or 140–750 °F) to remove adsorbed and absorbed gases on
the chamber wall. The bakeout temperature should be considerably below the tempering or aging temperature
of the test material to ensure that the microstructure and the mechanical properties of the test material are not
altered by the bakeout process. For example, the first-step artificial aging temperature for high-strength 7050-
T7451 aluminum alloy is 121 °C (250 °F). The bakeout temperature for the test chamber is thus normally kept
below 80 °C (175 °F).
Environment. Only high-purity, laboratory-grade gases should be used. Additional purification and
dehumidification of the gas is recommended by passing it through a molecular-sieve purifier and a cold trap (-
196 °C, or -320 °F) before allowing the gas to enter the test chamber. Gas pressure in the environmental test
chamber is usually controlled by admitting the gas through a variable-leak valve.
If the test environment contains a toxic gas (such as hydrogen sulfide) or a combustible gas (such as hydrogen
or methane), a protective hood with negative suction pressure should be used to enclose the test chamber or the
entire test system. The test chamber should be purged thoroughly with an inert gas, such as argon or nitrogen,
before it is reopened to the atmosphere.
If water vapor is used as the test environment, it can be drawn through the variable-leak valve from a high-
purity reservoir that is attached to the test chamber. Deionized distilled water in the reservoir should be purified
further by subjecting it to repeated freezing/pumping/thawing cycles to remove residual dissolved gases in the
water (Ref 104).
Certain gases can decompose or react with containment vessels over time. For example, hydrogen sulfide can
react with a stainless steel container to produce hydrogen. Provision must be made to remove the product gases
before the test gas is admitted into the test chamber.
Finally, if the environment consists of mixed gases, the gas at the lowest partial pressure should be admitted
first. If premixed gases are used, they must be thoroughly mixed in the supply reservoir to minimize
stratification.
If test conditions such as gas pressure, test frequency, or applied load are changed during fatigue testing, a
transient period may occur before the material assumes the steady-state fatigue crack growth rate that
corresponds to the new test condition. The duration of this transient period depends on several variables,
including the type of material, the test environment, and the magnitude of the change in test conditions.
References cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
104. Surf. Sci., Vol 64, 1977, p 617
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
High-Temperature Vacuum and Oxidizing Gases
Fatigue testing in elevated-temperature vacuum and oxidizing environments requires a carefully designed
vacuum test chamber. The chamber must keep the test specimen in a vacuum or oxidizing gas environment,
allowing forces to be applied to the specimen, a means to measure crack length, and a method of applying and
controlling the specimen temperature.
Variables that can affect fatigue crack growth rate at high temperature are time and rate dependent or structure
dependent. Examples of time- and rate-dependent variables are oxidation and creep. Structure-dependent
variables include phase transformations, nucleation and growth of new and existing phases, and grain growth.
When fatigue crack growth rate test data are reported for these environments, test temperature, vacuum
pressure, partial pressure of oxidizing gas, waveform type, waveform frequency, and stress ratio must be
reported. Additional information on high-temperature fatigue crack growth testing is given in the article
“Fatigue Crack Growth Testing” in this Volume.
Environment Chambers. Materials used in the test chamber should be selected to minimize outgassing in
vacuum. For example, many plastic materials contain plasticizers, which slowly outgas in vacuum. These types
of materials limit the ultimate vacuum obtainable. Stainless steel is suitable for the manufacture of the main test
chamber. Components in the chamber should be designed for fast outgassing. When threaded components are
used in the test chamber, channels should be machined in the threads to allow paths for fast outgassing.
For vacuum levels of 6.5 × 10
-5
Pa (5 × 10
-7
torr), O-rings provide sufficient sealing; for higher vacuum levels,
copper gaskets should be used. Electricity, water, radiofrequency, and the thermocouple can be input into the
chamber using standard vacuum feedthroughs.
