Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.

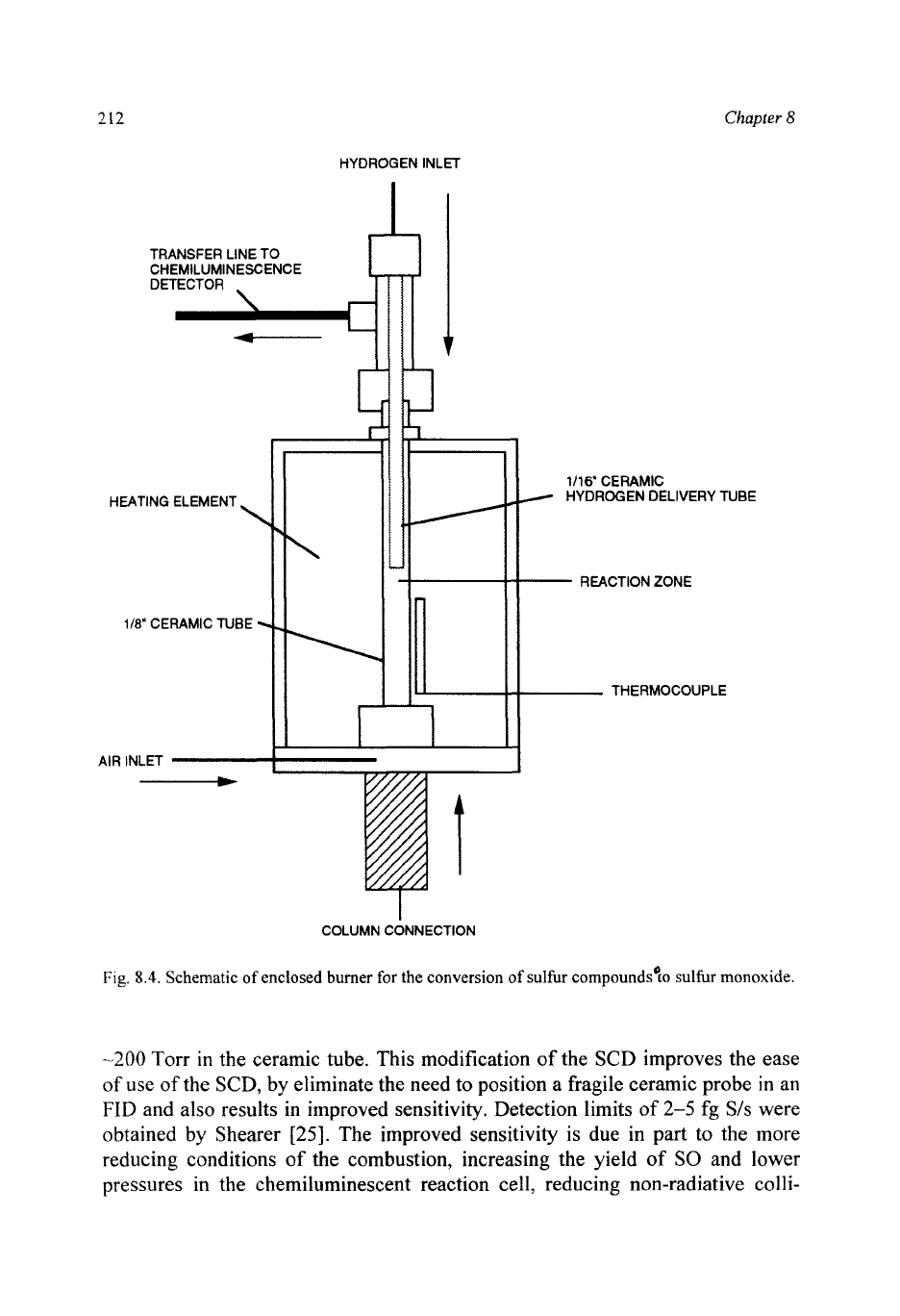
212
Chapter
8
HYDROGEN INLET
1/16'
CERAMIC
HEATING ELEMENT HYDROGEN DELIVERY TUBE
REACTION ZONE
1/8"
CERAMIC TUBE
THERMOCOUPLE
COLUMN CONNECTION
Fig.
8.4.
Schematic
of
enclosed burner
for
the conversion
of
sulfur compounds'[o sulfur
monoxide.
-200
Torr in the ceramic tube. This modification
of
the SCD improves the ease
of
use
of
the SCD, by eliminate the need to position
a
fragile ceramic probe in an
FID
and also results in improved sensitivity. Detection limits
of
2-5
fg
S/s
were
obtained by Shearer
[25].
The improved sensitivity is due in part
to
the more
reducing conditions
of
the combustion, increasing the yield
of
SO
and lower
pressures in the chemiluminescent reaction cell, reducing non-radiative colli-

The chemiluminescence detector
213
sional quenching of
SO2*.
As with the flame-based SCD, a nearly equimolar re-
sponse is observed for sulfur-containing compounds and good sulfur to carbon
selectivity
(>lo8)
is
obtained
[25].
The burner is available commercially, however the sensitivity is somewhat re-
duced compared with Shearer, with typical detection limits of
>0.5
pg
S/s
re-
ported
[26].
One drawback of the lower gas flow rates of the ceramic burner is
that quenching of the sulfur response due to co-elution of hydrocarbons can be
observed. In most cases, this quenching can be reduced or eliminated by increas-
ing the hydrogen and air flow rates, while maintaining the
5:
1
hydrogerdair ratio
(e.g.
