ASM Metals HandBook Vol. 8 - Mechanical Testing and Evaluation
Подождите немного. Документ загружается.


The K level used for precracking should not exceed about two-thirds of the intended initial K value. This
procedure prevents the forming of compressive stresses at the crack tip, which may alter the SCC behavior of
the alloys.
Aluminum alloys can also be precracked by the pop-in method, where the wedge-opening method is used to the
point of tensile overload. This method cannot be used for steels and titanium alloys, because of the strength of
these alloys.
Loading Procedures. Stress-corrosion crack growth in precracked specimens can be studied in K-increasing and
K-decreasing tests (Ref 84). In constant load or K-increasing tests, crack growth results in increased crack
opening, which keeps the environment at the crack tip and corrosion products from interfering with crack
growth. One of the problems with this mode of loading is that with increasing K, the plastic zone ahead of the
crack tip may increase and at some point interfere with crack propagation. Moreover, for this type of testing
bulky and relatively expensive equipment is required.
Constant displacement (K-decreasing) tests do not have the problems of the K-increasing tests indicated above.
The plastic zone ahead of the crack tip does not increase with increasing crack size, so that the stress condition
always remains in the plane strain mode. Also, the constant displacement tests can be self-loaded, and thus
external testing equipment is not needed. Because in these tests the stress-intensity factor decreases with
increasing crack growth, the stress-corrosion threshold stress-intensity factor (K
Iscc
) can be easily determined by
exposing a number of specimens loaded to different initial K
I
values. This can even be accomplished by crack
arrest in one specimen.
A major problem with this test method occurs when corrosion products form in the crack, blocking the crack
mouth and interfering with the environment at this crack tip. Moreover, the oxide can wedge open the crack and
change the originally applied displacement and load.
Measurement of Crack Growth. In order to quantify the crack growth behavior in precracked stress-corrosion
specimens, the crack length needs to be monitored, so that the crack velocity (da/dt) can be calculated, and the
relationship between the increasing K and the crack velocity can be determined. There are basically three
methods to monitor the growth of stress-corrosion cracks:
• Visual/optical measurements
• Measurement of the crack-opening displacement using clip gages
• The potential drop measurement, which monitors the increase in resistance across two points on either
side of the propagating crack
These methods are described further in ASTM E 647, “Standard Test Method for Measurement of Fatigue
Crack Growth Rates.”
References cited in this section
76. D. Sprowls, Evaluation of Stress Corrosion Cracking, Stress Corrosion Cracking, Materials
Performance and Evaluation, ASM International, 1992, p 363–416
84. G. Vogt, Werkstoff. Korros., Vol 29, 1978, p 721
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Corrosion Fatigue
Similar to SCC and/or hydrogen embrittlement under monotonic loads, environmentally assisted crack growth
occurs under fatigue conditions as well. Several mechanistic aspects are similar for these forms of

environmentally assisted crack growth, although several areas of corrosion fatigue are not fully clarified,
particularly with respect to the numerous variables and complex interactions that may cause crack initiation.
However, very useful information is available in the literature regarding computational mechanics and
advanced experimental techniques for corrosion fatigue (e.g., Ref 86 and 87). General reviews on SCC and
corrosion fatigue are also contained in Ref 88 and 89. This section is a brief overview on the key variables and
test methods of corrosion fatigue testing from Ref 90.
