Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.


62
Chapter
3
Then the injector body is rapidly heated
so
that each sample constituent is
eluted, in turn, onto the column inlet (which is
10-20°C
below the boiling point
of the solvent) where it condenses. After an initial period in which all the sample
components are transferred to the column, the oven temperature program is
started.
This technique avoids the interaction of the stationary phase with the injected
sample, associated with the splitless and on-column injectors.
Also,
after flash
vaporization, the solvent and sample are condensed as a narrow band within a
short distance of the column inlet. This optimizes the solvent effect and leads to
quantitative separation over
a
wide molecular weight distribution. There
is
no
need for a retention gap with its associated connector problems at high oven
temperatures. However, there can be
a
deposition
of
components in the split
outlet and septum purge if they are not heated
or
insulated correctly. These re-
quire regular cleaning with solvent followed by oven drying.
The advantages of the PTV injector are:
1.
Any
size of capillary column can be easily fitted into the glass insert.
These range from wide bore columns for rapid analysis of polywaxes, to
the latest microbore columns used to obtain detailed research information
regarding wax blends.
2.
An
ordinary domed tip syringe with a tapered needle (which does not
damage the prepierced high temperature septum during injection) can be
used. This minimizes the chance of septum pieces being flash vaporized
onto the column, causing ghost peaks. The problems associated with silica
needles (i.e. backflushing and needle contamination of the column) do not
arise and the system can be easily automated.
3.
The sample components vaporize sequentially, reducing the need
for
a
large volume glass injector liner (which is required for classical splitless
injection) and decreasing sample flashback, septum contamination, peak
splitting and solvent tailing to a minimum
[28].
Also
any involatile mate-
rial collects on the glass
or
quartz wool insert, which can easily be
changed.
4.
It
is
a versatile injector, being capable of split, splitless and solvent purge
modes. Therefore, a wide range of samples can be catered for. In the split
mode the response factor for alkanes appears to be linear over the whole
boiling point range.
As
there is a definite difference between the boiling
points of the solvent and the sample components, the solvent purge mode
can be used
for
large volume injections of dilute solutions.
The disadvantages of the PTV injector are:
1.
Dirt can accumulate in the insert and this needs to be regularly changed to
eliminate the occurrence of “ghost” peaks.
The chromatographic analysis ofrefined
and
synthetic waxes
63
2. If the PTV injector is used in the splitless mode, it is important that the
vaporizing chambers is not too small to take the large volume of solvent
vapour
from
a
1p1
injection. Otherwise the solvent vapour can flow
backwards into the septum region or the carrier gas supply. It has been
recommended that the vaporizing chamber should have
an
internal vol-
ume of 1 ml [29]. Another alternative is to initially vaporize most of the
solvent at a low temperature with the split valve open, then close the
valve for the sample vaporization stage [13]. However, this reduces the
solvent effect.
Comparison of on-column with PTV injection (both split and splitless) for the
quantitative analysis of alkane standards up to C44 shows little difference in the
relative response factors. Initial comparative investigations between the two in-
jection techniques used to separate compounds above C60 indicated that the
PTV in the splitless mode gave significant losses of higher boiling components
[
181. This was later attributed to opening the PTV split valve too soon after in-
jection (i.e. before complete vaporization of the high boiling components)
[30].
Hinshaw and Ettre [3
11
investigated this effect. They used Polywax
655
to show
that the PTV and on-column injectors can give equivalent recoveries of alkanes
up to C78 if the PTV split valve is initially closed for
4
min (although later re-
search found even this time too short).
