Vocking B., Alt H., Dietzfelbinger M., Reischuk R., Scheideler C., Vollmer H., Wagner D. Algorithms Unplugged
Подождите немного. Документ загружается.

390 Stefan N¨aher
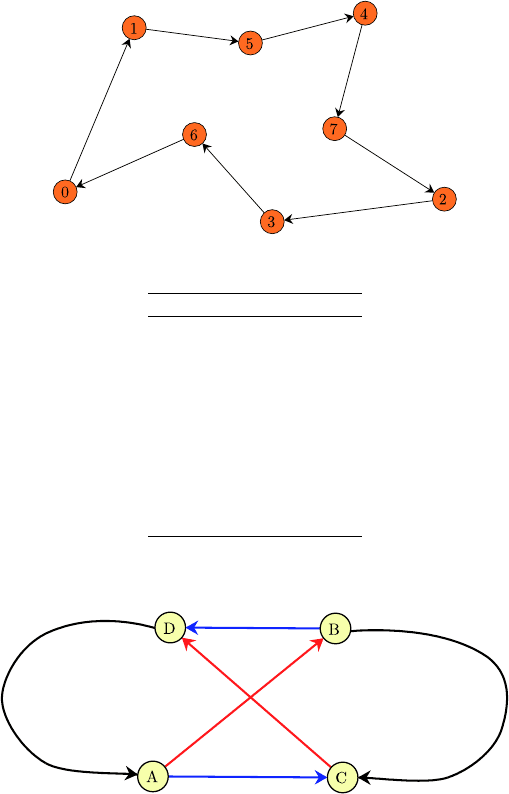
Fig. 40.5. The minimum spanning tour
Cities Running time
100 0.01 sec
200 0.08 sec
300 0.36 sec
400 1.30 sec
500 3.62 sec
600 8.27 sec
700 16.07 sec
800 29.35 sec
900 50.22 sec
1000 85.38 sec
Table 40.3. Running time of the MST-heuristics
Fig. 40.6. One step of the 2-OPT heuristics
and from D to A. Now check if these two parts can be combined to a shorter
tour by adding edges A −→ C and D −→ B.
Some Final Remarks
• There are many more heuristics for improving the quality of approximative
solutions. In many practical cases they are even able to find the optimal
tour.

40 The Travelling Salesman Problem 391
• Algorithms for the exact solution of TSP have been considerably improved
over recent years. They can solve problems with several thousand cities in
a few hours.
• In the worst case solving TSP remains difficult and requires an exponential
number of computation steps.
Further Reading
1. Wikipedia: Travelling Salesman Problem
http://en.wikipedia.org/wiki/Travelling
Salesman Problem.
2. An introduction by Martin Gr¨otschel and Manfred Padberg (in German)
http://elib.zib.de/pub/UserHome/Groetschel/Spektrum/index2.
html
3. The Travelling Salesman Problem Home Page
http://www.tsp.gatech.edu
4. The Knapsack Problem (Chap. 39)
An optimization problem with a similarly fast growing number of possi-
bilities.
5. Fast Sorting Algorithms (Chap. 3)
An introduction to recursive algorithms.
6. A demo program that provided the figures in this chapter
http://www-i1.informatik.rwth-aachen.de/
∼
algorithmus/
Algorithmen/algo40/tsp.exe
41
Simulated Annealing
Peter Rossmanith
RWTH Aachen, Aachen, Germany
Let us look at a simple combinatorial puzzle game: we can place several
quadratic tiles on a wooden board at 12 ×8 different positions. At the begin-
ning the tiles might be placed as indicated in Fig. 41.1. As you can see, each
tile has four sides that may be colored blue, yellow, green, and orange.