References cited in this section
86. R.P. Gangloff, Corrosion Fatigue Crack Propagation in Metals, EICM Proc., R.P. Gangloff and M.B.
Ives, Ed., National Association of Corrosion Engineers, 1990, p 55
87. D.J. Duquett, Corrosion Fatigue Crack Initiation Processes—A State of the Art Review, EICM Proc.,
R.P. Gangloff and M.B. Ives, Ed., National Association of Corrosion Engineers, 1990, p 45
88. R.W. Revie, Uhlig's Corrosion Handbook, 2nd ed., John Wiley & Sons, 2000
89. R. Baboian, Ed., Corrosion Tests and Standards: Application and Interpretation, ASTM, 1995
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Key Test Variables (Ref 90)
The specific types and influence rankings of experimental variables in corrosion fatigue can vary markedly with
specific alloy/environment systems. However, the following factors are crucial in most investigations of
corrosion fatigue:
• Stress-intensity amplitude (ΔK) or stress amplitude (Δσ)
• Loading frequency (ν)
• Load ratio (R= P
min
/P
max
or K
min
/K
max
)
• Chemical concentration and contaminants (e.g., for aqueous environments: ionic species, pH, and
dissolved species/gases, such as oxygen, hydrogen, and copper ion, that influence the corrosion
potential)
• Alloy microstructure, yield strength, and often inhomogeneities, such as MnS and other inclusions and
second phases, grain-boundary enrichment or depletion, and so forth
Other variables, such as load waveform, load history, and test temperature may also contribute, but they vary
substantially in importance from system to system. Electrode potential should be monitored and, if appropriate,
maintained constant during corrosion fatigue experimentation. Often, apparent effects of variables such as
solution dissolved oxygen content, flow rate, ion concentration, and alloy composition on corrosion fatigue are
traceable to changing electrode potential.
Stress-Intensity Amplitude (ΔK). While environmental crack growth rates increase with increasing ΔK, the
specific dependency varies greatly. In some environments, the effect of environment is merely to offset the
observed crack growth rate by some fixed factor above the inert rate. However, there is often a profound shift in
the dependence of ΔK, typically producing a reduced ΔK dependence in aggressive environments, at least in the
intermediate region where power-law behavior is observed. It is always important to examine the entire relevant
ΔK regime, not assuming the observed enhancement at a specific ΔK.
Environments do not always enhance the crack growth rate. The most common origins of crack retardation are
associated with increased crack closure and crack blunting. Crack closure is most often increased by thicker
oxides and perhaps the rougher (i.e., intergranular, with secondary cracks) fracture surface (Ref 91 and 92).
Crack blunting results from aggressive environments that result in inadequate passivity. If the flanks of the
crack are not adequately passive, then the crack tip will not remain sharp. This has been observed in low-alloy
and carbon steels in hot water (Ref 93) and in other systems.
Shifts in ΔK, K
max
, or load ratio during testing should be made very gradually, preferably continuously (e.g.,
under computer control). Changes in K should be limited to less than 10%, preferably much less. Any large
change in growth rate should be confirmed using increments of <1%. Data may differ for rising K versus K-
shedding conditions. Crack increments should be sufficient to provide statistically significant crack growth
rates (e.g., >10 times above the crack length resolution) and should account for effects of plastic zone size
under prior conditions during K-shedding. Shifts in frequency and hold time are not as restrictive, although
changes greater than 3 to 10 times can lead to anomalous results.
The presence of an environment can also shift the dependence on stress amplitude (Δσ) or plastic strain
amplitude (Δε), not only by decreasing the stress at which a certain cyclic life can be attained, but also by
eliminating the stress amplitude threshold altogether (Fig. 20). This, and increased scatter in the data, can lead
to differences in estimating environmental effects at different stress amplitudes (Fig. 21). Note also that there is
a consistent trend versus time in which the “bounding” curves are periodically shifted lower and to the left in
Fig. 21.