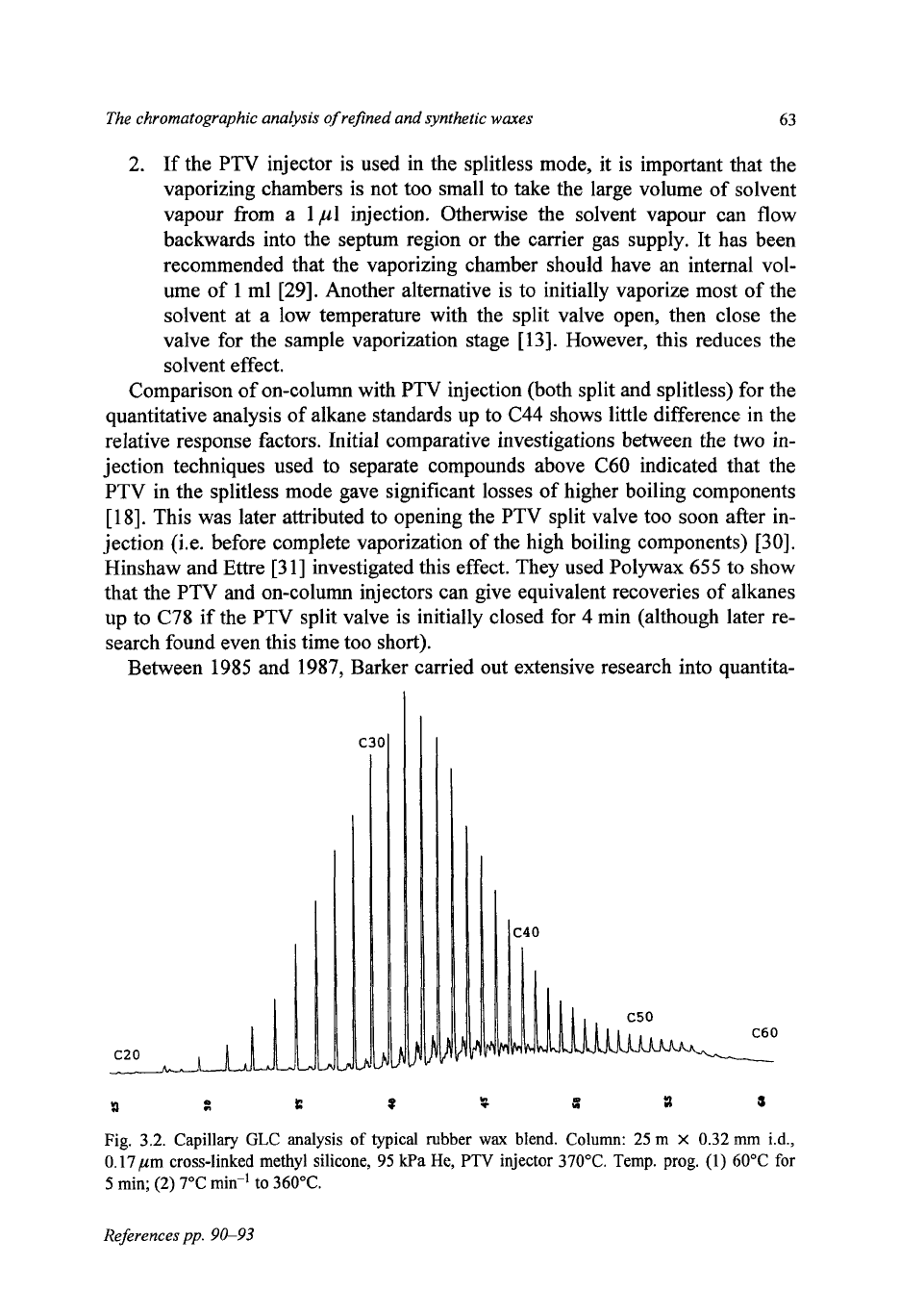
Between 1985 and 1987, Barker carried out extensive research into quantita-
*
P
c
k
t
u'
x
J
Fig.
3.2.
Capillary
GLC
analysis
of
typical rubber
wax
blend.
Column:
25
m
x
0.32
mm
i.d.,
0.17
,um cross-linked methyl silicone,
95
kPa
He,
PTV
injector
370°C.
Temp. prog.
(1) 60°C
for
5
min;
(2) 7°C
mid
to
360°C.
References pp.
90-93

64
Chapter
3
tive HTGLC. This included investigating the best operating conditions for using
the PTV injector
for
separating refined and synthetic waxes
[13,32].
A
PTV in-
jector was used up to 450°C in the split, splitless and solvent flush mode to sepa-
rate alkanes to above
C70
(Fig.