200
ml/min H2,
40
mVmin air), however decreased sensitivity for sulfur
compounds is obtained at these higher flow rates. For example, the sulfur re-
sponse is decreased by approximately a factor of
two
at
200
ml/min H2,
40
ml/min of air versus the response obtained at
100
ml/min
H2,
20
mVmin
of
air. Another drawback of the ceramic burner is that in order to obtain simul-
taneous SCD/FID signals it is necessary to use a post-column split. However,
for many routine applications and for low level detection of sulfur com-
pounds, the ceramic burner offers many advantages compared to the flame-based
SCD.
8.7
COLUMN SELECTION
AND
SAMPLING
TECHNIQUES
As
previously noted, sulfur compounds can be sorbed and lost in all compo-
nents of the chromatographic system and in sampling containers. Specially
treated gas bombs and cylinders have been developed to minimize the loss
of
sulfur compounds, but in most cases, passivation of the sampling devices by
treatment with high levels
of
sulfur compounds is required to avoid losses
of
low
levels of sulfur compounds.
In
many cases, the best sampling containers are
older ones that have been in use for long periods of time and thus have been
passivated through use. Gas-tight syringes can also be a source
of
problems.
Some syringes are more active than others and cause significant loss of low lev-
els of sulfur compounds. When high levels samples are analyzed, the sulfur
compounds can permeate through the Teflon and other syringe components, then
slowly outgas, resulting in contamination of future samples analyzed with this
syringe.
Passivation with high levels
of
sulfur compounds is also the most common
method for minimizing loss in the chromatographic system. Exposing inlet lines,
gas sampling valves, sample loops, and injection port liners to high levels of
H2S,
SO2,
mercaptans and other reactive sulfur compounds can reduce the loss of
sulfur compounds in the GC system. Decomposition and loss of sulfur com-
pounds can also occur in the chromatographic column. For example, porous
References
pp.
22
7-229
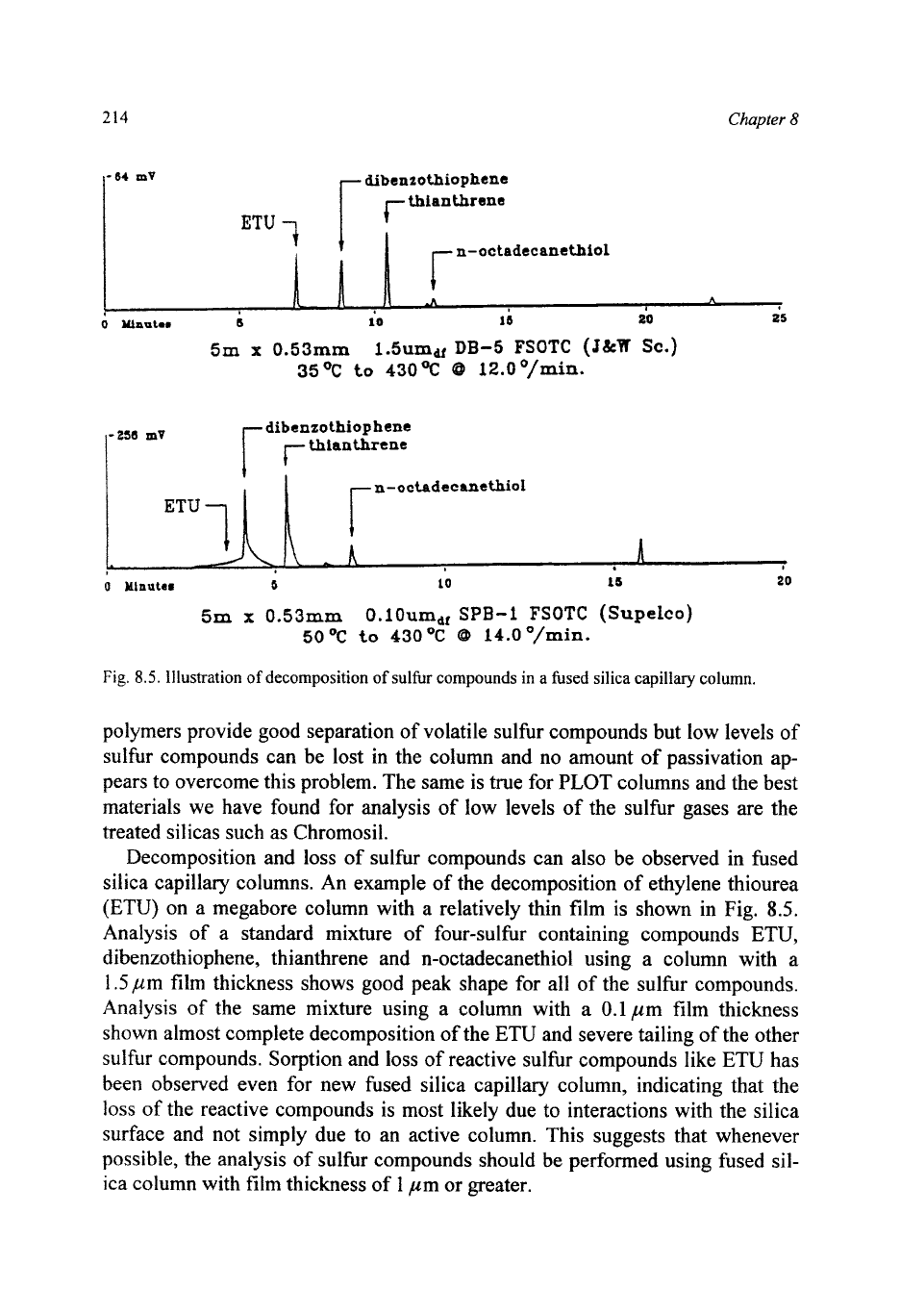
214
Chapter
8
dibenzothiophene
n-oc
tadecanethiol
h
5
10
16
20
25
-64
mV
ETU
3
0
YlnuU.
