Албертс Б., Брей Д. и др. Молекулярная биология клетки. Том 1
Подождите немного. Документ загружается.


171
(P.D. Boyer ed.), Vol. 1, pp. 213-240. New York, Academic Press, 1970.
37. Adam G., Delbruck M. Reduction of dimensionality in biological diffusion processes. InB Structural Chemistry and Molecular Biology (A.
Rich, N. Davidson eds.), pp. 198-215. San Francisco, Freeman, 1968.
Berg O. G., van Hippel P. H. Diffusion-controlled macromolecular interactions. Anna Rev. Biophys. Biophys. Biochem., 14, 131-160, 1985.
38. Cantor C.R., Schimmel P.R. Biophysical Chemistry. Part III: The Behavior of Biological Macromolecules. Chapters 15 and 17. New York, W.
H. Freeman, 1980.
Dickerson R. E., Geis I. Hemoglobin: Structure, Function, Evolution and Pathology. Menlo Park, CA. Benjamin-Cummings, 1983.
Edelstein S. J. Introductory Biochemistry. San Francisco, Holden-Day, 1973. (Chapter 10 on protein aggregates and allosteric interactions.)
Monod J., Changeux J.-P., Jacob F. Allosteric proteins and cellular control systems. J. Моl. Biol., 6, 306-329, 1963.
39. Koshland D.E., Jr. Control of enzyme activity and metabolic pathways. Trends Biochem. Sci., 9, 155-159, 1984. Newsholme E. A., Start C.
Regulation in Metabolism. New York, Wiley, 1973.
40. Koch K.-W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature, 334, 64-65, 1988.
Nishizuka Y. Protein kinases in signal transduction. Trends Biochem. Sci., 9, 163-166, 1984.
41. Kantrowitz E.R., Lipscomb W.N. Escherichia coli aspartate transcarbamoylase: the relation between structure and fanction Science, 241, 669-
674, 1988.
Schachman H.K. Can a simple model account for the allosteric transition of aspartate transcarbamoylase? J. Biol. Chem., 263, 18583-18586,
1988.
Sprang S., Goldsmith E., Fretterick R. Structure of the nucleotide activation switch in glycogen phosphorylase a. Science, 237, 1012-1019,
1987.
42. Hill T.L. Biochemical cycles and free energy transduction. Trends Biochem. Sci., 2, 204-207, 1977.
Hill T.L. A. proposed common allosteric mechanism for active transport, muscle contraction, and ribosomal translocation. Proc. Natl. Acad.
Sci. USA, 64, 267-274, 1969.
Johnson K.A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu. Rev. Biophys.
Biophys. Chem., 14, 161-188, 1985.
43. Hokin L.E. The molecular machine for driving the coupled transports of Na
+
and K
+
is an (Na
+
+ Reactivated ATPase. Trends Biochem. Sci., 1,
233-237, 1976.
Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature, 292, 201-204, 1981.
Tanford C. Mechanism of free energy coupling in active transport. Annu. Rev. Biochem., 53, 379-409, 1983.
44. Nicholls D. G. Bioenergetics: An Introduction to the Chemiosmotic Theory. New York, Academic Press, 1982.
45. Alberts В. М. Protein machines mediate the basic genetic processes. Trends Genet., 1, 26-30, 1985.

172
4. Как изучают клетки?
Клетки очень малы по размеру и сложно устроены: трудно рассмотреть их структуру, трудно определить молекулярный состав и еще
труднее установить, как функционируют их отдельные элементы. Для изучения клеток разработано множество экспериментальных методов,
возможности которых определяют уровень наших знаний в этой области, Успехи в изучении биологии клетки, включая наиболее удивительные
достижения последних лет, как правило, связаны с применением новых методических подходов. Поэтому для понимания клеточной биологии
необходимо иметь некоторое представление о соответствующих экспериментальных методах.
В этой главе мы вкратце рассмотрим современные методы, используемые для изучения клеток. Мы начнем знакомиться с теми из них,
которые позволяют изучать клетку как единое целое, и затем обратима к анализу составляющих клетку макромолекул. Отправной точкой станет
микроскопия, поскольку клеточная биология началась со световой микроскопии, и этот метод до сих пор остается весьма эффективным
инструментом исследования, наряду с более современным устройствами для получения изображения, основанными на электронных пучках или
иных формах излучения. От пассивного наблюдения мы постепенно перейдем к методам, предполагающим активное вмешательство: рассмотрим,
как клетки различных типов могут быть отделены от ткани и при этом сохранять способность расти, узнаем, как клетки можно разрушить, а
клеточные органеллы и составляющие их макромолекулы выделить в чистом виде. И наконец, мы изложим суть технологии рекомбинантных ДНК,
благодаря которой стало возможным выделять, секвенировать и манипулировать генами и, следовательно, изучать механизмы их действия в клетке.
4.1. Микроскопия [1]
Диаметр типичной клетки животных составляет 10-20 мкм, что в пять раз меньше мельчайшей видимой частицы. Только с появлением
совершенных световых микроскопов в начале XIX века удалось установить тот факт, что все ткани животных и растений состоят из отдельных
клеток. Это открытие, обобщенное в форме клеточной теории Шлейденом и Шванном в 1838 году, знаменует собой начало клеточной биологии.