Specimen Heating and Temperature Control. Induction heating is the only suitable method to heat test
specimens in vacuum and oxidizing environments. Radiofrequency generators with frequencies of 200 to 500
kHz are used for induction heating of test specimens. The induction coils should be made of copper and have no
insulating coating. When oxidizing gases are introduced into the test chamber, a certain pressure range exists at
which the gases will be ionized between the specimen and induction coils. In this pressure range, it is
impossible to heat the specimen, because the radiofrequency field arcs and shuts off the radiofrequency
generator. To continue testing, the gas pressure must be either increased or decreased.
Two types of temperature controllers that are suitable for induction heating are thermocouple and infrared
controllers. Each controller type has advantages and disadvantages. Infrared temperature controllers measure
and control temperature from the spectral energy density emitted from the test specimen over a certain
wavelength range. These measurements are noncontacting, but require a clear optical path from the sensor head
to the test specimen. Infrared temperature controllers have a minimum temperature measurement capability of
approximately 350 °C (660 °F). Two-color infrared controllers eliminate errors due to transmission loss and
emissivity changes, but they have a minimum temperature measuring capability of 700 °C (1290 °F).
Thermocouple temperature controllers are also used in vacuum test chambers. A variety of thermocouple types
can be used, depending on the temperature range and the required durability of the thermocouple. For example,
American National Standards Institute type S and type K thermocouple temperature ranges overlap, but for
long-term tests of more than one week, type S thermocouples are preferred because they are more oxidation
resistant. This would not be a consideration in a high-vacuum environment. Thin thermocouple wire less than
0.25 mm (0.01 in.) in diameter must be used to eliminate inductive heating of the thermocouple wire. With
some temperature controllers, it is necessary to filter out radiofrequency noise in the thermocouple with a
passive inductor/capacitor-type filter.
Test Specimens. Because high-temperature vacuum requires specimen heating by induction, many of the
standard fracture mechanics test specimens cannot be used. Center-cracked tension and single-edge notched
specimens are commonly used, because it is relatively easy to maintain the specimen gage section at uniform
temperature with induction heating. When tests are conducted at high vacuum levels or low oxidizing gas
partial pressures, specimen thickness may affect crack growth rate, because transport of the oxidizing gas to the
crack tip may be the rate-limiting factor.
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Aqueous Solutions at Ambient Temperature (Ref 90)
Fatigue studies in aqueous solutions at ambient temperatures present fewer problems experimentally than many
of the other environments considered in this article. Nevertheless, it is often the case that the most frequent
problem in determining the validity of corrosion fatigue data lies with the control and monitoring of the bulk
water chemistry and the monitoring and recording of the electrochemical potential.
Environment Containment. Glass and plastics are suitable materials for environmental test chambers and
ancillary pipework for aqueous solutions at ambient temperatures. At elevated temperatures (>60 °C, or 140
°F), however, dissolution of silicates from glassware can inhibit corrosion. Dissolution of plasticizers from
certain plastics (e.g., polypropylene) is also a concern. Flexible plastics, such as twin-pack casting silicone
rubber, have proved to be useful in the vicinity of the fatigue specimen.