We can exchange the positions of any two tiles, but we are not allowed to
rotate them. The goal of the game is to obtain as many neighboring sides of
tiles of the same color as possible: You score one point for each such neigh-
boring pair. In that way it is not possible to score more than 172 points: Each
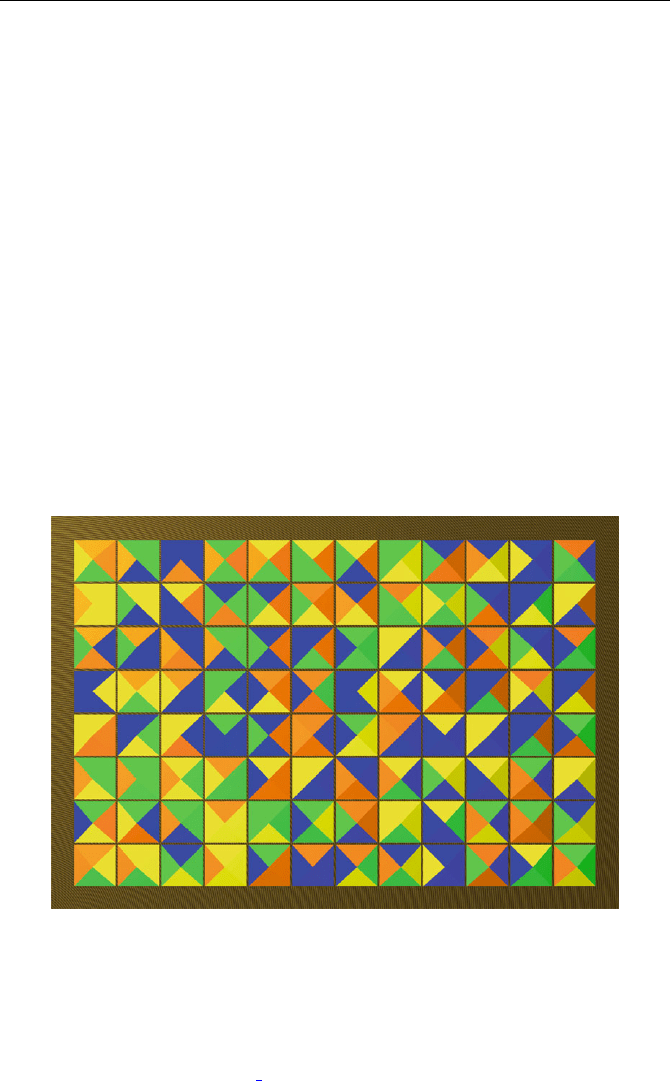
Fig. 41.1. Background of the domino game: The pieces are arranged at random in
the box. Even in this situation we see 36 points
B. V¨ocking et al. (eds.), Algorithms Unplugged,
DOI 10.1007/978-3-642-15328-0
41,
c
Springer-Verlag Berlin Heidelberg 2011
394 Peter Rossmanith
row contains 11 tile pairs and each column contains 7. There are 8 rows and
12 columns making altogether 11 · 8+7· 12 = 172.
Throughout this chapter we will continue to consider this tiling game as a
typical example that is similar to many problems in combinatorial optimiza-
tion. Some of the optimization problems mentioned in this book can be solved
quite efficiently (Chaps. 32, 33, 34,and35), while we can exactly solve others
only for relatively small input sizes (Chaps. 39 and 40). Here we are interested
more in the latter kind, in particular problems that can neither be solved by
trying out all possibilities nor with the help of backtracking. One method that
works well in many of those cases is simulated annealing, which is the topic
of this chapter.
What Is Simulated Annealing?
Simulated Annealing means simulating a process that involves first heating
and then slowly cooling some material. There are several technological pro-
cesses based on a similar principle. For example, cooling red hot iron in met-
alworking quickly or slowly leads to materials with quite different properties.
So why do that? Let us think about what happens to the particles (atoms) of
the metal:
The atoms are bound in a rigid crystal structure. When we start to heat
the metal they start to break free from their bonds and move about. If we
let the metal cool again, the particles will find new bonds. Interestingly, often
their new distribution will be more regular than before. Doing it the right way
leads to metal that is softer, more flexible, and contains fewer irregularities.
To better understand the effects of slowly cooling, we can think of the
particles as small balls. If you just throw them into a vessel, they will lie
around higgledy-piggledy. What can we do to put them into order? Shake
them! At first their disorder increases and the balls fly about, but soon they
get into order all by themselves. If we, however, stop shaking too suddenly,
the balls will not be packed very closely.