Будучи чрезвычайно малыми по размерам, животные клетки к тому же бесцветны и прозрачны; следовательно, открытие их основных
структур стало возможным благодаря разработке набора красителей в конце XIX столетия. Именно красители обеспечили достаточный контраст
для наблюдения субклеточных структур. Сходная ситуация наблюдалась в начале 40-х годов нашего столетия, когда изобретение мощного
электронного микроскопа потребовало новых методов сохра-
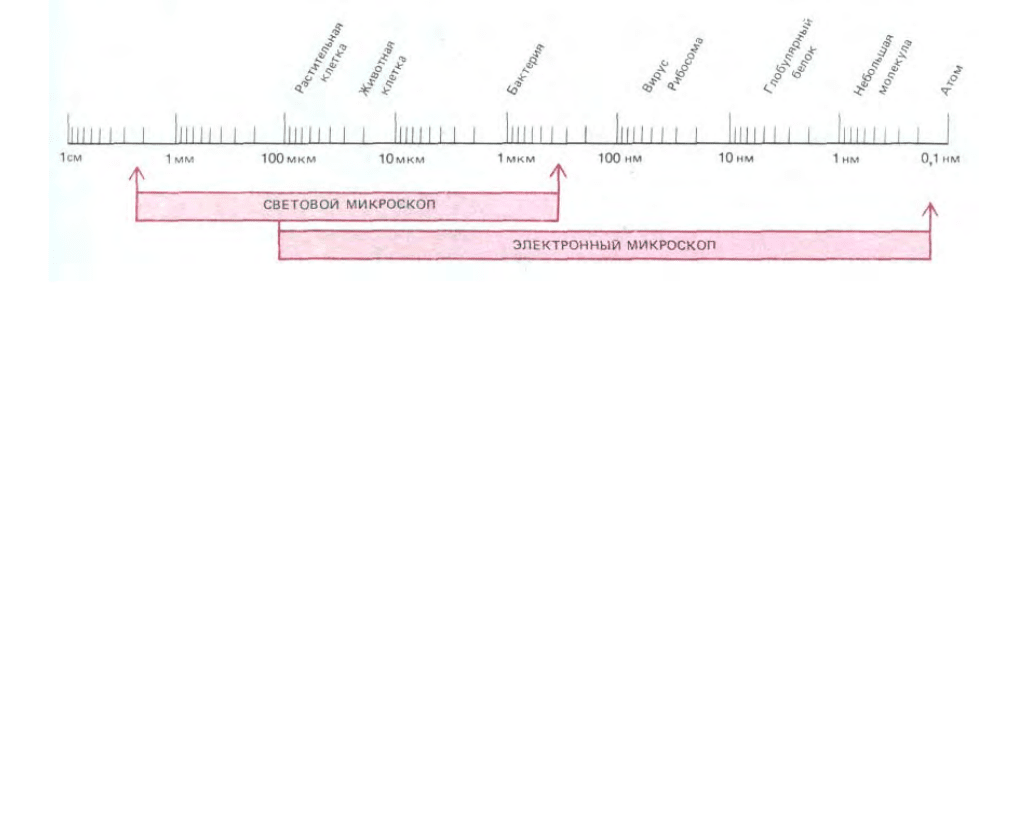
173
Рис. 4-1. Размеры клеток и клеточных компонентов, а также рабочие диапазоны светового и электронного микроскопа, изображенные в
логарифмической шкале. В микроскопии принято пользоваться следующими единицами длины: мкм (микрометр) 10
-6
м, нм (нанометр) - 10
-9
м,
0
A
(ангстрем) - 10
-10
м.
Таблица 4-1. Основные вехи в истории световой микроскопии
1611 - Кеплер (Kepler) предложил принцип создания сложного светового микроскопа
1655 - Гук (Hook) использовал сложный микроскоп для описания небольших пор в срезах пробки, названных им «клетками»
1674 - Левенгук (Leeuwenhoek) сообщил об открытии им одноклеточных. Спустя 9 лет он впервые увидел бактерии
1833 - Браун (Brown) опубликовал свои микроскопические наблюдения над орхидеями, в которых он четко описал ядро клетки
1838 - Шлейден и Шванн (Schleiden, Schwann) предложили клеточную теорию, согласно которой структурной и функциональной
единицей строения растений и животных является клетка, содержащая ядро
1857 - Колликер (Kolliker) описал митохондрии в мышечных клетках
1876 - Аббе (Abbe) проанализировал влияние дифракции на формирование изображения и показал возможность усовершенствования
конструкции микроскопа
1879 - Флемминг (Flemming) с большой точностью описал поведение хромосом во время митоза у животных клеток
1881 - Ретциус (Retzius) наиболее подробно описал многие ткани животных. В течение следующих 20 лет он, Кахал (Cajal) и другие
гистологи разработали методы окрашивания тканей и заложили основы микроскопической анатомии
1882 - Кох (Koch) для окрашивания микроорганизмов использовал анилиновые красители и идентифицировал бактерии, вызывающие
туберкулез и холеру. В течение последующих 20 лет другие бактериологи, в том числе Клебс и Пастер (Klebs, Pasteur),
выявили и описали возбудителей многих болезней, изучая окрашенные препараты под микроскопом
1886 - Цейсе (Zeiss), используя идею Аббе (Abbe), изготовил серию линз. Благодаря этому усовершенствованию, микроскописты смогли
различать структуры, размеры которых были соизмеримы с теоретическим пределом разрешения для видимого света
1898 - Гольджи (Golgi), окрашивая клетки азотнокислым серебром, впервые наблюдал и описал аппарат Гольджи
1924 - Лакассань (Lacassagne) и его сотрудники разработали первые методы радиоавтографии для выявления радиоактивного полония в
биологических образцах
1930 - Лебедев разработал и создал первый интерференционный микроскоп. В 1932 г. Зернике (Zernicke) изобрел фазово-контрастный
микроскоп. Эти два изобретения позволили наблюдать неокрашенные живые клетки и изучать их строение
1941 - Кунс (Coons) для выявления клеточных антигенов использовал антитела, связанные с флуоресцирующими красителями
1952
- Номарский (Nomarski) разработал и запатентовал систему дифференциального интерференционного контраста для светового
микроскопа, которая до сих пор носит его имя

174
Рис. 4-2. Интерференция световых волн. Если две световые волны совпадают по фазе, амплитуда результирующей волны возрастает и,
следовательно, яркость увеличивается. Если две световые волны не совпадают по фазе, они гасят друг друга и образуют волну, амплитуда которой
(а следовательно, и яркость) уменьшается.
Рис. 4-3. Эффект интерференции, который можно наблюдать при большом увеличении по краям твердого объекта, расположенного между
источником света и наблюдателем.
нения и окраски клеток. И только после того, как они были разработаны, начала проявляться вся сложность клеточной структуры, В основе
микроскопии как методологии до сих пор лежат способы приготовления образца и возможности самого микроскопа.
На рис. 4-1 сравниваются степени разрешения в современном световом и электронном микроскопах.
Основные этапы развития современной микроскопии перечислены в табл. 4-1.
4.1.1. С помощью светового микроскопа можно различить объекты, отстоящие друг от друга на 0,2 мкм [2]
В общем случае излучение данной длины волны может быть использовано для изучения только таких структур, минимальные размеры
которых еще сопоставимы с длиной волны самого излучения. Этот фундаментальный принцип ограничивает возможности любого микроскопа.