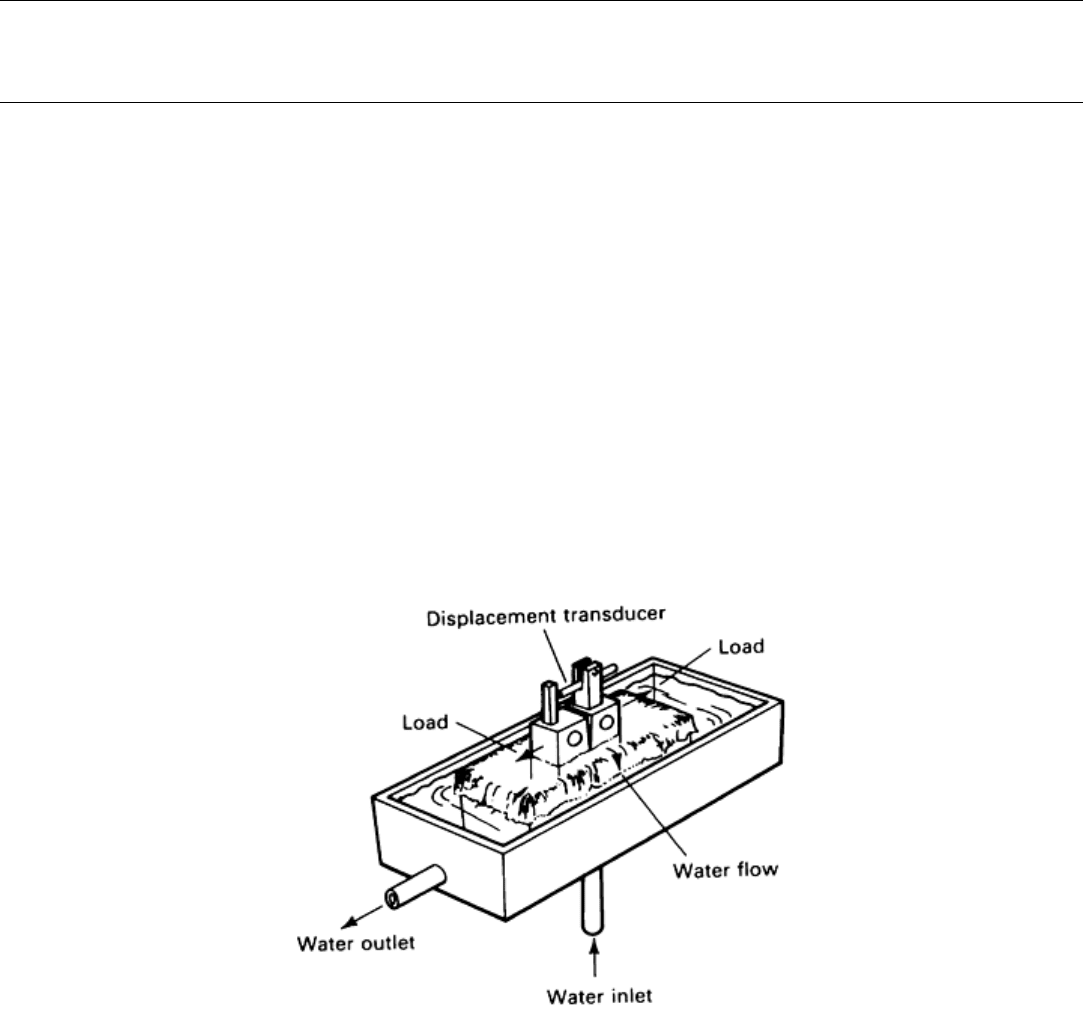
A corrosion fatigue test cell that avoids the need for a water-tight seal at the specimen is shown in Fig. 24.
Normal specimen movement and any sudden fracture event can be accommodated without catastrophic
consequences. Highly effective seals between plastic and metal surfaces can be made with silicone rubber
caulking compounds, if necessary, although sufficient time must be allowed for escape of the acetic acid
solvent base.
Fig. 24 Typical corrosion fatigue test cell. Maintenance of the equilibrium oxygen concentration is
ensured by cascading the solution in the circulation rig.
Fatigue specimens of passive metals such as aluminum, titanium, and stainless steel may be subject to crevice
corrosion under the caulking compound unless a primer and epoxy paint coat are applied initially to the metal
surface. Gasket seals using O-rings, for example, can also form a satisfactory seal, but generally are more
expensive to engineer and can also be subject to crevice corrosion in some configurations. The decision to
circulate the environment depends on the application and the extent of any problems in controlling water
chemistry.