5m
x
0.53mm
1.5umu
DB-5
FSOTC
(J&W
SC.)
35OC
to
430%
Q
12.O0/min.
dibenzothiop
hene
r
rthianthrene
n-octadecanethiol
A
5
10
16
20
O.lOum,,
SPB-1
FSOTC
(Supelco)
0
Mlnutsr
5m
x
0.53mm
50°C
to
43OoC
Q
14.Oo/min.
Fig.
8.5.
Illustration
of
decomposition
of
sulfur compounds in a fused silica capillary column.
polymers provide good separation of volatile sulfur compounds but low levels of
sulfur compounds can be lost in the column and no amount of passivation ap-
pears to overcome this problem. The same is true for
PLOT
columns and the best
materials we have found for analysis of low levels of the sulfur gases are the
treated silicas such as Chromosil.
Decomposition and
loss
of sulfur compounds can also be observed in fused
silica capillary columns. An example of the decomposition of ethylene thiourea
(ETU) on a megabore column with a relatively thin film is shown in Fig.
8.5.
Analysis of a standard mixture of four-sulfur containing compounds
ETU,
dibenzothiophene, thianthrene and n-octadecanethiol using a column with
a
1.5
pm film thickness shows good peak shape for all of the sulfur compounds.
Analysis of the same mixture using a column with a
0.1
pm film thickness
shown almost complete decomposition of the ETU and severe tailing of the other
sulfur compounds. Sorption and
loss
of reactive sulfur compounds like ETU has
been observed even for new fused silica capillary column, indicating that the
loss
of the reactive compounds is most likely due to interactions with the silica
surface and not simply due to an active column. This suggests that whenever
possible, the analysis of sulfur compounds should be performed using fused sil-
ica column with film thickness of
l
pm or greater.

The chemiluminescence detector
215
A particularly useful capillary column has been developed for the analysis of
sulfur compounds. The column (30 m
X
0.32
mm i.d.
SPB-I
4,um film thick-
ness, Supelco Inc.) provides separation
of
H2S
from co-eluting COS/S02 at
35°C
and can be used for samples up through the diesel range. At
-1O"C,
COS and
SO2
can be separated. Methyl silicone columns with 4
,urn
film thickness are now
also available from other column manufacturers. The combination of a thick film
methyl silicone and special cross-linking to minimize column bleed permits
separation of most sulfur compounds and applications for a wide range of petro-
leum samples.
The combination of high sensitivity and selectivity of the SCD has led to the
development of a number of applications for this detector in the measurement of
sulfur compounds in petroleum and petroleum products. Some representative
examples
are
given below.
8.8
APPLICATIONS
8.8.1
Refinery
gases
A major application for the analysis of sulfur compounds is natural gas,
LPG,
refinery gases and other process gas streams. Since these streams are usually
upgraded or sold for heating purposes, accurate measurement of the levels of
sulfur compounds is required. Figure
8.6
shows the FID and SCD chroma-
tograms obtained from the analysis
of
a refinery gas
(LP)
sample using the
flame-based SCD. A 0.1-ml sample was injected onto a capillary column, using a
split injection technique (split ratio 1O:l). The FID chromatogram shows that
propylene and propane are the major constituents of the sample with low levels
of
C4
and C5 hydrocarbons. Hydrogen sulfide is the major sulfur contaminant,
with lower levels of COS,
SO2,
mercaptans, sulfides disulfides and thiophenes
present in the sample.
Not all of the sulfur compounds were identified, however, the equimolar re-
sponse of the SCD for sulfur compounds can be used to determine the sulfur
content of these unidentified components and the total sulfur content of the
sample. A response factor (pg S/area) can be determine from the analysis of a
standard containing one or more sulfur compounds and this response factor used
to quantitate unknown sulfur compounds and total sulfur content can be calcu-
lated from the total area.
Another important application is the measurement of sulfur compounds in
polymer
grade ethylene, propylene and other olefins used in the production
of
plastics. Due to the complications from low levels of sulfur compounds in the
polymerization reactions, the desired level of total sulfur in the feedstock is
References
pp.