This is also an important principle in the manufac-
turing of silicon semiconductors, of which computers’
processors and memory chips consist. In that case we
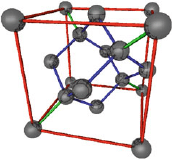
require very pure silicon crystals that do not contain
any defects. Usually silicon is polycrystalline: similarly
to grains of sand, many small monocrystals are placed
close to each other. Each monocrystal itself consists of
very many small elementary cubes that are flawlessly
stacked next to each other. On the left side you can
see such a silicon elementary cube. On the outside eight silicon atoms form a
cube in whose interior ten more silicon atoms reside. In semiconductor pro-
duction we need relatively large monocrystals. To this end a monocrystalline
“pillar” is slowly drawn out of a bath of molten silicon. Consequently, with

41 Simulated Annealing 395
the help of a saw the monocrystal is cut into wafers, which finally are turned
into electronic semiconductors.
The monocrystalline state of silicon has the small-
est energy possible: the bonds between individual
atoms are strongest. From this perspective, the dif-
ference from our tiling game is not that big anymore.
Here, too, we are concerned with elementary par-
ticles whose relative position can be changed. The
bond between two neighboring tiles is stronger if
sides with the same color face each other. Again,
we are looking for a tessellation with smallest pos-
sible energy. If our game were a heated crystal, the
tiles would wildly jump about. The lower the tem-
perature gets, the harder it becomes to tear a tile
from its position, and the more stable bonds they
have with their neighbors, the stronger they stick to
their position.
It seems to be very hard to modify the board
game in order to make it behave just like that, al-
though it might be possible: Perhaps we could en-
force stronger bonds between sides of same color
by cleverly located magnets and mount the whole
board on a vibrating table. On the other hand, it
is much easier to simulate the whole process using a computer. Shaking the
board corresponds to swapping the positions of two tiles in the simulation.
Doing so, it is quite easy to compute how much the number of points scored
increases or decreases. At high temperatures we tolerate swaps that decrease
the number of points, while at low temperatures we get stricter and will tend
to allow only “good” changes to the board. The following algorithm can easily
be implemented in all usual programming languages:
Tiling Game
Repeat quite often:
1. Decrease the temperature a little
2. Swap two randomly chosen tiles
3. If the number of points has decreased:
• Randomly decide whether to keep this swap
• The probability for keeping the swap decreases with the temperature
• Undo the swap in case of a negative decision
This algorithm works surprisingly well for our tiling game. During the
execution of the algorithm the point score both increases and decreases. In
the beginning its fluctuation is quite big, but the longer we wait – and the
lower the temperature gets – the smaller it becomes. Finally, we cannot notice
396 Peter Rossmanith
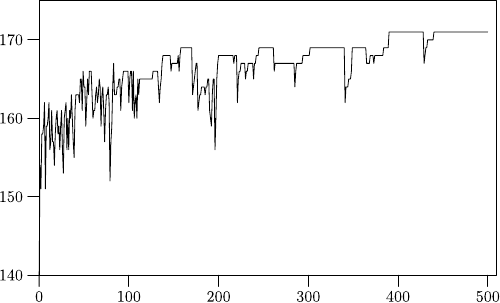
Fig. 41.2. How the point score changes by repeatedly swapping tiles: The y-axis
indicates the number of points and the x-axis shows the number of steps in units of
10,000. Altogether 500,000 steps are executed. In the end the score amounts to 171
points
any change in the point score anymore. Figure 41.2 shows how the point score
changes in the course of time, showing only scores higher than 140. This area
is reached very quickly, while further improvements become more infrequent
toward the end.
At this point, the question why we do not always take back swaps that
decrease the point score arises – in that way we would never voluntarily give
up earned points? Very often such a strategy works quite well and, therefore,
bears its own name: method of steepest descent (in our metaphor of reaching
the highest mountain, method of steepest ascent would make more sense, but
some optimization problems happen to be maximization problems, while oth-
ers are minimization problems, and the name was chosen based on the latter
ones). This strategy only climbs upwards, never downwards. If we want to
climb the highest mountain, we choose a direction that leads upwards, until
no such direction exists anymore, which is only the case on a summit. Can we
be sure that it is the highest summit? Not necessarily!
One who wants to climb the highest mountain, must be also willing to
descend along the way.