Water Chemistry. The prevailing water chemistry and the electrode potential of the material in its environment
in the field are essential factors in any simulation experiment. Accelerated fatigue cracking can occur in a
number of environments, including seawater, salt water/salt spray, and body fluids. These must be reproduced
as closely as possible in the laboratory, although limitations are necessarily imposed in simulating aspects of
complex environments, such as the biological activity of seawater.
The importance of reproducing the service environment as closely as possible is illustrated by comparing the
behavior of metals in sodium chloride and in seawater. The buffering action of seawater associated with
dissolved bicarbonate/carbonate can result in the formation of calcareous scale under cathodic protection, which

can precipitate in cracks and influence the cyclic crack opening and closing, thus affecting crack growth rates.
Substitute ocean water, as described in ASTM D 1141, usually is a satisfactory substitute for seawater, but
some differences have been observed in relation to the rate of calcareous scale formation and the rate of
corrosion fatigue growth.
Laboratory solutions should be prepared using the purest chemicals available in distilled or deionized water.
Concentrations at the level of parts per million can have profound effects on electrochemistry and corrosion.
Several variables must be measured and controlled when simulating an aqueous environment: solution purity,
composition, temperature, pH, dissolved oxygen content, and the flow (circulation) rate of the solution.
Reference cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Acidified Chloride (Ref 90)
Investigations performed in acidified chloride, particularly at high temperature, pose unique problems. These
include not only experimental barriers, such as suitable containment and seal materials and sensitivity to low-
level oxidizing species, but also interpretational complexities, such as the effects of pitting and crevice
processes on enhancement or retardation (by blunting) of crack initiation and growth. Care must be exercised in
designing and conducting experiments to ensure personnel and equipment safety and to ensure proper
simulation, control, and monitoring of environmental parameters.
Below 100 °C (212 °F). Materials and techniques for solution containment depend on the test-temperature
regime. Below the boiling point in solutions containing dissolved oxygen, a primary design concern is to
prevent leaks that can damage equipment. A horizontal loading frame helps ensure that sensitive components
are not readily damaged by leaks. Additionally, some specimen configurations (such as compact tension) permit
the loading linkage to be placed above the solution, simplifying the choice of materials and seal designs.
Testing in deaerated solutions may require careful selection of materials, depending on the sensitivity of the test
to low oxygen concentration. For example, the clear, flexible tubing often used in laboratories is very
permeable to oxygen. Additionally, some plastics degrade in acidic environments.
Above 100 °C (212 °F), the propensity for pitting and crevice attack increases, the internal pressure rises, the
design strength of some materials (e.g., titanium) begins to decrease, and good seal design (particularly for
sliding seals) is crucial. Pitting and crevice potential studies show that the resistance of iron- and nickel-base
alloys in environments containing chloride decrease from room temperature to about 200 °C (390 °F).
The best approach for selecting pressure boundary materials is to combine published data with
recommendations from autoclave manufacturers and metals producers. No assumptions should be made
regarding the performance of materials with varying environment. For example, commercial-purity titanium,
which is often used in neutral and acidified chloride environments, performs very poorly in acidified chloride
under reducing conditions, in acidified environments containing sulfate, and in caustic environments at high
temperature. Addition of a small amount (0.2%) of palladium (grade 7) greatly improves resistance in acidified
environments that contain sulfate.
Above 200 °C (390 °F), materials selection is particularly difficult. In general, for acidified chlorides,
commercial-purity titanium is favored under oxidizing conditions (containing oxygen, iron ion, or copper ion),
while zirconium (for example, UNS R60702) is favored for reducing environments. Zirconium alloys are highly
intolerant of fluoride. In some cases, high-strength materials, such as Ti-6Al-4V or the Hastelloy C series

alloys, are required, although there is generally a loss in corrosion resistance. Liners of Teflon or tantalum are
options in some instances.