227-229
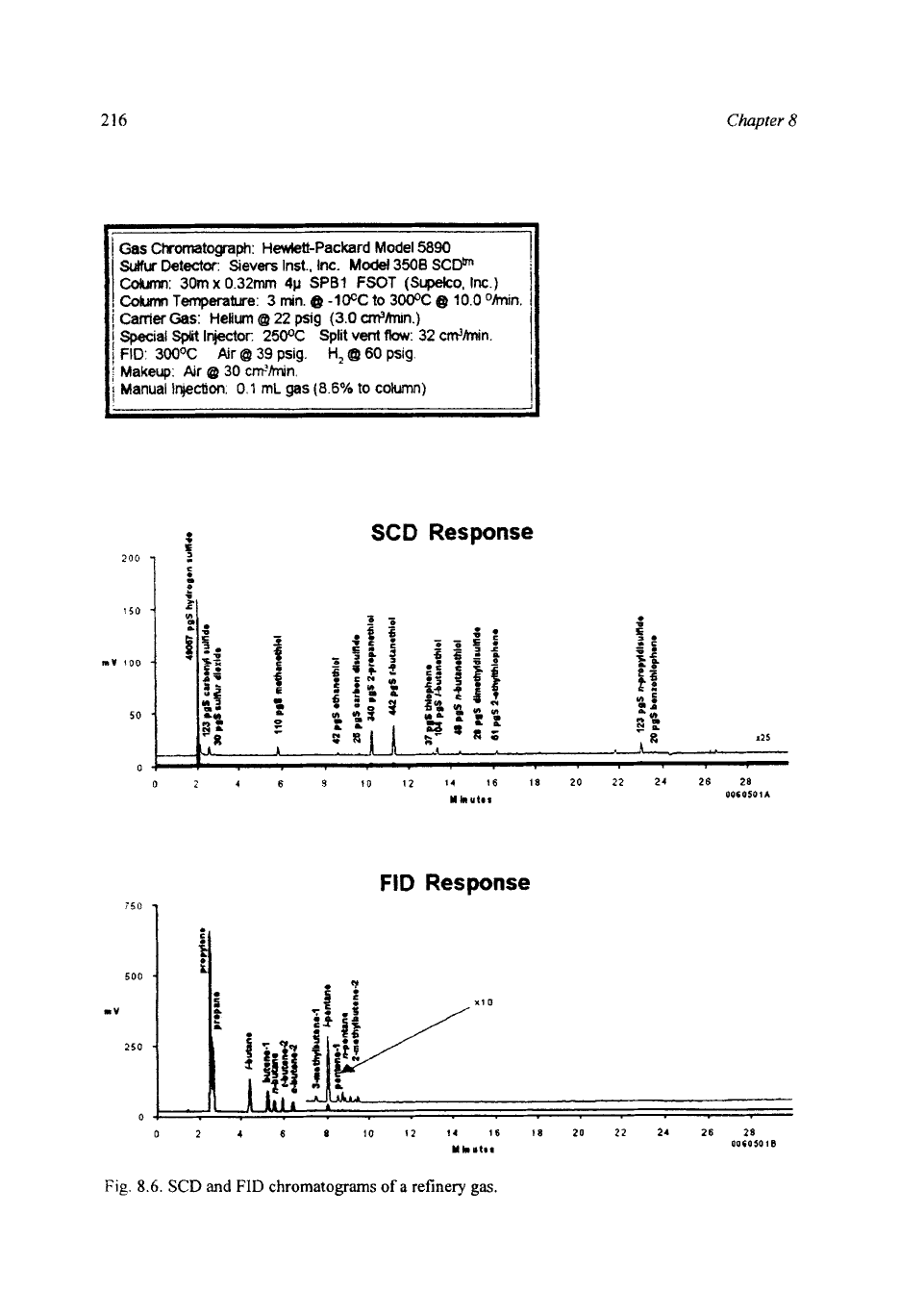
216
Chapter
8
Gas
Chomatograph Hervlet-Packard
Model
5890
Sulfur
Detector
Slevers
lnst,
Inc
Model
3506 SCDbn
Cokmn
30m
x
0
32mm
4p
SPBl
FSOT
(Supelco.
Inc
)
Column
Temperature
3
mn
@
-10%
to
3ooOC
@
10
0
Ohin
Carrier
Gas
Hekum
Q
22
png
(3
0
dhn
)
Speaal
Spkt
lryector
250%
Split
vent
Row
32
cdhn
FID 300OC
kr
@
39
psig
H2
@
60
psig
Makeup
Air
Q
30
cm?lMn
Manual
Injecbon
0
1
mL
gas
(8
6Oh
to
column)
SCD
Response
FID
Response
0
2
4
6
0
10
I2
14
I6
18
20
22
24
26
28
00605018
Yhrtaa
Fig.
8.6.