Предел разрешения светового микроскопа задается длиной световой волны, которая для видимого света лежит в пределах от 0,4 мкм (фиолетовый)
до 0,7 мкм (темно-красный). Из этого следует, что самыми маленькими объектами, которые еще можно наблюдать в световой микроскоп, являются
бактерии и митохондрии (их ширина ~ 0,5 мкм). Более мелкие элементы клетки искажаются эффектами, вызванными волновой природой света.
Чтобы понять природу этих эффектов, мы должны проследить за тем, что происходит со световыми волнами по мере их прохождения сквозь линзы
микроскопа.
Вследствие волновой природы света его луч не движется по идеально прямому пути, предсказываемому законами геометрической
оптики. В реальной ситуации световые волны перемещаются сквозь оптическую систему по множеству слегка отличающихся путей. Оптическая
дифракция обусловлена интерференцией световых волн, пути прохождения которых через оптическую систему несколько различаются. Если
световые волны точно совпадают по фазе, т. е. гребень одной соответствует гребню другой, а впадина одной - впадине другой, то они взаимно
усиливаются, и яркость возрастает. С другой стороны, если фазы волн не совпадают, они будут взаимно погашаться (рис. 4-2). Тень прямого края,
например, освещенного светом одной длины волны, при большом увеличении будет выглядеть как набор параллельных линий, тогда как округлое
пятно проявится в виде набора концентрических окружностей (рис. 4-3). По этой же причине отдельная точка выглядит в микроскопе, как яркое
пятно, а два ближайших точечных объекта дают перекрывающиеся изображения, которые сливаются в одно. Повышение точности обработки линз
не позволяет преодолеть это ограничение, поскольку оно задано волновой природой света.
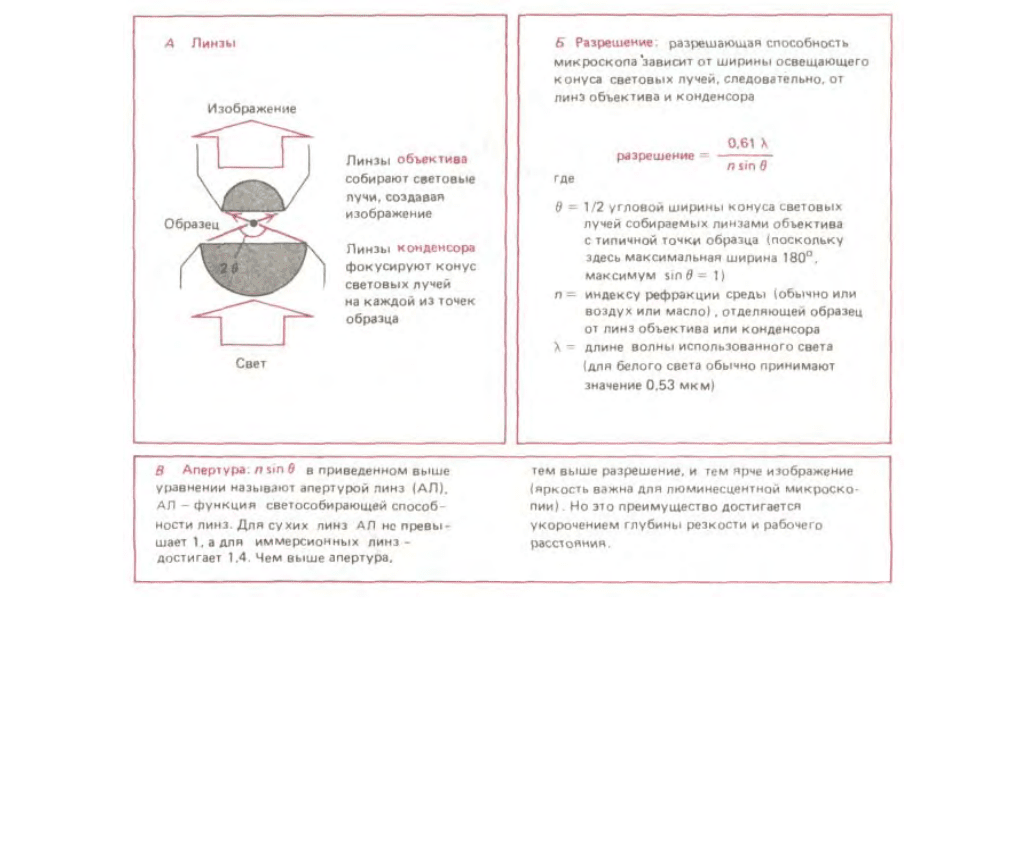
Предельное разрешение, при котором два объекта могут наблюдаться в отдельности, так называемый предел разрешения - зависит как от
волновой природы света, так и от апертуры использованной системы линз (рис. 4-4). В наиболее благоприятных условиях - при фиолетовом свете
(длина волны = 0,4 мкм) и апертуре 1,4 можно достигнуть теоретически возможного предела разрешения светового микроскопа около 0,2 мкм. Этот
предел был достигнут конструкторами микроскопов в конце XIX столетия (однако в современных микроскопах, производимых серийно,
достигается очень редко). И хотя изображение можно увеличить как угодно, например, проецируя его на экран, все же в световой микроскоп нельзя
разрешить два объекта, если они разделены расстоянием менее 0,2 мкм: такие объекты будут выглядеть как один объект.
Волновая природа света не всегда является помехой в изучении
175
Рис. 4-4. Направление движения световых волн, проходящих сквозь прозрачный образец в микроскопе. Иллюстрирует концепцию апертуры и ее
связь с ограничением разрешения.
клеток, позже мы увидим как интерференция и дифракция могут быть использованы для изучения живых неокрашенных клеток. Но сначала
необходимо обсудить методы получения постоянных препаратов клеток и то, как с помощью химических красителей можно улучшить
возможности наблюдения клеточных структур в таких препаратах.
4.1.2. Для проведения микроскопических исследований ткани обычно фиксируют и режут [2]
Для приготовления постоянного препарата, который можно окрасить и наблюдать в микроскоп, необходимо сначала обработать клетки
фиксирующим агентом с тем, чтобы иммобилизировать, убить и сохранить их. Используя химические термины можно сказать, что фиксация
повышает доступность клеток красителям; макромолекулы клеток скрепляются поперечными сшивками, что стабилизирует и закрепляет их в
определенном положении. Некоторые ранние методы фиксации включали обработку кислотами или органическими растворителями, например,
спиртом. В современных методах, как правило, используется обработка альдегидами, например, формальдегидом или глутаральдегидом, которые
формируют ковалентные связи со свободными аминогруппами белков и, таким образом, сшивают соседние молекулы.