Because of its effect on the autoclave and test results, control of the oxidizing nature of the environment is often
critical. In addition to oxidizing species, such as oxygen, iron ions, and copper ions, care in the use of externally
applied potential is required. The autoclave may be polarized into a harmful regime if ground loops exist, or if
it is used as the counterelectrode. A similar result can occur if the autoclave contacts a dissimilar metal.
Because of the rate and extent of expansion on leakage, hot pressurized water poses a serious safety hazard.
Each autoclave must have a pressure-relief device attached to it, preferably in a fashion that does not permit
bypassing or isolation. Selection of the pressure-relief device must account for the pressure, environment (often
gold-coated elements are used in rupture disks), and temperature at which the device actually operates.
Additionally, autoclaves, particularly when used in aggressive environments, must be examined regularly for
damage resulting from pitting, crevice attack, general corrosion, hydriding, and so forth.
Pressure testing coupled with dimensional checks must also be performed. Manufacturers offer this service and
will usually provide the test details. Test pressure and dimensional tolerances are a function of autoclave
design, material, and temperature of use. Leaks may also occur in tubing and in valves, which are often difficult
to inspect or test. Leaks almost always develop slowly. Nevertheless, a relatively rapid, controlled method for
depressurizing the system should be included in the system design.
For some applications, inexpensive miniature autoclaves can be custom fabricated. The small internal volume
of these devices is an advantage if a leak occurs in the system.
Reference cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Liquid Metal Environments (Ref 90)
Liquid metals (sodium, potassium, and lithium, for example) are frequently used in heat-transport applications
at elevated temperatures. Such applications include liquid-metal-cooled nuclear reactors, first-wall coolant for
fusion devices, and heat-transport systems in solar collectors. These applications often involve cyclic
temperature and/or pressure fluctuations, as well as other sources of cyclic stresses. For this reason, knowledge
of the fatigue crack propagation behavior of structural alloys in the liquid metal environments is sometimes
necessary.
Generally, liquid metals react (in some cases, quite violently) with air and/or water vapor; therefore, testing
systems must be designed to exclude both air and water. Three basic designs have been developed to expose the
specimen (or crack region of a specimen) to the liquid metal environment, while excluding air, water, and other
contaminants.
The simplest method uses a sealed environmental chamber attached to the specimen that completely surrounds
the notch and crack extension plane in a compact-type specimen. The small environmental chamber contains
the liquid metal, but does not extend to the region of the loading holes; hence, the loading pins, clevis grips, and
remainder of the load train are not subjected to the liquid metal environment.
Relative motion across the notch and crack area is accommodated by bellows. This type of system has the
advantages of simplicity and low cost. The main disadvantage is that the liquid metal is static; hence, the
characteristics of large heat transport systems (e.g., mass transport due to nonisothermal operation) cannot be
studied.

The second type of system, a circulating loop, is much more costly to build and operate, but it can be used to
study potential effects on fatigue crack propagation such as mass transport, which occurs during carburizing,
decarburizing, and dissolution of alloying elements. A third type of system consists of an open crucible
(containing the test specimen immersed in static liquid metal) that is located within an inert gas cell or
glovebox. This type of system is relatively inexpensive to build and operate, but it has the greatest potential for
exposure to air and other contaminants.
Austenitic stainless steels generally have been used in the construction of current systems, and their use has
been satisfactory. System designers should consider, however, that under some conditions mechanical
properties (tensile, stress rupture, etc.) can be influenced by long-term exposure to liquid metals.
In most cases, fatigue crack propagation rates are lower in sodium environments than in elevated-temperature
air environments. The relatively benign nature of sodium environments also leaves the fracture faces in
excellent condition for viewing with optical microscopes, scanning electron microscopes, or transmission
electron microscopes.
Reference cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Steam or Boiling Water with Contaminants (Ref 90)
Corrosive environments, such as steam or boiling water with contaminants, come in contact with many
structural components. To assess the structural integrity of machine hardware, testing in the environments of
concern is essential. Fatigue crack growth testing in corrosive environments requires special care because of the
presence of corrosive media and testing complexity.