SCD
and
FID
chromatograms
of
a
refinery
gas
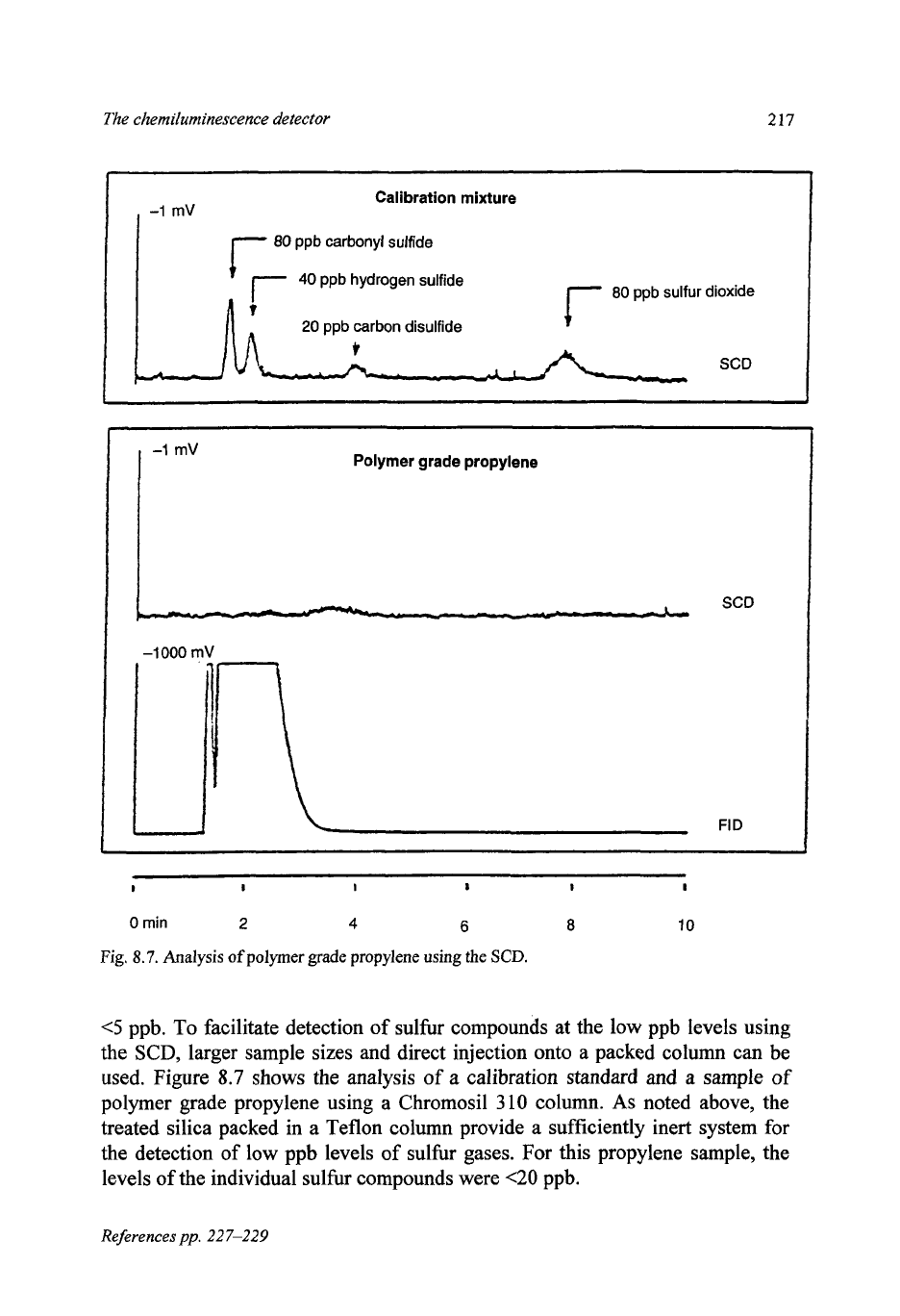
The chemiluminescence detector
217
Calibration
mixture
80
ppb
carbonyl sulfide
40
ppb
hydrogen sulfide
20
ppb carbon
disulfide
80
ppb
sulfur
dioxide
t
SCD
SCD
FI
D
I
1
I
I
I
I
I
0
min
2
4
6
8
10
Fig.
8.7.
Analysis
of
polymer grade propylene using the
SCD.
<5
ppb. To facilitate detection
of
sulfur compounds at the low ppb levels using
the SCD, larger sample sizes and direct injection onto a packed column can be
used. Figure
8.7
shows the analysis of
a
calibration standard and a sample of
polymer grade propylene using a Chromosil
310
column.
As
noted above, the
treated silica packed in a Teflon column provide a sufficiently inert system for
the detection of low ppb levels of sulfur gases. For this propylene sample, the
levels of the individual sulfur compounds were
<20
ppb.
References
pp.
22
7-229

218
Chapter
8
8.8.1.2 Gasoline
The concentration of total sulfur in motor gasolines typically ranges from
300
to
500
mg
Skg
(ppm). The development of new reformulated gasolines is now
underway in an effort to improve ambient air quality. Included in the regulations
governing fuel reformulation are new limits for the concentration of total sulfur
in
the gasolines. For example, the California Air Resources Board regulations
call for
a
total sulfur content of less than
40
mgkg in gasoline. These lower sul-
fur concentrations present many problems, not only in the refining and blending
of the fuels, but also to the analytical chemist.
The SCD and FID chromatograms obtained from the analysis of a sample of a
commercial motor gasoline is shown in Fig.