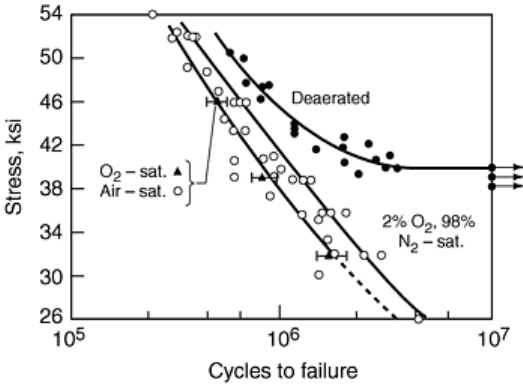
Fig. 20 Stress amplitude versus cycle to failure for corrosion fatigue of 0.18% C steel in 3% NaCl at 25
°C, showing the strong effect of dissolved oxygen in accelerating cracking and eliminating the stress
threshold. Source: Ref 94
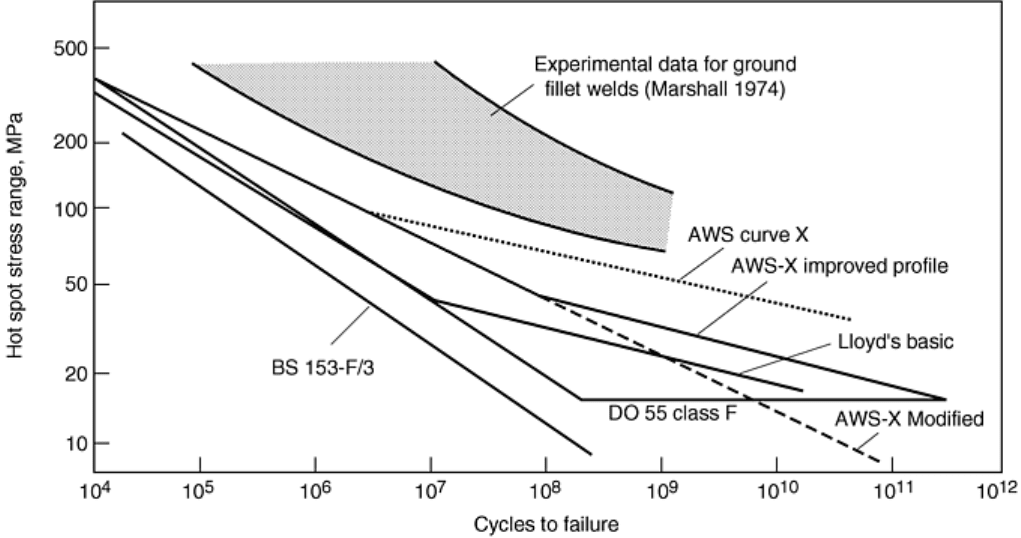
Fig. 21 Empirically derived design codes for corrosion fatigue of offshore welded tube structures,
illustrating their invalidity under specific test conditions and their constantly changing formulation. Hot
spot stress range refers to local peak stress amplitudes at specific locations of the structure. Source: Ref
95
Loading Frequency (ν). Because the environment induces a significant time-dependent response, environment
enhancement can vary markedly with loading frequency. At high frequency, it is common for the
environmental enhancement to be substantially eliminated because of inadequate time available for associated
chemical reaction and mass transport kinetics. Transitions in significant environmental enhancement are often
apparent when plotting crack growth rate versus frequency or hold time. Predictive modeling has been quite
successful in accounting for the transition between cycle- and time-dependent behavior as a function of
corrosion potential, water purity, and degree of sensitization of the stainless steel (Ref 96 and 97).
Load Ratio (R). At higher load ratios (P
min
/P
max
), corrosion fatigue crack growth rates are usually higher than in
inert environments. This can be viewed as a mean stress effect, and the greater environmental enhancement can
be considered to result from the expected increase in contribution of time-dependent crack advance that would
occur even under static load conditions.
Test Environment and Chemical Contaminants. Besides the obvious concern of primary species (such as NaCl
concentration for salt water) in corrosion fatigue, small amounts of contaminants are also a key variable. A
striking example (Ref 98) of an environmental-purity effect is illustrated in Fig. 22 for gaseous hydrogen
embrittlement of a low-strength carbon steel. Relative to vacuum, crack growth is accelerated by factors of 3
and 25 for moist air and highly purified low-pressure hydrogen gas, respectively. Small additions of oxygen to
the hydrogen environment essentially eliminate the brittle corrosion fatigue component to crack growth,
consistent with a trend first reported by Johnson (Ref 99). Similar effects have been reported for carbon
monoxide and unsaturated hydrocarbon contamination of otherwise pure hydrogen environments. In aqueous
environments, the effects of bulk ionic concentration and pH are often quite pronounced (especially in
unbuffered systems), although dissolved oxidants are often of greater consequence (e.g., dissolved oxygen,
hydrogen peroxide, and copper and iron ions), as are contaminants (e.g., dissolved sulfur, chloride, lead,
mercury).