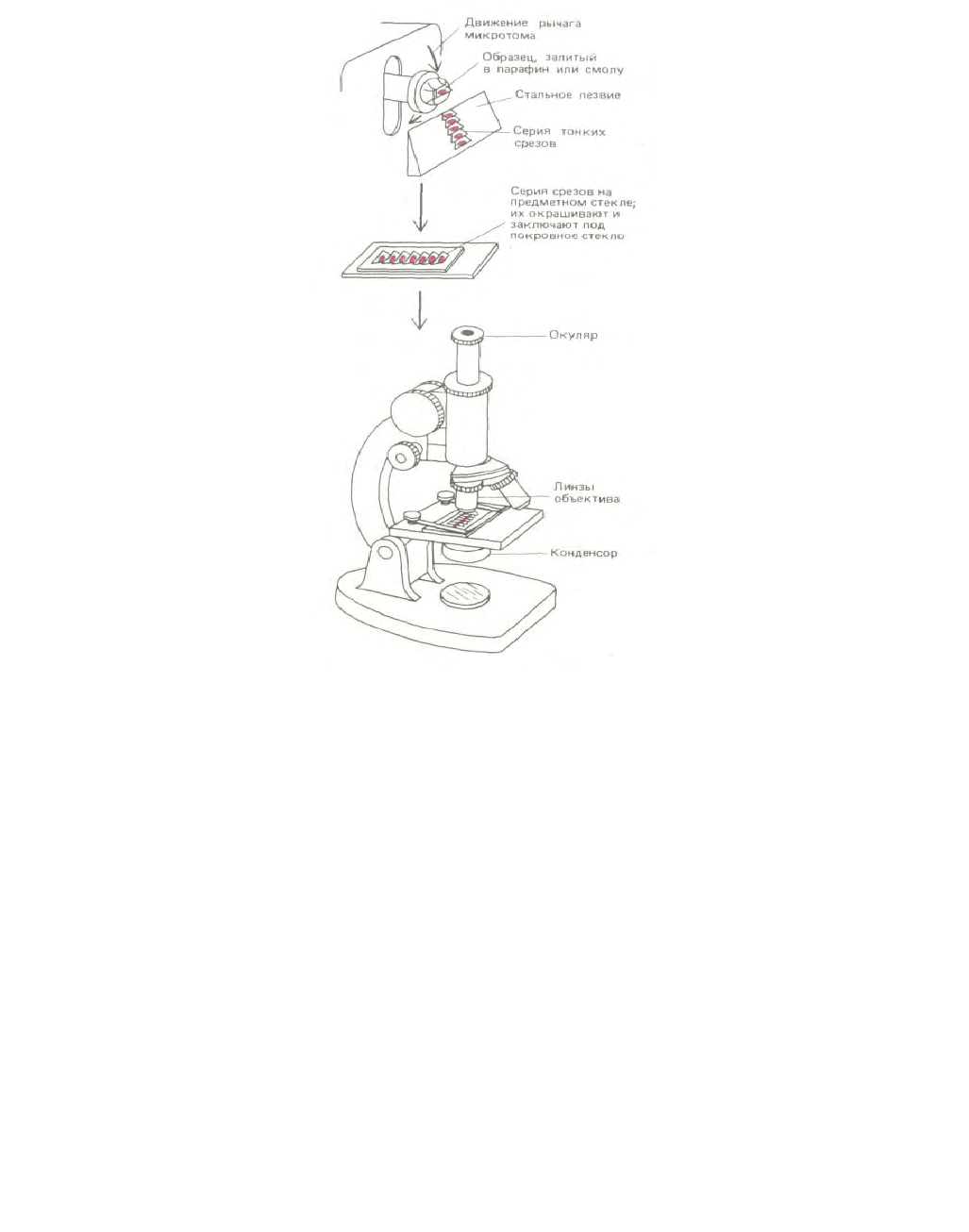
Толщина большинства образцов тканей слишком велика, чтобы можно было непосредственно изучать отдельные клетки при высоком
разрешении. Поэтому после фиксации ткани обычно режут на очень тонкие «ломтики»
(срезы) на микротоме: это прибор с очень острым
металлическим лезвием, который действует подобно хлеборезке (рис. 4-5). Срезы толщиной от 1 до 10 мкм помещают на поверхность предметного
стекла. Обычно ткани очень мягки и нежны даже после фиксации и их необходимо перед разделением на срезы
заключать в поддерживающую
среду. Как правило, в качестве заключающих сред используют парафин или специальную смолу. В жидком виде эти среды пропитывают и
окружают фиксированную ткань; затем они затвер-
176
Рис. 4-5. Приготовление среза на микротоме после заливки ткани. Срез предназначен для исследования с помощью светового микроскопа.
девают при охлаждении или за счет полимеризации, образуя твердый блок, который удобно резать на микротоме.
Существует серьезная опасность того, что процедуры фиксации или заключения могут повредить структуру клеток или клеточных
макромолекул. Вот почему предложен другой метод приготовления срезов, уменьшающий эту опасность, - быстрое замораживание. Здесь можно
обойтись без фиксации и заливки. Замороженную ткань просто режут на криостате - специальном микротоме, установленном в холодной камере.
Полученные таким образом срезы позволяют избежать некоторых артефактов, и в то же время обладают определенными недостатками: отдельные
структуры индивидуальных макромолекул, таких, например, как белки, при замораживании сохраняются хорошо, но структура самой клетки может
оказаться поврежденной. Следующий этап после приготовления срезов (любым из методов) - их окраска.
4.1.3. Различные компоненты клетки можно окрашивать по-разному [3]
В содержимом большинства клеток, состоящих, как правило, на 70% из воды, практически отсутствуют компоненты, способные
помешать прохождению световых лучей. Поэтому в естественном состоянии большинство клеток даже после фиксации и приготовления срезов
практически невидимы в обычном световом микроскопе. Одна из возможностей их увидеть состоит в окраске клеток красителями.