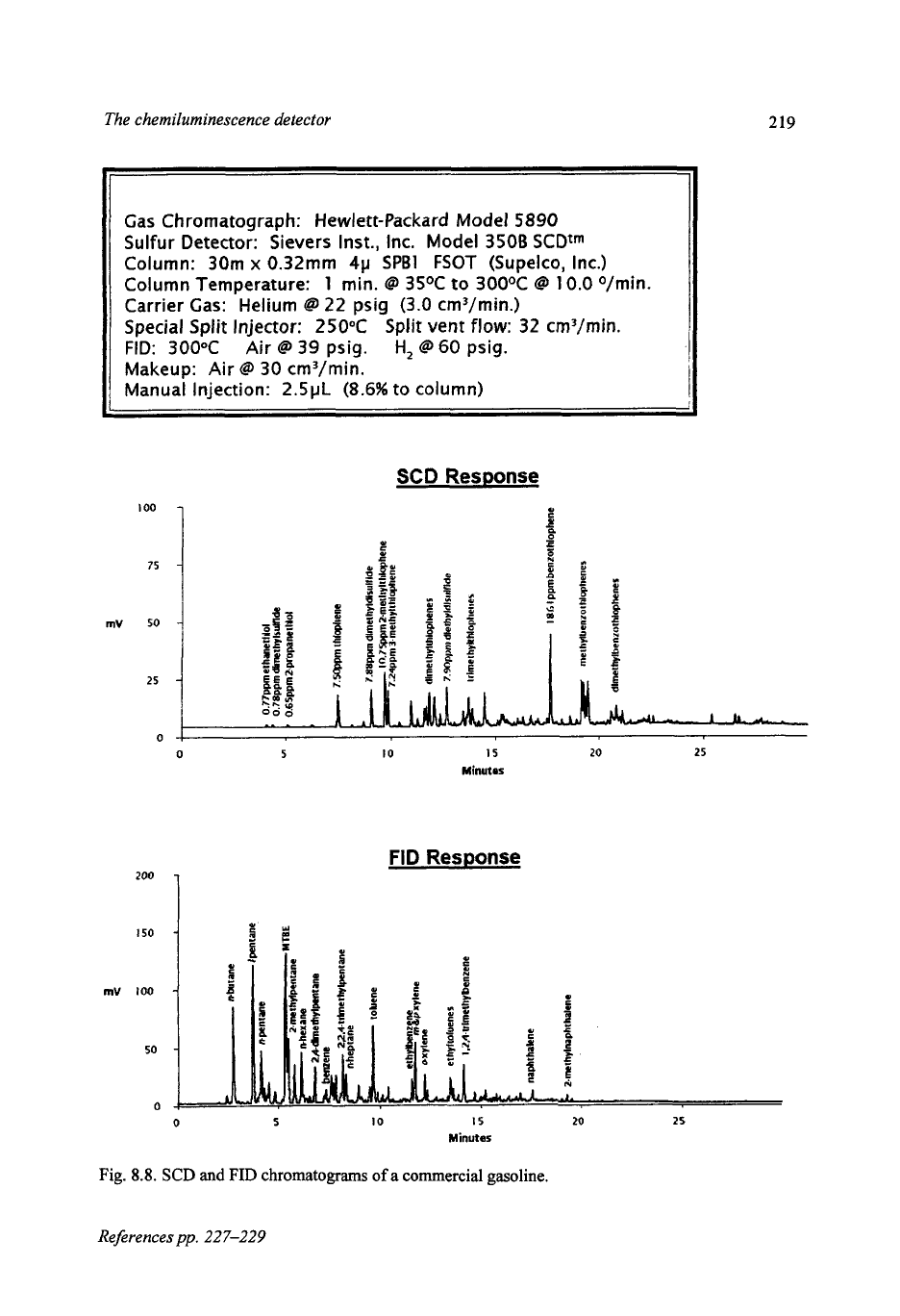
8.8.
This oxygenated gasoline is
used in the winter months in the Denver, Colorado, area as part of a program to
reduce atmospheric carbon dioxide levels. The SCD chromatogram shows the
typical sulfur compounds present in gasoline, thiophene and alkyl thiophene,
benzothiophene and the alkyl derivatives. In addition, lower levels of thiols and
sulfides are also present in this sample, with a total sulfur content of
337
mg
S/kg.
The FID chromatogram shows the typical hydrocarbon pattern expected for
gasoline, aromatic and aliphatic hydrocarbon, and also shows methyl tert-butyl
ether (MTBE), the additive used for the oxygenated fuel program.
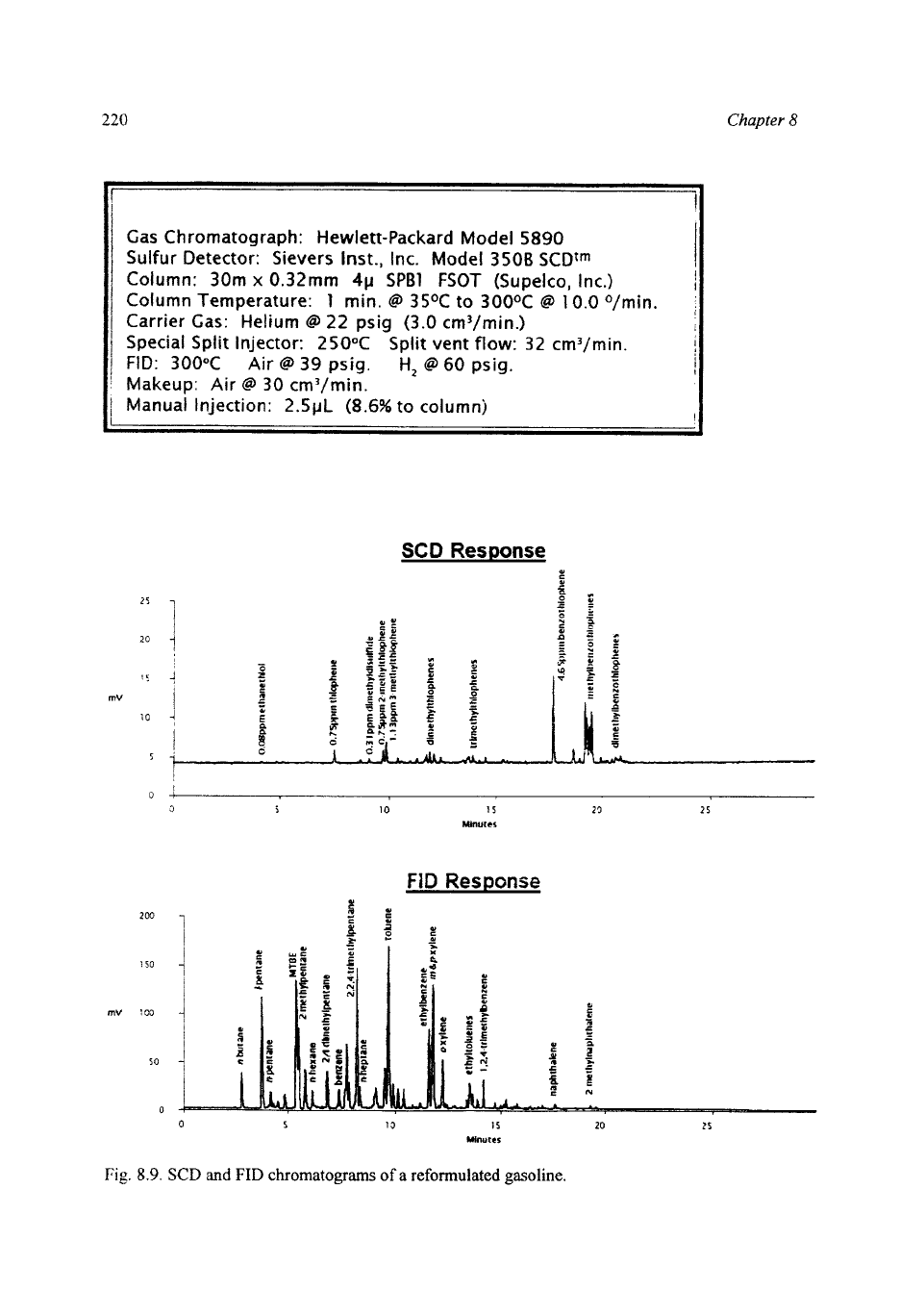
An