3.2).
It was found that the PTV had to be set to
20°C
above the maximum column temperature for
4
min to ensure that all the
components of any hydrocarbon wax were transferred onto the column (which
was kept at
60°C
for
this stage).
To
improve this situation additional insulation
was placed around the injector body (particularly around the split outlet from the
injector body).
For
quantitative analysis in the splitless mode, the split valve was
opened only after
9
min.
For
the analysis
of
dilute samples
of
synthetic waxes,
the solvent flush mode was used. Initially the solvent is removed through the
split valve (using
a
PTV temperature of 80°C for
1
min) before vaporizing the
components
onto
the column. This allowed the injection
of
a
large sample vol-
ume without the solvent tail affecting the baseline in the region of the first com-
ponent peaks.
This work was amplified and extended by Tipler and Johnson
[33],
who used
Polywax
500
and a microcrystalline wax to investigate the optimization of a
PTV
in the split mode. They found that, as well as PTV temperature, the PTV
heating period definitely affects mass discrimination, peak shape and peak dis-
persion (for Polywax
500
an optimum
of
20
min was required). The importance
of only opening the split valve after a significant time (at least
10
min) and other
effects (detector temperature, make-up gas, temperature program and carrier gas
flow) were investigated. Recently, a practical guide to using the PTV has been
published
[34].
Ai Instruments, Cambridge, have produced
a
PTV injector ca-
pable of use up to 600°C. This now enables all the components of Polywax
1000
to be rapidly eluted onto a high temperature column without discrimination
[35].
3.2.2.2
Detection
The flame ionization detector (FID.)
is
commonly used for the analysis of
waxes. However, the new atomic emission detectors has been used
for
the GC
analysis of polymer additives
[36].
Mass-spectroscopy could also be used, but is
limited by the maximum temperature
of
the transfer line from the GC, the maxi-
tnum mass number detectable by the instrument and by the expense. The FID is
mass flow sensitive and the response of hydrocarbons
is
proportional to the
number of carbon atoms passing through the flame at a given time.
For
quantita-
tive analysis it is important that the FID conditions are controlled to give linear-
ity
of
response for all the eluted alkanes (i.e. no mass discrimination).
GC
instrumentation designed for packed columns can be converted for capil-
lary
GC.
However, make-up gas (usually nitrogen) is required to obtain the best
flow
for unbiased detection.
For
accurate quantitative analysis of high melting
waxes, the capillary
GC
FID requires
to
be designed for low flow at high tem-

The chromatographic analysis
of
refned and synthetic waxes
65
peratures. Adding make-up gas to the FID may improve the chromatography of
microcrystalline waxes
[3
I].
The column should end near to the tip of the detector to minimize any high
temperature catalytic cracking on metal surfaces. It has been claimed [37] that, at
high FID temperatures, thermionic emission also takes place on the metal tip
leading to increased background noise. The same paper also suggests that the
higher boiling components
of
synthetic waxes may be adsorbed onto the metal
FID surface, leading to peak tailing. However, this seems to be improbable con-
sidering the high detector temperatures used. Some detectors now have ceramic
tips to overcome these problems.
The FID should be kept at least 10°C higher than the maximum programmed
column temperature to avoid the higher boiling components from condensing out
on any detector surfaces. To eliminate condensation,
it
is also important that the
detector is well insulated
so
that there are no cold spots before the FID jet. Most
FID detectors are only designed to reach a maximum temperature of 450"C, even
although the associated GC oven can be programmed to 500°C. The practical
temperature program maximum
is
440°C. In
1988,
Carlo Erba produced the Wax
Analyser with an FID detector designed for HTGC, and
a
few other companies
have now followed their lead.
Wax materials are complex, containing many constituents that elute close to-
gether and are often not completely resolved. These rapidly eluting components
require a detector with a short time constant
so
that all the peaks are detected
without distortion. A time constant of 0.1
s
or less is required to separate waxes.
Signal filtration
is
then important to remove detector noise [38], particularly
when it is used at high temperatures.