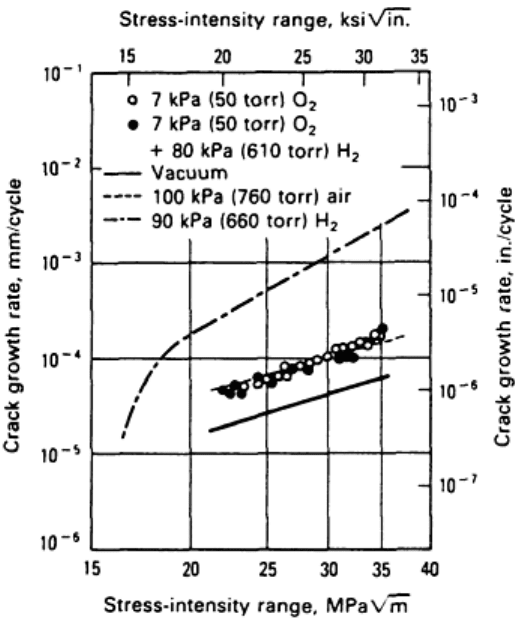
Fig. 22 Effect of oxygen (O
2
) contamination on gaseous hydrogen embrittlement of a low-strength
AISI/SAE 1020 carbon steel. Frequency: 1 Hz. Source: Ref 98
The primary role of oxidizing and reducing species, especially dissolved oxygen and hydrogen, is in shifting the
corrosion potential. Some species, such as nitrate, may also directly influence crack chemistry and, if reduced to
ammonia, can be directly responsible for environmental enhancement (e.g., of brasses). In many cracking
systems, the role of oxidants (elevated corrosion potential) is an indirect one, because inside the crack the
oxidants are generally fully consumed and the corrosion potential is low. In such systems, the role of oxidants is
to create a potential gradient, usually near the crack mouth, that causes anions (e.g., Cl
-
) to concentrate in the
crack and causes the pH to shift.
Oxidants increase the corrosion potential in aqueous environments, which can have very pronounced effects on
environmental enhancement. This can occur at exceedingly low concentrations; in high-temperature water,
crack growth rates can increase by orders of magnitude merely from the presence of parts-per-billion levels of
dissolved oxygen in water. Similar enhancements are observed for small concentrations of aqueous impurities
(e.g., <10 ppb of sulfate or chloride) or MnS inclusions in low-alloy and carbon steels, which dissolve within
the crack to form sulfides. This usually is associated with the formation of a differential aeration cell by
complete oxygen consumption within the crack (Fig. 23). Thus, even very small cracks usually advance under
deaerated conditions, and the gradient in corrosion potential that is formed from crack mouth to crack tip causes
an increase in anion concentration and a shift in pH in the crack. The shift is often acidic, but not necessarily so,
because it requires the presence of non-OH
-
anions to balance the acidity (H
+
). Thus, if only OH
-
is present
(e.g., from NaOH), the pH shift can only be in the alkaline direction.
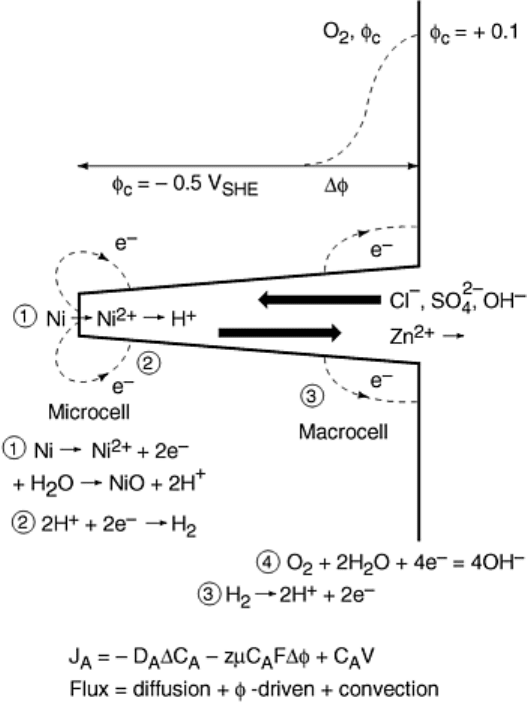
Fig. 23 Schematic of crack showing the differential aeration macrocell that establishes the crack-tip
chemistry and the local microcell that is associated with metal dissolution and crack advance. Because
the differential aeration macrocell is not essential to elevated crack growth rates, some coupling of the
currents associated with these two cells may occur, but this is unnecessary. Source: Ref 100
Potentiostats can be used to control the specimen potential, although their use (which rarely directly simulates
the real situation) can provide misleading data. Primary concerns are associated with:
• Voltage drop in solution: The reference electrode reading may be biased by the potential distribution in
the solution associated with passage of ionic current.