example of the SCDKID analysis of a reformulated fuel is shown in Fig.
8.9.
The SCD chromatogram again shows the thiophenic sulfur compounds, but
lower levels
of
thiols and sulfide, with a total sulfur content of
36
mg S/kg. The
FID chromatogram from the reformulated fuel shows MTBE and higher levels of
2,2,4-trimethylpentane and other iso-paraffins compared with typical gasolines.
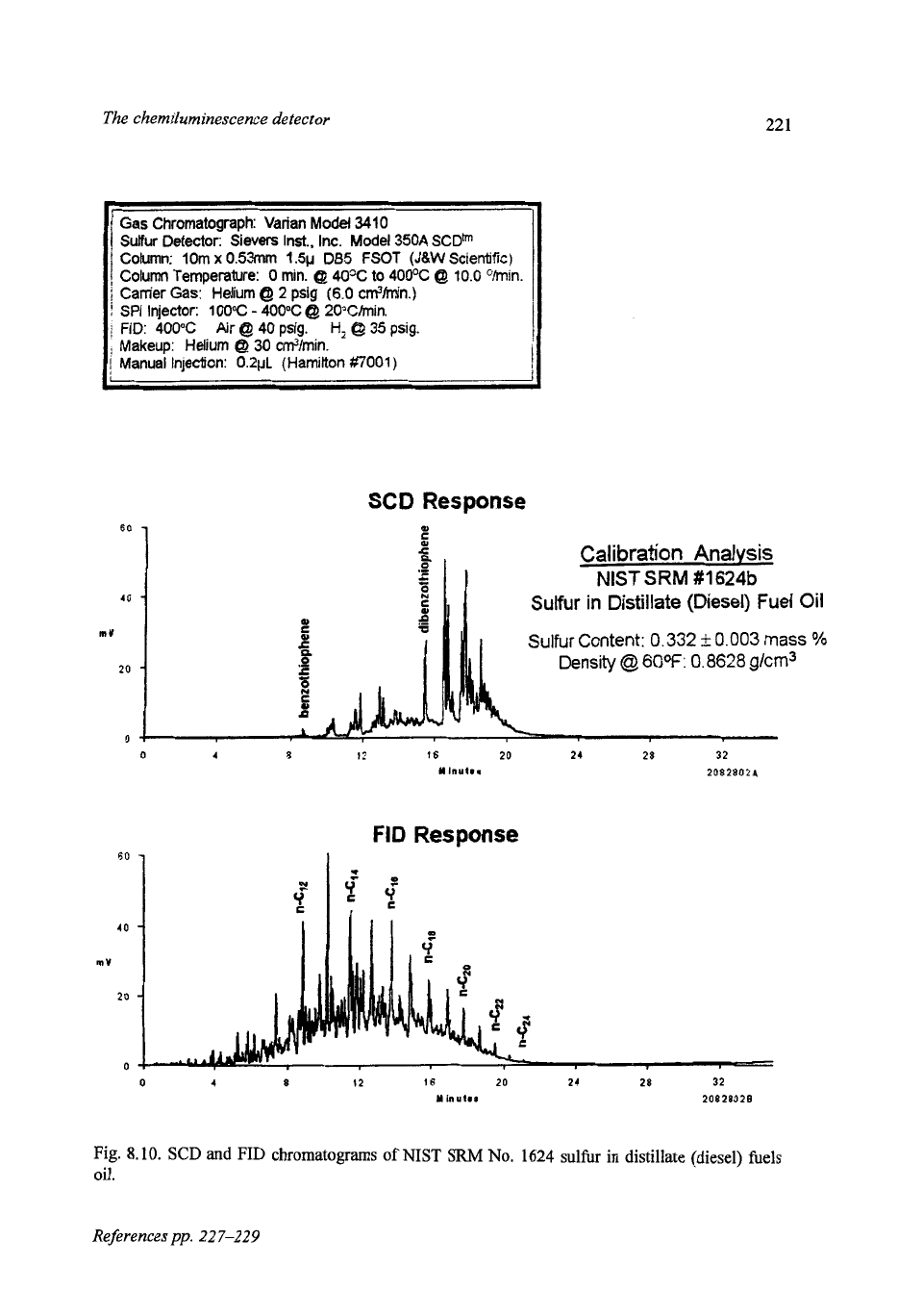
8.8.1.3 Diesel fuels
The total sulfur content of diesel fuels is also subject to current and pending
limitations, with proposed limit of
500 ppm. Some example of the sulfur and
hydrocarbon profiles for diesel fuels are shown. The FID and SCD chroma-
tograms from the analysis of NIST Standard Reference Material
No.