3.2.2.3
High
temperature
GLC
columns
The only limitation to the GLC separation of refined waxes and synthetic
waxes is thermal cracking, which starts under normal atmospheric conditions at
400°C [39,40]. At 500"C, up to
90%
of
an alkane component in a wax may de-
compose (the thermal stability of alkanes decreases with increase in molecular
weight). However, as GLC analysis uses hydrogen, helium or nitrogen, this ef-
fect
is
reduced and the maximum practical separation temperature of alkanes is
probably between 450 and 500°C.
The main problems associated with the HTGLC separation of alkanes are the
stability of the stationary phase and the column during temperature program-
ming. Above 250"C, packed column non-polar stationary phases start to degrade
and above 300°C, bonded non-polar stationary phases on capillary columns tend
to degrade. This column bleed is reduced by deactivating the surface of the sup-
port material (which has to be a high purity silica) and by using a thermally sta-
ble stationary phase. High temperature non-polar bonded phases can now be
References
pp.
90-93

66
Chapter
3
used to greater than
450°C.
Metal capillary columns were used originally to
separate high boiling petroleum fractions, but high stationary phase bleeding
above
350°C
limited their use. These were followed by pyrex columns, but the
impurities in the glass led to a rapid increase in stationary phase bleed above
400°C.
Simultaneously, fused silica columns (made of high purity silica) were
introduced, externally coated with a polyimide. Polyimide coating has been im-
proved over the years
so
that fused silica columns can now be used up to
420°C.
However, columns coated with aluminium have now extended working tempera-
tures beyond
440°C.
During the early
1980s,
the major advances in column technology were made
by those researching into simulated distillation techniques. In
1982,
Szafranek et
a1 investigated the separation of high boiling crude oil alkane fractions using
Dexil
300
coated on a pyrex capillary column, with mass spectrometry detection
1411.
They tested the thermal stability of this type of column to
410°C
and es-
tablished that the separation for each alkane below
C30
was in the sequence
branched chain hydrocarbons before straight chains, followed by cycloalkanes.
In
1984,
Luke and Ray
[42]
used a short pyrex column, coated with a thin film of
OV-1,
to separate Polywax
500
up to n-C70 by programming the
GC
oven to
400°C.
It
was found that above
400"C,
the stationary phase bleed levels in-
creased rapidly, until at
420°C
decay occurred. This technique was developed
further by Munari
et
al.
[18,43].
They coated a thin film
of
dimethyl silicone
onto
5-10-m
long, wide-bore pyrex columns to separate Polywax
655
up to n-
C
120
at
430°C.
Pyrex columns tend to lose stationary phase at an exponential rate as the col-
umn temperature reaches
400°C.
This is due to the trace metallic oxides in the
pyrex causing instability at the stationary phase interface. With high purity fused
silica columns this effect is removed and any bleed problems are mainly due to
instability in the stationary phase itself. Although fused silica columns and
chemically bonded stationary phases were introduced in
1979 [44,45],
it was not
until
1986
that wax chromatography methods using these columns above
350°C
were reported.
Perkin Elmer published
[46]
the results of an investigation into the use of
their PTV injector, combined with a
25
m
x
0.25
mm i.d. fused silica column
(coated with a
0.1
ym methyl silicone bonded phase). The
GC
was temperature
programmed to
3 70°C
to qualitatively separate microcrystalline waxes into
straight and branched chain alkanes up to approximately
C55,
and total alkanes
to
C68.
However, the results were not much of an improvement on the work
carried out
13
years previously by Tulloch
[9],
who used a
short
column packed
with Dexil
300
and a temperature program to
400°C
to separate alkanes to the
same carbon number. Above
370"C,
the standard polyimide coatings tend to
thermally degrade leaving small bare patches
of
fused silica, causing the capil-

The chromatographic analysis
of
reJined and synthetic
waxes
67
lary column
to
disintegrate. Few standard stationary phases could be used in
fused silica columns above 370°C at this time.
At this point, Lipsky and Duffy published their work on the manufacture and
use of aluminium-coated hsed silica columns containing a new bonded phase,
capable of application up to
450°C
for a limited time
[47,48].
As
the fused silica
column was drawn from its furnace, it entered a molten metal coating chamber.