Environment Containment. Special designs are required to accommodate fatigue crack growth testing in steam
or boiling water with contaminants. If the environmental pressure and temperature are moderate, for example at
a pressure of 500 kPa (72.5 psi) and a temperature of 100 °C (212 °F), simple stainless steel O-ring sealed
chambers can be clamped to each side of the specimen in which cracking will occur. If necessary, the test
environment can be circulated through the chamber at a controlled flow rate.
If the environmental pressure and temperature are high, for example in steam at a pressure of 7.2 MPa (1040
psi) and a temperature of 288 °C (550 °F), a chamber that encloses the test specimens must be constructed.
Composition of the test environment must be carefully analyzed before and after the experiment, given the
variety of possible chemical effects on crack growth rates.
Dissolved Oxygen. Control and measurement of dissolved oxygen levels in the steam environment are of prime
importance, because oxygen can affect fatigue crack propagation rate properties. Oxygen content can be
controlled by bubbling argon or nitrogen through the water reservoir, or by maintaining a hydrogen
overpressure. Oxygen content can be measured by using a colorimetric technique or by using oxygen analyzers
that continuously monitor oxygen in the parts per billion range.
Reference cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209

Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Environmentally Assisted Cracking of Nonmetallic Materials
This section briefly reviews the evaluation of environmentally assisted crack growth in ceramics and plastics.
However, another important manifestation of environmentally assisted cracking is the interfacial failures
between two materials such as metal-polymer (Ref 105 and 106), ceramic-polymer (Ref 107), or metal-ceramic
(Ref 108 and 109) interfaces. Evaluating interfacial failures from environmental degradation requires an
understanding of adhesion; and further information on this topic is contained in the article “Adhesion Testing”
in this Volume.
References cited in this section
105. A. Carre and J. Shultz, Polymer-Aluminum Adhesion. III. Effect of a Liquid Environment, J.
Adhes., Vol 18, 1984, p 171–184
106. J.D. Venables, Review: Adhesion and Durability of Metal-Polymer Bonds, J. Mater. Sci., Vol
19, 1984, p 2431–2453
107. H. Wu, J.T. Dickinson, and S.C. Langford, Dynamic Measurements, of Humidity Attack on
Polymer/Glass Interfaces Under Stress, J. Adhes. Sci, Vol 11, 1997, p 695–717
108. S.X. Mao and A.G. Evans, The Influence of Blunting on Crack Growth at Oxide/Metal
Interfaces, Acta Mater., Vol 45, 1997, p 4263–4270
109. T.S. Oh, J. Rodel, R.M. Cannon, and R.O. Ritchie, Ceramic/Metal Interfacial Crack Growth
Toughening by Controlled Microcracks and Interfacial Geometries, Acta Metall., Vol 36, 1988, p 2083–
2093
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Environmentally Assisted Cracking in Ceramics