В начале XIX столетия ввиду потребности в красителях для текстильной промышленности, органическая химия переживала очень
плодотворный период. Оказалось, что некоторые из этих красителей также способны окрашивать биологические ткани. К удивлению
исследователей, некоторые из этих красителей обладали определенным сродством к специфическим компонентам клетки - ядру или митохондриям,
окрашивая их внутренние структуры и делая их доступными для изучения под микроскопом. В настоящее время известен широкий набор
органических красителей. Многие из них обладают весьма колоритными названиями: малахитовый зеленый, судан черный, кумасси голубой;
каждый краситель характеризуется сродством к определенным субклеточным компонентам. Например, краситель гематоксилин имеет сродство к
отрицательно заряженным молекулам и поэтому выявляет распределение в клетках ДНК и кислых белков. Химическая природа специфичности
многих красителей до сих пор неизвестна.
Накопление опыта в клеточной химии сопровождалось отбором наиболее рациональных и избирательных методов окраски и, в
частности, методов, позволяющих отличать специфические белки либо иные макромолекулы клеток. Здесь же возникла проблема
чувствительности. Поскольку большинство макромолекул представлены в клетках относительно незначительным числом копий, одна или две
молекулы красителя, связанные с макромолекулой, могут оставаться незамеченными. Один из путей разрешения этой проблемы состоял в
увеличении числа молекул красителя, ассоциированных с отдельными клеточными молекулами. По каталитической активности в клетках удалось
локализовать многие ферменты: при достаточном обеспечении соответствующим субстратом каждая молекула фермента создавала множество
молекул видимого продукта реакции. Альтернативный подход к проблеме чувствительности состоит в использовании флуоресценции. В данном
случае можно на темном фоне выявлять специфические красители по свету, который они и только они излучают, будучи соответствующим образом
возбуждены. Далее мы переходим к объяснению этого феномена.
177
4.1.4. Специфические молекулы могут быть локализованы в клетках с помощью флуоресцентной микроскопии [4]
Флуоресцирующие красители поглощают свет одной длины волны и излучают свет другой длины волны, более длинной. Если такое
вещество облучить светом, длина волны которого совпадает с длиной волны света, поглощаемого красителем, и затем для анализа использовать
фильтр, пропускающий свет с длиной волны, соответствующей свету, излучаемому красителем, флуоресцирующую молекулу можно выявить по
свечению на темном поле. Высокая интенсивность излучаемого света является характерной особенностью таких молекул. Применение
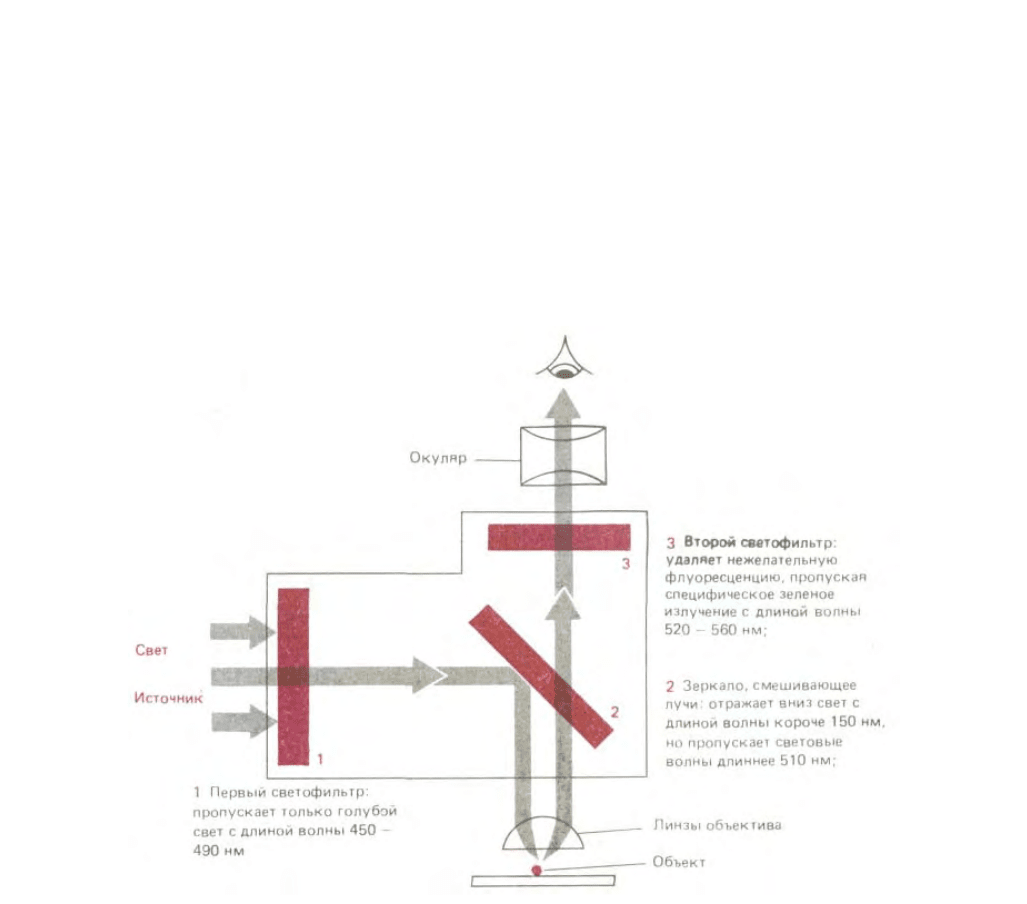
флуоресцирующих красителей для окраски клеток предполагает использование специального флуоресцентного микроскопа. Такой микроскоп
похож на обычный световой микроскоп, но здесь свет от осветителя, излучаемый мощным источником, проходит через два набора фильтров - один
для задержания света перед образцом и другой для фильтрации света, полученного от образца. Первый фильтр выбран таким образом, что он
пропускает свет длины волны, возбуждающей определенный флуоресцирующий краситель; в то же время второй фильтр блокирует этот падающий
свет и пропускает на окуляр свет длины волны, излучаемой красителем при его флуоресценции (рис. 4-6). Флуоресцентная микроскопия часто
используется для выявления специфических белков или других молекул, которые становятся флуоресцирующими после ковалентного связывания с
флуоресцирующими красителями. Например, флуоресцирующие красители могут быть связаны с молекулами антител, что сразу же превращает их
в высокоспецифические и удобные красящие реагенты, селективно связывающиеся со специфическими макромолекулами на поверхности живой
либо внутри фиксированной клетки (см. разд. 4.5.3). Для этой цели обычно используют два красителя - флуоресцеин, который дает интенсивную
желто-зеленую флуоресценцию после возбуждения светло-голубым светом, и родамин, обусловливающий темно-красную флуоресценцию после
возбуждения желто-зеленым светом (рис. 4-7). Применяя
Рис. 4-6. Оптическая система современного флуоресцентного микроскопа состоит из двух избирательных фильтров и дихроического
(смешивающего лучи) зеркала. Здесь указан набор фильтров, используемых для выявления свечения флуоресцеина. Для микроскопа этого типа
важно наличие в объективе линз, характеризуемых высокой апертурой, так как для данного увеличения яркость изображения пропорциональна
одной четвертой апертуры (см. также рис. 4-4).