On cooling this gave a smooth, bonded coating of aluminium approximately
20,um thick.
A
new bonded methyl polysiloxane phase and coating procedure
was developed to enable separations to be carried out up to
450°C.
They used a
15
m
x
0.2
mm i.d. column coated with a
0.1
pm bonded phase to separate
Polywax
655
to above
C90.
A
1986
Quadrex Update Bulletin showed the same
type of column, but only
8
m long, being used to separate Polywax
1000
up to
approximately
C 120.
There were initial problems with this technology, due to columns becoming
brittle after continuous use and spontaneously breaking. Improvements have
been made in the coating process
so
that the columns are less likely to disinte-
grate (only tending to break if repeatedly heated and cooled at a rapid rate).
SGE
have developed
a
carborane-modified polysiloxane stationary phase which cross-
links on being coated inside an aluminium clad fused silica column
[19].
Useful
work was carried out in investigating the changes in gas velocity up to
450°C
for
0.22,
0.32
and
0.53
mm i.d. columns. It was found that, at 70 kPa hydrogen pres-
sure, an optimum flow of
50
cm/s at
100°C
produced maximum resolution over
the whole temperature range. These stable
HT5
series of columns are capable of
continuous operation at
460°C.
They have been programmed to
480°C
to sepa-
rate Fischer-Tropsch wax (near to theoretical maximum temperature for the
GLC analysis of alkanes).
SGE
have shown that the
0.53
mm i.d. columns can be
used to separate Polywaxes and the
0.32
mm i.d. columns can separate mi-
crocrystalline wax. However, as with most HTGLC column research, no atten-
tion has been given to the temperature program and flow conditions for quanti-
tative analysis.
Barker found that high quality polyimide columns could be externally condi-
tioned, by slowly increasing the maximum column temperature,
so
that the
polyimide further cross-linked. In this way, it was possible to regularly tempera-
ture program a
15
m
X
0.25
mm diameter polyimide-coated column to
400°C
for
accurate quantitative analysis of microcrystalline waxes and some synthetic
waxes
[13].
Chrompack U.K. then announced their new high temperature poly-
imide column, coated with a high temperature methyl silicone stationary phase
[49].
This was produced as an alternative to the metallized columns for HTGLC
up to
450°C.
In comparison to aluminium coated columns, these columns were claimed to
be less fragile after repeated use at high temperature. AIso it is claimed that
References
pp.
90-93

68
Chapter
3
aluminium coated columns tend to work loose in ferules (due to the expansion
coefficient). Aluminium coated columns are conductive, therefore they have to
be carefully placed in the FID
(or
a silica column placed between the aluminized
column and the detector). It
is
also impossible to judge the internal quality of
used aluminium coated columns, whereas polyimide columns are transparent.
However, above 40OoC, polyimide hardens and becomes brittle, giving it a lim-
ited life-time. These factors are important in making a final, practical choice of
column.
A
recent development in column technology has been the re-introduction of
metal capillaries, following on from the work of Takayama
et
al.
[50]. They pro-
duced a metal column coated with a polydimethyl siloxane with hydroxyl end
groups, capable
of
use up to 430°C (although it bleeds heavily when used above
350°C). Buyten
er
a/.
[51]
improved the technology by coating
a
deactivated
stainless steel metal surface with
a
series of bonded layers, culminating in the
high temperature stationary phase (see Fig. 3.3). This research resulted in the
Chrompack Unimetal columns
[52],
which can be continuously used for 32 h at
450°C with little effect on the capacity factors. They were designed for simu-
lated distillation use (e.g. the separation of Polywax
1000
up to 450°C). How-
ever, a 25
m
X
0.25 mm column coated with a 0.1 pm
SIM.
DIST. phase has
been used to separate slackwax to 375°C. Metal columns used for HTGLC are
now
a serious alternative to both aluminium and polyimide-coated columns.
In
1992,
the Restek Corporation introduced a new type of HTGLC column
(Fig.
3.3).
It was produced by depositing a thin film of fused silica onto the in-
side of stainless steel tubing followed by a thin deactivating polymeric layer,
which
is
then coated with a bonded high temperature stationary phase. This pro-
duces a tough, inert, thermally conductive column that can be continuously used
above 400°C.