• Failure to polarize the crack tip: Even in highly conductive solutions, the crack-tip potential is rarely
significantly affected by external polarization, and therefore crack advance does not occur at the
potential that is controlled on the specimen surface.
• A reversal of surface reactions and shifts in local pH: In solutions containing oxygen, the reaction on
the external surface is cathodic, and inside the crack, anodic reactions occur. With a potentiostat, as the
specimen is polarized to more positive potentials, it becomes more anodic and causes oxidation
reactions to take place predominantly on the metal surface, which can alter the local pH. Cathodic
reactions occur predominantly on the relatively remote counterelectrode.
To accurately measure potentials, commercial or custom-built reference electrodes are used. Both to measure
potentials accurately and to prevent galvanic coupling, it is desirable to electrically isolate the specimen from
the linkage and surrounding metal surfaces. If the environment is not very conducting or the potentials of
surrounding metal surfaces are not too different from the specimen, electrical isolation may not be critical.
However, it is then necessary to place the reference electrode much closer to the test specimen than to other
(electrically connected) metal surfaces.
Other concerns for the environment include:
• Specimen and grip design (e.g., to avoid failure at crevices by minimizing stress in creviced regions)
• Proper design of environmental cells (e.g., to avoid contamination from leachants from or diffusion of
oxygen through plastics)
• Maintenance of proper chemistry, which often requires refreshed/flowing systems for controlling the
chemistry in gases or liquids
• Proper stability and measurement of temperature (near the specimen)
• Proper and thorough monitoring/recording of all relevant chemical and electrochemical parameters
Metallurgical Variables. Microstructure and alloy strength influence fatigue crack propagation in embrittling
gases and liquids. In general, brittle corrosion fatigue cracking is accentuated by:
• Impurity (e.g., phosphorus or sulfur) segregation at grain boundaries
• Solute depletion or sensitization (e.g., chromium) about grain boundaries
• Planar deformation associated with ordering or peak-aged coherent precipitates
• Increased yield strength or hardness
• Large inclusions (e.g., MnS)
The effects of alloy composition, grain size, and microstructure (e.g., bainitic versus martensitic steel) vary with
environment and brittle cracking mechanism. Laboratory experiments are necessary to establish specific trends.
Yield strength plays a large role in environmental cracking, which has been attributed both to enhanced crack-
tip strain rate as well as to complete shifts in the crack advance mechanism. The importance of hydrogen
embrittlement in higher-strength materials has been confirmed by experiments in gaseous hydrogen. Its direct
role in environmental crack advance is considered to be limited to about 150 °C (300 °F) in iron- and nickel-
base alloys, although it may have an indirect role at higher temperatures.
Similar effects have been observed for bulk or surface cold work, which raises the yield strength. Thus,
machining and surface treatments such as shot peening can significantly affect cracking. Shot peening and
related treatments that produce surface compressive stresses can be very beneficial, provided that cracks do not
exist (or form) and that tensile stresses do not exceed them. If sufficient strain occurs, transgranular cracks
often nucleate in the surface-hardened region.
Other important microstructure factors include γ′ or δ phases on grain boundaries of nickel alloys, martensite
formation in steels, carbide formation (sensitization) in stainless steel and nickel alloys, and inhomogeneities
(e.g., MnS and nonmetallic inclusions). These often have an even larger role in corrosion fatigue than under
inert conditions, and a uniform microstructure or distribution of inhomogeneities can rarely be assumed.
Crack Closure Effects. Premature crack surface contact during unloading, or “crack closure,” can greatly reduce
rates of fatigue crack propagation. The true (or effective) crack-tip driving force is reduced below the applied
ΔK because of the reduced crack-tip displacement range. Closure phenomena are produced by a variety of
mechanisms and are particularly relevant to fatigue crack propagation in the near-threshold regime, after large
load excursions, or for corrosive environments.