178
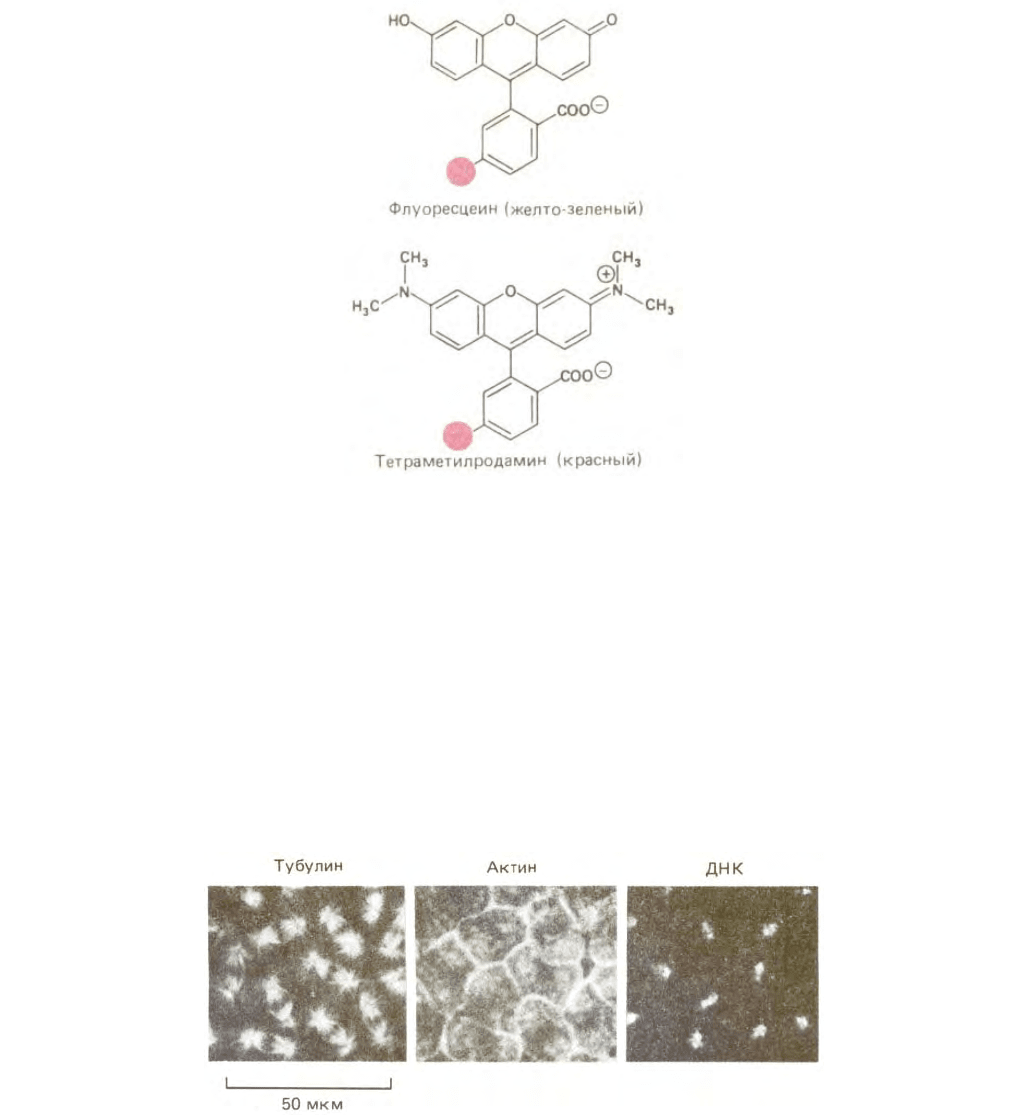
Рис. 4-7. В флуоресцентной микроскопии обычно используют два красителя флуоресцеин и тетраметилродамин, структуры которых представлены
на данном рисунке. Флуоресцеин излучает желто-зеленый свет после активации светом соответствующей длины волны. Родамин излучает красный
свет. Часть молекулы, обозначенная цветом, указывает на расположение химически активной группы; в этом положении, как правило, формируется
ковалентная связь между красителем и белком (или иной молекулой). В настоящее время промышленность выпускает несколько вариантов этих
красителей с различными типами реакционно-активных групп, что позволяет «нацелить» этот краситель либо на SH-группы, либо на NН
2
-группы
белка.
для окраски флуоресцеин и родамин, можно изучать распределение различных молекул, два вида молекул будут выявляться в микроскопе после
простого переключения двух наборов фильтров, каждый из которых специфичен для одного из красителей (рис. 4-8).
Далее мы переходим к обсуждению новых важных методов, которые позволяют использовать флуоресцентную микроскопию для анализа
изменений концентрации и расположения специфических макромолекул в живых клетках (разд. 4.1.9).
4.1.5. Фазово-контрастный и интерференционный микроскопы позволяют изучать живые клетки[2]
Возможность потери или нарушения образцов в процессе их приготовления всегда беспокоила микроскопистов. Единственный способ
решить эту проблему состоит в изучении живых клеток без фиксации или замораживания. Для этой цели очень полезны микроскопы со
специальными оптическими системами.