A
paper by Schuyler
et
al.
1531 showed that, as wide bore col-
umns, they can be used to separate Polywax
655
and
1000
up to 445°C.
A
0.28 mm i.d. column coated with a 0.25
,urn
non-polar high temperature phase
is
available, but nothing has yet been published on the use of these columns for
quantitative wax analysis.
METAL
TUB
PRIMARY
CO
FUSED
SILI
NTERMEDIATE
EACTIVATION LAYER
CHEMICALLY BONDED PHASE
Fig
3
3.
Cross-sections
of
current metal columns. (a) Chromopak unimetal column;
(b)
Restek
MXT
column

The chromatographic analysis
of
refined and synthetic waxes
69
Desty
et
al.
[54]
were the first to use long open tubular columns to carry out
high speed separation of petroleum products. Since the early
1980s
there has
been an interest in microbore columns (ranging from 0.18 to
0.02
mm. i.d.) for
high speed GC
[S].
In
1988,
MicroQuartz introduced polyimide coated fused
silica microbore columns, from
0.02
mm internal diameter upwards, for use with
high temperature stationary phases
[56].
They are claimed to endure continuous
use for
80
h at 400°C to
10
h at
450°C.
Although there are many advantages in
using these columns, there are also problems associated with their practical ap-
plication. The advantages are:
Increase in column efficiency with decrease in internal diameter.
A
10
m
x
0.10
mm i.d. column may have up to
100
000
theoretical plates. Highly
complex microcrystalline waxes can be resolved to the baseline, leading
to
accurate quantitative analysis.
Increase in sensitivity, because of the sharp peaks obtained. This means
that low solubility synthetic waxes can be injected at lower con-
centrations, reducing injection problems.
Decrease in column bleed due to the small amount
of
stationary phase in
the column. This reduces baseline problems at high temperature to a
minimum.
High speed analysis, which reduces the retention time of components.
With narrow bore columns, the theoretical plate height
(H)
varies little
with increase in linear velocity
(u).
Therefore, high carrier gas velocities
can be used to reduce the elution temperature of high boiling point al-
kanes (lowering the maximum program temperature of the column).
Compatible with GCMS systems due to the low carrier gas flow rate.
The disadvantages are:
1.
2.
3.
-
High component concentrations overload the column capacity. Therefore
more solvent has to be used to prepare the dilute solutions required for
injection.
The narrower the column diameter, the more difficult it is
to
fit into the
injector and detector. For on-column injectors there is a large drop in di-
ameter from the
0.32
mm diameter retention gap to the microbore column.
This leads to connector problems and extra-column band broadening.
A
solution to this problem has been the invention by Hagman
and
Roeraarde
of
the “at-column” injector
[57],
where the injection
is
made into
a
small
cavity directly above the column entrance.
The swift elution of many highly resolved peaks requires a detector and a
data handling system that can cope with the large number of rapidly
eluting components.
It is only recently that microbore columns have been used for HTGLC. Dam-
asceno
et
al.
[58]
evaluated their
own
borosilicate microbore column
(6
m
X
References pp.
90-93

70
Chapter
3
0.1
1
mm) up to
390°C,
combined with on-column injection. They separated out
the porphyrin fraction of a Serpiano oil shale. Microbore columns have great
potential for separating microcrystalline and synthetic waxes.
A
PTV injector
with a narrow insert
is
probably the most effective injector. The flow through the
detector is obviously much reduced and requires make-up gas.
3.2.3
Quantitative
gas
liquid
chromatography separation
of
waxes
Over the past
10
years there has been a steady growth in understanding the
quantitative
GC
separation of refined and synthetic waxes. Several papers have
been written on the use of the carbon number distribution of waxes
[3-51.
A
pa-
per by Case
[59]
graphically illustrates carbon number distribution, the chroma-
tographic conditions that can be used to separate microcrystalline and synthetic
waxes, and their use in hot melt adhesive formulation. In these papers, no expla-
nation is given of the chromatographic separation
or
the means of obtaining ac-
curate carbon number distribution.