1624b
(Sulfur in Distillate (Diesel) Fuel Oil) are shown in Fig.
8.10.
This material is
certified to contain
332
f
3
mgkg sulfur and is useful for calibrating the SCD
for
total sulfur determinations. Benzothiophene, dibenzothiophene and alkyl de-
rivatives of these compounds are the major sulfur components of this sample and
most diesel fuels. For comparison, the FID and SCD chromatograms for a low
sulfur diesel
(26.5
mg
S/1)
are shown in Fig.
8.1
1.
8.8.1.4 High temperature
gas
chromatography
High temperature GC is widely used to determine the boiling range
of
crude
oils and petroleum feedstocks. The flame-based SCD permits simultaneous de-
termination
of
the hydrocarbon and sulfur compounds boiling range distribu-
The chemiluminescence detector
219
Gas Chromatograph: Hewlett-Packard Model 5890
Sulfur Detector: Sievers Inst., Inc. Model 3506 SCD'm
Column: 30m
x
0.32mm
41.1
SPBl
FSOT
(Supelco, Inc.)
Column Temperature:
1
min.
@
35OC
to
3OOOC
@
10.0
Ohin.
Carrier Gas: Helium
@
22 psig
(3.0
cm3/min.)
Special Split Injector: 250°C Split vent flow: 32 cm3/min.
FID:
300°C Air
@
39 psig.
H,
@
60
psig.
Makeup: Air
@
30 cm3/min.
Manual Injection: 2.5pL (8.6% to column)
SCD
Response
loo
1
u
t
o!
0
5
10
15
20
25
minutes
FID
Response
0
5
10
IS
20
25
Minutes
Fig.
8.8. SCD
and
FID
chromatograms
of
a commercial gasoline.
References
pp.
22
7-229
220
Chapter
8
Gas Chromatograph: Hewlett-Packard Model
5890
Sulfur Detector: Sievers
Inst.,
lnc. Model 3508 SCDtm
Column: 30m
x
0.32mm
4p
SPBl
FSOT
(Supelco, Inc.)
Column Temperature:
1
rnin.
@
35OC
to 3OOOC
@
10.0
O/min.
Carrier Gas: Helium
@
22 psig (3.0 cm3/min.)
Special
Split
Injector: 250°C Split vent flow: 32 cm3/min.
FID:
3OOOC
Air
@
39 psig. H,
@
60
psig.
Makeup: Air
@
30
cm3/rnin.
Manual Injection: 2.5pL
(8.6%
to columnj
SCD
Response
I
O!
3
1
10
IS
20
23
mutes
FID
Response
0
1
13
13
20
23
mrts
Fig.
8.9.
SCD
and
FID
chromatograms
of
a
reformulated
gasoline.
The chemiluminescence detector
22
I
Gas Chromatograph: Varian
Model
3410
Sulfur Detector: Seven Inst.,
lnc.
Model
350A
SCDm
Column: 1Om
x
0.53mm 1.5~ DB5
FSOT
(J&W
Scienbfic)
Column Temperature:
0
min.
Q
40°C
to
4OOOC
Q
10.0
Vmin.
Carrier Gas: Helium
Q
2
psig
(6.0
CmVtnin.)
SPI
Injector: 100°C
-
400°C
Q
20*C/min.
FID: 400°C
Air
Q
40 psig. HZ
Q
35 psig.
Makeup: Helium
Q
30 cm3/min.
Manual Injection:
0.2pL
(Hamiiton
#lOOl)
SCD
Response
60
-
0
f
c a
f
8
Calibration Analysis
NIST
SRM
#1624b
Sulfur
in
Distillate (Diesel) Fuel
Oil
Sulfur
Content:
0.332
k
0.003
mass
YO
Density
@
6G°F:
0.8628
g/cm3
.-
40
-
mV
20
-
0
0
4
8
12
16
20 24 28 32
Ylnuirr
20828021
FID
Response
0
4
8
12
16
20
24
28
32
Yinulrr
20828020
Fig.
8.10.
SCD
and
FID
chromatograms
of
NIST
SRM
No.
1624
sulfur
in
distillate (diesel) fuels
oil.
Referencespp.
227-229