При прохождении света через живую клетку фаза световой волны меняется согласно коэффициенту рефракции клетки: свет, проходящий
через относительно тонкие или относительно толстые участки клетки, такие, как ядро, задерживается, и его фаза соответственно сдвигается по
отношению к фазе света, проходящего через относительно тонкие
Рис. 4-8. Флуоресцентная микрофотография участка поверхности ранних эмбрионов Drosophila, микротрубочки которых были помечены
антителами, связанными с флуоресцеином
(слева), а актиновые филаменты - антителами, меченными родамином (в центре). Кроме того,
хромосомы были помечены третьим красителем, который флуоресцирует, только связавшись с ДНК
(справа). На этой стадии все ядра эмбриона
расположены в общей цитоплазме и не разделены клеточными стенками; они находятся в метафазе митоза. Все три микроснимка были сделаны с
одного участка фиксированного эмбриона с использованием трех различных наборов фильтров в флуоресцентном микроскопе (см. также рис. 4-6).
(С любезного разрешения Tim Carr).
179
Рис. 4-9. Два способа увеличения контраста в световой микроскопии. А. Окрашенные участки клетки уменьшают амплитуду проходящих световых
волн определенной длины. В результате можно получить окрашенное изображение, видимое при прямом наблюдении. Б. Амплитуда световых
волн, проходящих через неокрашенную живую клетку, практически не меняется; поэтому многие детали нельзя увидеть при прямом наблюдении.
Здесь, однако, имеет место изменение фазы проходящего света явление, используемое в фазово-контрастном и интерференционном микроскопах
для получения высококонтрастного изображения.
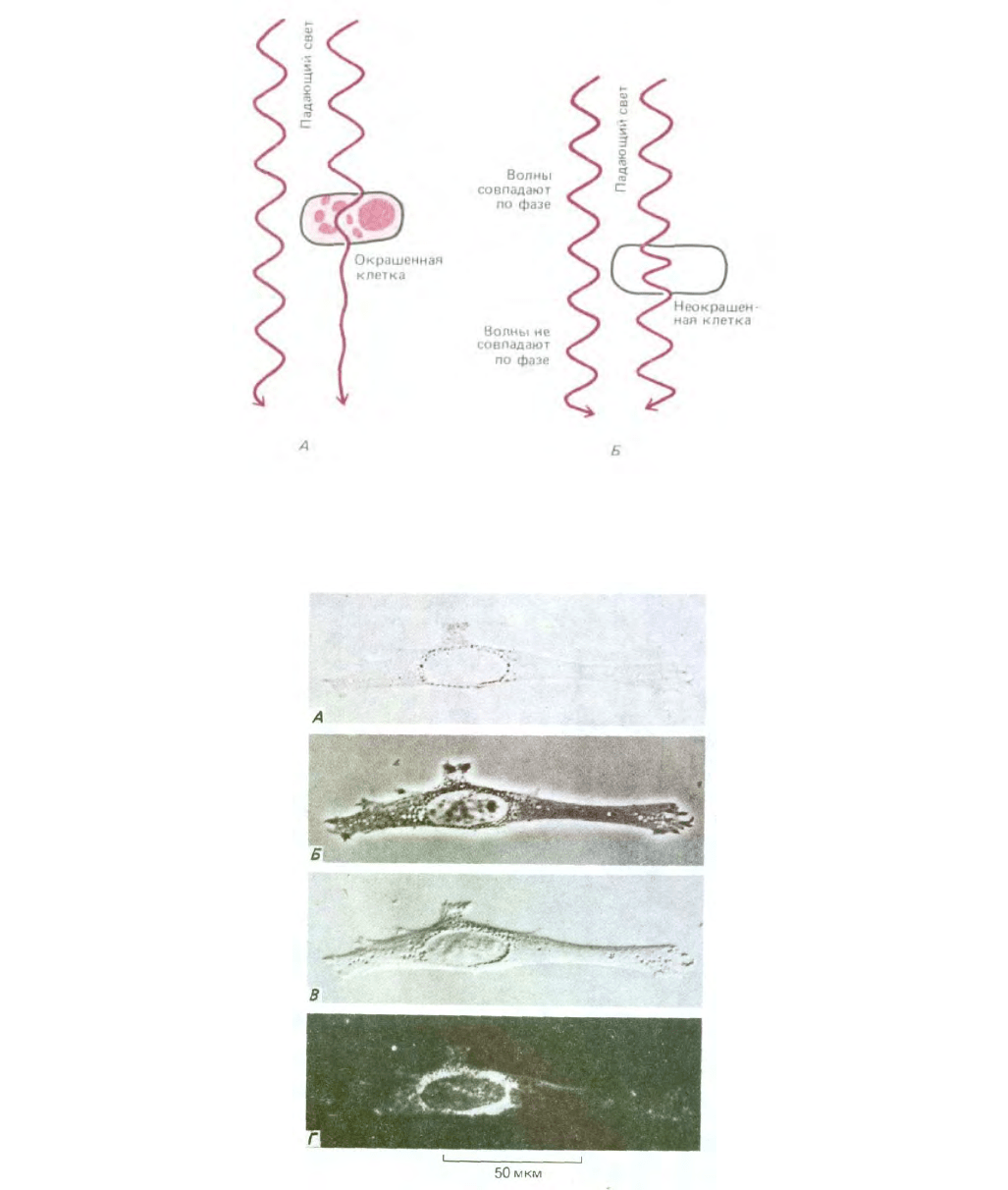
Рис. 4-10. Фибробласт в культуре ткани при наблюдении с помощью четырех различных типов световой микроскопии. А. Изображение получено
при прямом прохождении лучей через клетку (микроскопия в светлом поле). Остальные изображения получены с помощью методов,
рассматриваемых в тексте: Б
-фазово-контрастная микроскопия; В -интерференционная микроскопия; Г микроскопия в темном поле. Простая замена
компонентов оптики большинства современных микроскопов позволяет получать все четыре типа изображения.