Nakagawa
et
al.
[60] tried to characterise a whole range of waxes using solid
vaporization injection at
550°C
onto a capillary column coated with Dexil
300GC.
Their pioneering work on carbon number distribution analysis unfortu-
nately suffered from the then common problems of injection cold spots and high
split flow ratios. These problems caused distortion of the high molecular weight
response factors for Polywaxes. However, their analysis of paraffin waxes and
correlation
of
these results to physical properties were of a high standard.
In
advancing new injector or column technology to separate materials to the
highest carbon number, most authors have neglected chromatographic integrity.
There has been little attempt to obtain baseline separation of n-alkanes. Also
it
is
questionable whether some of the higher carbon number peaks reported for
Poljwax 655 actually exist [61]. It is feasible that the high boiling components,
on
injection, partly condensed out
in
the cold on-column injector only to be
eluted when the oven was hot enough to heat the injector. Thomson and Rynaski
[62] observed that, during the chromatography of Polywax
655
using
a
IOm
wide-bore column, baseline perturbations occurred during the final isothermal
period giving many more apparent peaks than occur with
a
shorter column.
These additional “peaks” they attributed to column bleed produced by oven
cycling during
the
final isothermal period. The interpretation of
HTGLC
quanti-
tative analysis and the calculation of carbon number distribution are probably the
most
complicated chromatographic procedures.
Much of the basic information leading to accurate quantitative wax analysis
has
been gained from simulated distillation research
(see
previous sections). This
type
of
analysis does not require high resolution, whereas the quantitative anal-
ysis of refined waxes needs detailed separation
of
the branched and straight

The chromatographic analysis ofrefined and synthetic wares
71
chain alkanes. Short wide-bore thick-film columns can be used for quantitative
analysis
of
synthetic waxes (e.g. Polywaxes) if the simulated distillation tem-
perature program is adjusted to obtain baseline resolution. But the quantitative
analysis of refined wax materials requires capillary columns that are long and
narrow with thin films of stationary phase to sufficiently resolve the main com-
ponents.
The prime factors in optimising the separation of waxes are:
1.
sample solvent;
2.
mode of injection and detection;
3.
type and treatment of the column support material;
4.
stationary phase used;
5.
stationary phase film thickness;
6.
carrier gas purity, type and flow;
7.
column size (length and internal diameter);
8.
temperature conditions of oven, injector and detector;
The first four factors have been covered in previous sections, but there are
some practical points to obtaining precise and accurate HTGLC analysis. Par-
ticular attention should be paid to flushing the syringe with sample before injec-
tion, the technique of injection, and thoroughly rinsing it out afterwards. This
reduces the chances of contamination and leads to
m accurate quantitative
analysis. The nearer that manual injection mimics aut
J~J-
lted injection, the better
the precision and accuracy. With HTGLC, it is imif ‘ant that the injection
points are regularly checked for cleanliness (i.e. on-column, retention gap or
PTV insert, and the injector components kept clean or changed). Otherwise
higher boiling components can build up in the system and be eluted with later
injections. If a septum is used, then
it
should be of the highest quality and regu-
larly checked for leaks. Any air leaks into the system during HTGLC will
in-
crease the column bleed and distort the baseline, as well as reducing the col-
umn’s useful life. Therefore all connections should be regularly leak tested.
The carrier gas used affects most of the other listed factors in some way or
other.
In
HTGLC, it is not just important that a clean, dry and oxygen-free car-
rier gas is used, but it also has to be the most practical carrier gas for the separa-
tion required. Nitrogen has commonly been used to separate waxes by packed
column GLC
[12],
but van Deemter plots show that helium and hydrogen are
much better mobile phases for capillary GLC. Hydrogen has a flatter van Deem-
ter curve than helium and a higher optimum flow rate can be achieved. Compo-
nents separated with this gas can therefore be eluted with shorter retention times
and at lower temperatures during temperature programming. This
is
very impor-
tant for the separation of higher boiling components. However, the use of hydro-
gen is considered by some to be dangerous and helium may be the preferred gas
(particularly for quality control).
References
pp.
90-93