Two mechanisms of crack closure are relevant to corrosion fatigue. Rough intergranular crack surfaces (typical
of environmental embrittlement) promote crack closure, because uniaxially loaded cracks open in a complex
three-dimensional mode, thus allowing for surface interactions and load transfer. Roughness-induced closure is
most relevant to corrosion fatigue at low ΔK and at stress-ratio levels where absolute crack opening
displacements (0.5–3 mm, or 0.02–0.12 in.) are less than fractured grain heights (5–50 mm, or 0.20–2 in.).
Alternately, crack closure is impeded by dense corrosion products within the fatigue crack. For mildly
oxidizing environments, such as moist air, this closure mechanism is relevant at low stress-intensity levels and
contributes to the formation of a “threshold,” as described in Ref 101.
For corrosive bulk environments or localized crack solutions, cracking at high ΔK values may be retarded
below the growth rates observed for air or vacuum due to corrosion product formation within the crack. The
engineering significance of beneficial crack closure influences depends on the stability of the corrosion product
during complex tension-compression loading and fluid conditions.
References cited in this section
90. P.L. Andresen, Corrosion Fatigue Testing, Fatigue and Fracture, Vol 19, ASM Handbook, 1996, p 193–
209

91. J.C. Newman, Jr. and W. Elber, Ed., Mechanics of Fatigue Crack Closure, STP 982, ASTM, 1988
92. P.L. Andresen and P.G. Campbell, The Effects of Crack Closure in High Temperature Water and Its
Role in Influencing Crack Growth Data, Proc. Fourth International Symp. on Environmental
Degradation of Materials in Nuclear Power Systems—Water Reactors, National Association of
Corrosion Engineers, 1990, p 4-86 to 4-110
93. F.P. Ford, “Mechanisms of Environmental Cracking in Systems Peculiar to the Power Generation
Industry,” Final Report NP-2589, Electric Power Research Institute, 1982
94. D.J. Duquette and H.H. Uhlig, Trans. Am. Soc. Met., Vol 61, 1968, p 449
95. P.L. Andresen, R.P. Gangloff, L.F. Coffin, and F.P. Ford, Overview—Applications of Fatigue Analysis:
Energy Systems, Proc. Fatigue/87, EMACS, 1987
96. F.P. Ford, D.F. Taylor, P.L. Andresen, and R.G. Ballinger, “Corrosion Assisted Cracking of Stainless
and Low Alloy Steels in LWR Environments,” Final Report NP-5064-S, Electric Power Research
Institute, 1987
97. P.L. Andresen and F.P. Ford, Use of Fundamental Modeling of Environmental Cracking for Improved
Design and Lifetime Evaluation, J. Pressure Vessel Technol. (Trans. ASME), Vol 115 (No. 4), 1993, p
353–358
98. H.G. Nelson, Hydrogen Induced Slow Crack Growth of a Plain Carbon Pipeline Steel under Conditions
of Cyclic Loading, Effect of Hydrogen on the Behavior of Materials, A.W. Thompson and I.M.
Bernstein, Ed., The Metals Society—American Institute of Mining, Metallurgical, and Petroleum
Engineers, 1976, p 602–611
99. H.H. Johnson, Hydrogen Brittleness in Hydrogen and Hydrogen-Oxygen Gas Mixtures, Stress
Corrosion Cracking and Hydrogen Embrittlement of Iron Based Alloys, J. Hochmann, J. Slater, R.D.
McCright, and R.W. Staehle, Ed., National Association of Corrosion Engineers, 1976, p 382–389
100. P.L. Andresen and L.M. Young, Characterization of the Roles of Electrochemistry, Convection
and Crack Chemistry in Stress Corrosion Cracking, Proc. Seventh International Symposium on
Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, National
Association of Corrosion Engineers, 1995, p 579–596
101. S. Suresh and R.O. Ritchie, Int. Metals Rev., Vol 29, 1984, p 445–476
Evaluation of Environmentally Assisted Crack Growth
Y. Katz, N. Tymiak, and W.W. Gerberich, University of Minnesota
Corrosion Fatigue Crack Growth Test Methods (Ref 90)
Standard methods of fatigue crack growth (as defined in ASTM E 647 and described in the article“Fatigue
Crack Growth Testing” in this Volume) are generally applicable to corrosion fatigue crack growth tests. ASTM
E 647 also contains an appendix specific to crack growth in marine environments. Procedures for other
corrosion fatigue environments are not standardized, but various methods have evolved. Some general aspects
of corrosion fatigue crack growth are described below, and additional background is provided in Ref 102.