180
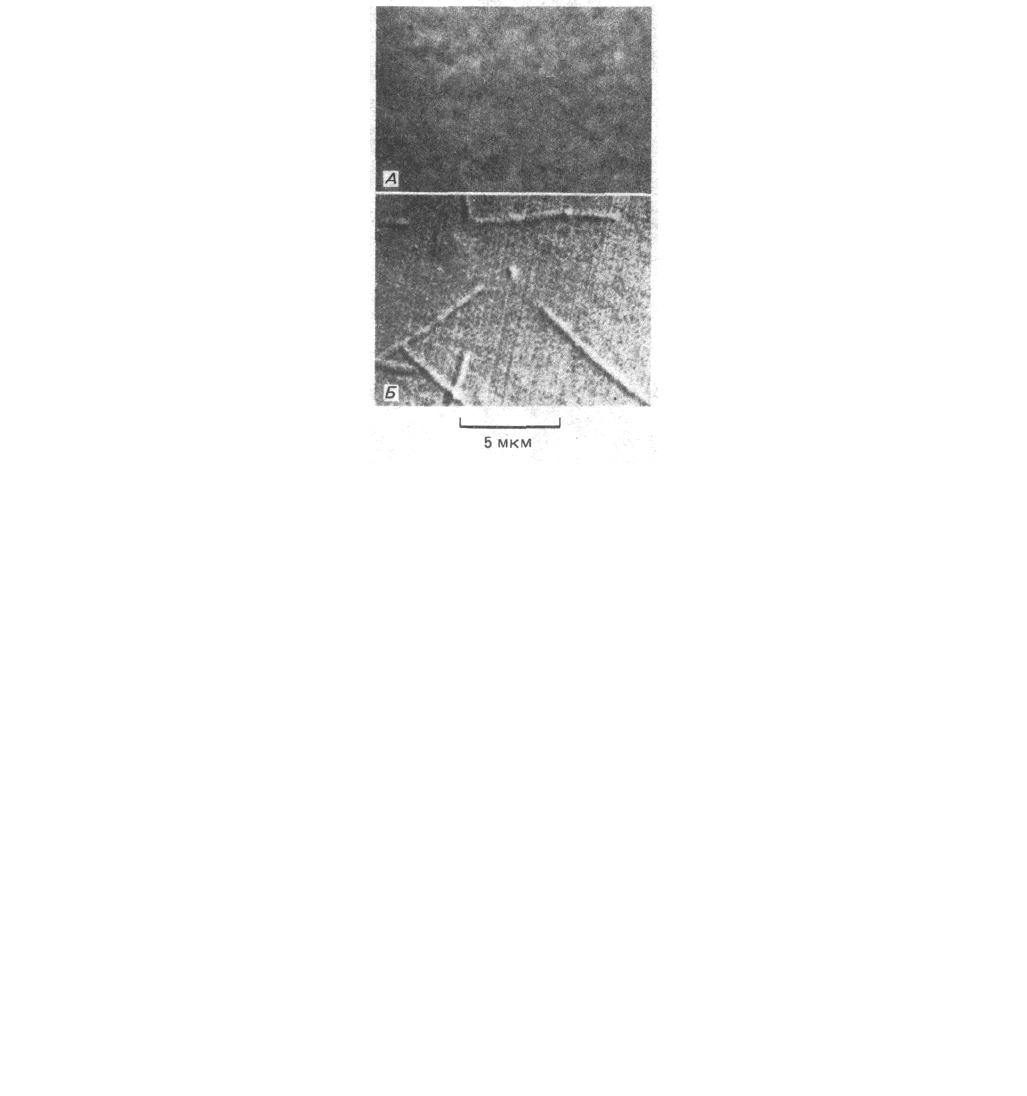
Рис. 4-11. Изображение неокрашенных микротрубочек, наблюдаемое в интерференционном микроскопе. А. Исходное необработанное изображение;
Б. Изображение, полученное после электронной обработки, существенно увеличивающей контраст и снижающей «шум». Хотя диаметр
микротрубочек всего 0,025 мкм, вследствие дифракции они выглядят, как значительно более толстые филаменты. (С любезного разрешения Вruсе
Sohnapp.)
участки цитоплазмы. Как в фазово-контрастном, так и в интерференционном микроскопе используются эффекты интерференции, возникающие
при рекомбинации двух наборов волн, которые и создают изображение клеточных структур (рис. 4-9). Оба типа световой микроскопии широко
используются для наблюдения живых клеток.
Простейший способ разглядеть детали клеточной структуры - наблюдать свет, рассеивающийся различными компонентами клетки. В
темнопольном микроскопе лучи от осветителя направляются сбоку и при этом в линзы микроскопа попадают только рассеянные лучи.
Соответственно клетка выглядит как освещенный объект на темном поле. Изображение одной и той же клетки, полученное четырьмя способами
световой микроскопии, показано на рис. 4-10.
Одним из основных преимуществ фазово-контрастной, интерференционной и темнопольной микроскопии является возможность
наблюдать движение клеток в процессе митоза и миграции. Клеточные движения, как правило, совершаются очень медленно и их сложно
наблюдать в реальном времени. В этом случае используют покадровую (цейтраферную) микрокиносъемку или видеозапись. Последовательные
кадры при этом разделены во времени, но при воспроизведении записи с нормальной скоростью картина реальных событий ускоряется.
4.1.6. Изображение можно усиливать или анализировать с помощью электронных методов [5]
В последние годы видеокамеры и соответствующие технологии обработки изображения значительно увеличили возможности световой
микроскопии. Благодаря их применению удалось не только преодолеть трудности, связанные с несовершенством оптической системы, но и решить
проблемы, обусловленные особенностями физиологии человека. Они состоят в том, что:
1) глаз не регистрирует очень слабый свет;
2) глаз не способен фиксировать небольшие отличия в интенсивности света на ярком фоне.
Первая из этих проблем была преодолена после присоединения к микроскопу сверхвысокочувствительных видеокамер (подобных тем,
которые используются при ночных съемках). Это позволило наблюдать клетки в течение длительного времени при низкой освещенности, исключая
длительное воздействие яркого света (или тепла). Системы усиления изображения особенно важны для изучения в живых клетках
флуоресцирующих молекул.
Поскольку изображение создается видеокамерой в форме электронных сигналов, его можно соответствующим образом преобразовать
в числовые сигналы, направить в компьютер и затем подвергнуть дополнительной обработке для извлечения скрытой информации. Эти и
подобные методы обработки изображения позволяют компенсировать оптические недостатки микроскопов и практически достичь предела
разрешения. Более того, используя современные видеосистемы, контраст может быть усилен настолько, что преодолеваются ограничения глаза
в детектировании небольших отклонений интенсивности света. Хотя этот процесс усиливает случайные отклонения фона в оптической
системе, такой «шум» может быть устранен специальными методами. Таким образом, благодаря современным подходам мы получили
возможность анализировать прозрачные объекты, ранее не отличимые от фона.
Высокий контраст, достижимый с помощью компьютерной интерференционной микроскопии, позволяет наблюдать даже очень мелкие
объекты, как, например, отдельные микротрубочки (рис. 4-11), диаметр