Ceramics are increasingly being used as either dielectrics or protective barriers where stress and environment
interact to pose severe conditions. Being very brittle, ceramics in general exhibit very limited toughness
compared to materials capable of substantial plastic deformation such as metals and polymers. Moreover, some
classes of ceramic materials are extremely susceptible to environmental stress cracking. In glasses, this is
manifested by stable slow crack growth at stress intensities substantially lower than fracture toughness
determined from fast fracture or in an inert media. This phenomenon, frequently referred to as static fatigue,
SCC, or subcritical crack growth, may be observed in almost every ceramic material providing an appropriate
environment. Crack-growth kinetics curves for ceramics generally exhibit three distinctive regions as shown in
Fig. 25. Region I is controlled by environment crack-tip reactions and may be described with a power-law
relationship:
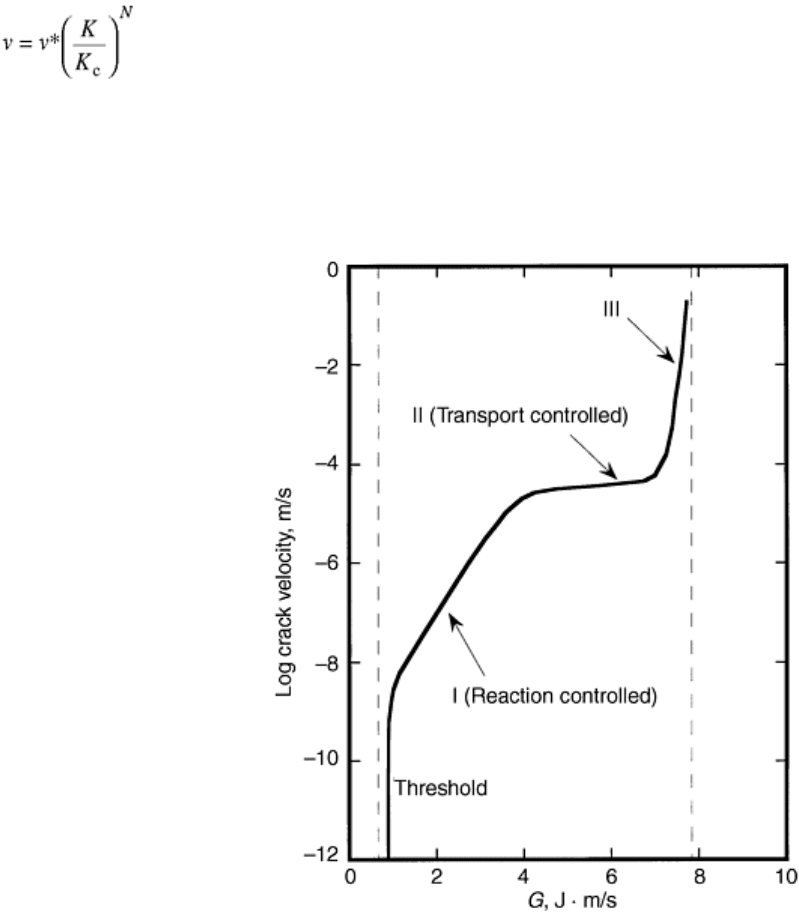
v = AK
N
(Eq 1)
where v is crack velocity and A and N are constants. Equation 1 may be rewritten in a slightly different form:
(Eq 2)
where v* is an empirical constant; the smaller N is, the higher the susceptibility to a static fatigue. Region I is of
primary importance for design considerations. A linear region II is governed by transport of reactive species
toward a crack tip. Finally, region III is determined by an “inert” fracture toughness with possible effects of
electrostatic interactions (Ref 110). Controlling mechanisms of crack growth processes in these regions in
different ceramic/environment systems are discussed here followed by a review of testing methods and a
summary of crack growth data for selected systems.
Fig. 25 Crack growth kinetics curve showing three characteristic regions
Environment/Ceramic Interactions with No Stress
Although frequently regarded as inert, most ceramic materials are reactive when exposed to appropriate
environment/temperature combination. Several examples are discussed here with the consideration for
governing mechanisms.
Glasses. Glass dissolves slowly in aqueous solutions under ambient temperature. Here, different mechanisms
dominate at different pH levels. In acidic solutions, ion exchange between the alkali ions in the glass and
hydrogen atoms in the solution occurs (Ref 111). Generally, the exchange rate increases with increasing
concentration of alkali ions in the glass composition. An exchange process produces more basic solution near
the glass surface. Similarly, the presence of alkaline earth elements such as calcium and magnesium would also
result in increase of a local pH. In contrast, for a pure silica glass, hydrolysis of silanol groups adds hydrogen
ions to produce a slight acidification (Ref 112).
In basic environments, hydroxyl ions react with the bonds of the network (Ref 113). In general, dissolution
rates increase with increasing pH. The increase is particularly steep for pH > 9, where a direct attack on the