Three problem areas are relevant to corrosion fatigue experimentation. First, the environment must be contained
about the cracked specimen without affecting loading, crack monitoring, or specimen-environment
composition. Parameters such as environmental purity, composition, temperature, and electrode potential must
be monitored and controlled frequently.
Second, the deleterious effect of low cyclic frequency dictates that crack growth rates must be measured at low
(often <0.2 Hz) frequencies, which lead to long test times, often from several days to weeks. Load-control and
crack-monitoring electronics and environment composition must be stable throughout long-term testing.
Third, crack length must also be measured for calculations of stress intensity and crack growth rate. Optical
methods are often precluded by the environment and test chamber. Indirect methods, based on specimen
compliance or electrical potential difference, have been applied successfully to monitor crack growth in a wide
variety of hostile environments and are described in more detail below. Experimental and analytical
requirements, however, are complex for indirect crack monitoring.
Finally, specimen thickness, as it influences the degree of plane-strain constraint, and crack size, as it influences
the chemical driving force, may affect corrosion fatigue crack speeds. Currently, such effects are unpredictable;
specimen thickness and crack geometry must be treated as variables. In corrosion fatigue, the electrochemistry
within the crack is mass transport dependent and can vary with crack depth, and possibly also with specimen
geometry and with accessibility of solution in the through-thickness direction via the crack sides. These factors
can influence crack growth rates despite the constancy of the range of the stress-intensity factor.
Because corrosion fatigue testing is often performed at low cyclic frequencies, multiple test stations are
desirable. For this reason and for general economy, compact tension specimens are frequently used. Such
specimens minimize the applied load required to achieve a given crack-tip stress intensity, thus permitting the
use of low-load capacity and less expensive test machines. In applying load to specimens in a test cell, cell
friction must not affect load in sealed systems. This is generally not a significant factor in most ambient-
temperature applications, however. Insulation between specimens and grips, pin assemblies, and so forth is
essential to avoid galvanic effects, but greases should not be used.
Electrode Potential
Monitoring and reporting the electrode potential during corrosion fatigue experiments is important. The
potential should be measured using a reference electrode located in the bulk solution adjacent to the specimen.
When impressed currents are applied to the specimen, measurement should be made adjacent to the surface
using a Luggin capillary to minimize the potential drop between the reference electrode and the metal surface,
the magnitude of which will depend on the solution conductivity and flow of current.
Selection of a reference electrode depends on the particular application, but those most commonly used in
laboratory room-temperature tests are the SCE and the silver/silver chloride electrode. For some solutions in
which contamination with chloride is undesirable, use of a mercury/mercurous sulfate reference electrode is an
option. Contamination can be reduced by using commercially available double-junction electrodes, in which the
outer jacket is filled with test solution.
In quoting measured potentials, the potential should be referred to a standard scale such as the standard
hydrogen electrode (SHE) or the SCE at 25 °C (75 °F). In tests remote from 25 °C (75 °F), allowance must be
made for the fact that the half-cell potential of the reference electrode varies with temperature. A high-
impedance meter (>10
12
W), such as an electrometer or a pH meter, should be used for monitoring potential,
although periodic (short-term) measurements can usually be successfully performed using digital voltmeters
whose input impedance is ≥10
9
Ω (usually limited ≤2 V full-scale direct-current, dc, ranges).
Near room temperature, it generally is possible to use commercial reference electrodes such as calomel and
silver chloride electrodes; some electrode designs permit use near boiling. Designs that place the reference
electrode in a separate chamber at a different temperature than the test solution are complicated by formation of
a thermal junction potential in the electrolyte, the magnitude of which may be large (above 0.1 V).
At temperatures above boiling, a custom reference electrode generally is necessary. Most investigators use
internal or external silver/silver chloride reference electrodes. For internal electrodes, the silver chloride
reaction occurs at the test temperature. For external electrodes, the silver chloride reaction occurs at room
temperature, but system pressure is applied (so no streaming potentials form), with a temperature gradient
occurring in the potassium chloride electrolyte as it enters the autoclave. A porous junction in the autoclave
isolates the potassium chloride electrolyte from the autoclave solution. This thermal junction potential has been
well characterized over a range of temperatures and potassium chloride concentrations (Ref 103).
Potentials should be reported on the SHE scale, particularly for elevated-temperature tests, for which the
conversion factors to V(SHE) are not widely known. However, when comparing results as a function of
temperature, it may be helpful to eliminate the contribution of the standard hydrogen cell, because as in other
reactions, it has a potential that varies with temperature. It is by convention that the standard hydrogen cell is 0
V at any temperature; relative to the standard hydrogen reaction at 25 °C (75 °F), the potential of the standard
hydrogen reaction is about 0.021 V at 50 °C (120 °F), 0.057 V at 100 °C (212 °F), 0.086 V at 150 °C (300 °F),
and about 0.105 V between 210 and 300 °C (410 and 570 °F).
Application of imposed potential using a potentiostat requires electrical isolation of the specimen. In some
cases, it may be difficult to insulate the specimen from the loading linkage; instead, the linkage must be
insulated from the autoclave, and the measured current flow cannot be attributed only to reactions at the
specimen. Ground loops present perpetual problems, because most potentiostats are designed to hold the
specimen at ground (or virtual ground). With necessary mechanical and plumbing connections, the autoclave is
usually connected to ground; thus, the specimen is effectively connected to the autoclave. The problem is
compounded if the autoclave is used as the counterelectrode, because the ground loop shorts out the
potentiostat. Options include thorough electrical isolation of the autoclave from ground and use of a fully
floating potentiostat.
Attachment of a lead to the specimen to permit measurement or application of potential can be a challenge in
aggressive environments. Recommendations include use of wire that is either identical to the specimen or a
very noble metal, such as platinum. Attachment using a weld bead (e.g., by gas-tungsten arc welding) usually is
superior to spot welding. Covering the lead wire with heat-shrink Teflon and, at low test temperatures, covering
the weld with an organic “stop-off” coating helps maintain a good connection and minimizes the effects of the
wire (via galvanic coupling or its contribution to the measured current). Another technique involves the use of a
commercially available plasma sprayed insulating coating, which can also be used in high-temperature water.
Errors in the potential applied by a potentiostat can occur in solutions of low conductivity. These iR drops,
which result from current flow between the counterelectrode and working electrode in the high-resistance
solution, are detected by the reference electrode and summed with the electrode potential of the specimen.
Electronic compensation is possible, but not straightforward in most high-resistivity media. Partial
compensation is possible by placing the reference electrode near the specimen, although for small specimens
the measured potential becomes very sensitive to electrode positioning. A rough estimate of the possible error
can be made by multiplying the resistivity of the solution (preferably determined by measuring the alternating
current, ac, flowing between the counterelectrode and working electrode when a known 1000 Hz ac voltage is
applied) by the potentiostat current that flows during a test.
Monitoring Crack Length
The electrical potential technique and the compliance method are frequently used to monitor fatigue crack
growth in solution and in gaseous environments. Visual methods generally are not practical; often, the crack
and the test specimen are obscured by the test chamber, or a microscope with a long focal length is needed.
The electrical potential technique is preferred over the compliance method for use inside an environmental test
chamber, because the compliance gage may outgas and is a potential source of test environment contamination.
Its use in a corrosive environment is also unsuitable. The electric potential technique, however, is
noncontaminating and can be used in most environments.
Use of the compliance method is generally limited to compact tension (CT, or compact type) and wedge-
opening load specimens. It is not used for center-cracked tension specimens because of limitations in sensitivity
and accuracy. The electrical potential technique can be readily applied to all three specimen types. The
principal drawback of the electrical potential technique is that the specimen must be electrically conductive;
thus, it cannot be applied directly to specimens made of nonelectrically conducting materials, such as polymer-
based composites and ceramics. In addition, electrical shorting across the crack surfaces may affect its
measurement accuracy, particularly for tests in vacuum. Both the electrical potential and compliance techniques
can be readily interfaced with a computer for real-time control of the experiment and for online data acquisition
and reduction.