An application of the method of steepest descent to our tiling problem
leads to a solution with 167 points, from which point on it is impossible to
further increase the score by swapping tiles. This score is quite good. Simu-
lated annealing, however, leads to the better solution with 171 points, shown
in Fig. 41.3. Only one possible point is missing, whose location is not at all
easy to spot with just a quick glance on the board. (Hint: Tilt the picture by
45
◦
and look along the visually emerging diagonal lines.)
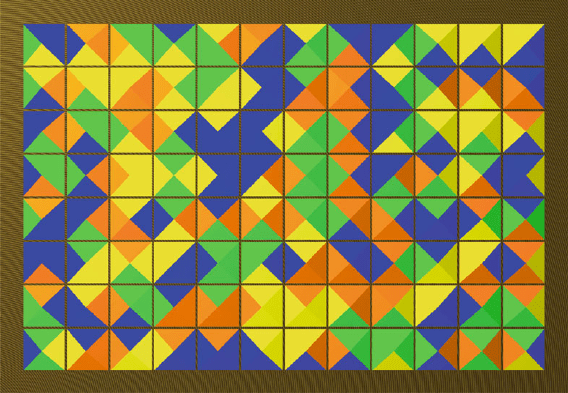
41 Simulated Annealing 397
Fig. 41.3. Positions of the tiles after application of simulated annealing: The score
is 171 points, and a closer look reveals that we got almost all possible points
Scoring 171 of theoretically possible 172 points is an excellent result
achieved by simulated annealing. We leave the natural question of whether a
perfect solution with 172 points exists as an open question to the interested
reader.
The Travelling Salesperson Problem
Let us have another look at a typical and famous problem in computer sci-
ence, the travelling salesperson problem, which is also a topic in Chap. 40
in this book: A salesperson likes to visit a number of cities, spending as lit-
tle time as possible for the trip. So the goal is to fix the sequence in which
he or she visits the cities in such a way that the total mileage traveled be-
comes as small as possible. Surprisingly, this turns out to be a very hard
problem.
Maybe we can find a nearly optimal solution by employing simulated an-
nealing. To find some solution to start with is easy: Just put the cities in some
arbitrary order! So we start with a random tour and, of course, it is highly
improbable that this tour is short.
How can we improve this tour by incorporating only small adjustments?
Well, one possibility is to choose randomly a segment of the tour and traverse
it in the opposite direction:
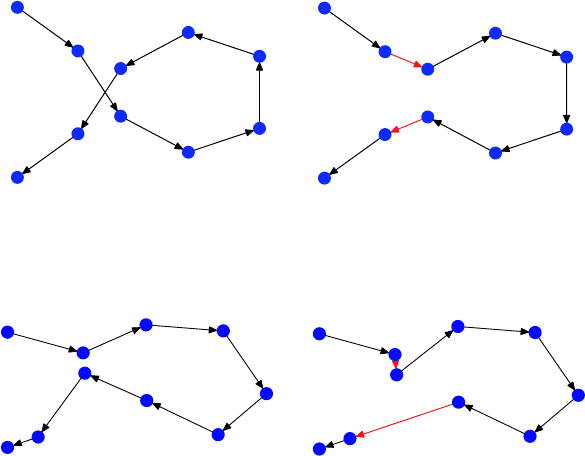
398 Peter Rossmanith
Another possibility is to visit some city at a different point in time, while
preserving the order in which we visit the remaining cities:
After adjustments of either kind, the new tour might be better or worse
than the old one. If the new one is better, we keep it, otherwise we return to
the old tour.
A big tour connecting 200 cities is depicted in Fig. 41.4. Of course, it was
found by using the two kinds of adjustments mentioned above and simulated
annealing.
Further Applications
We can employ simulated annealing if the following conditions are met:
1. The quality of a solution can be expressed as a number.
2. An initial solution can be easily computed.
3. There are simple adjustment rules that locally change a solution.
4. Every solution can be turned into every other solution by application of
these adjustment rules.
As these conditions are by no means very restrictive, it turns out that a
surprisingly large number of problems can be successfully solved by simulated
annealing.
Let us finally note that computer scientists were not the first to use crys-
tal growing by slow cooling as a model for solving technical problems. If you
take this book into your hand, for example, you might notice the accurate
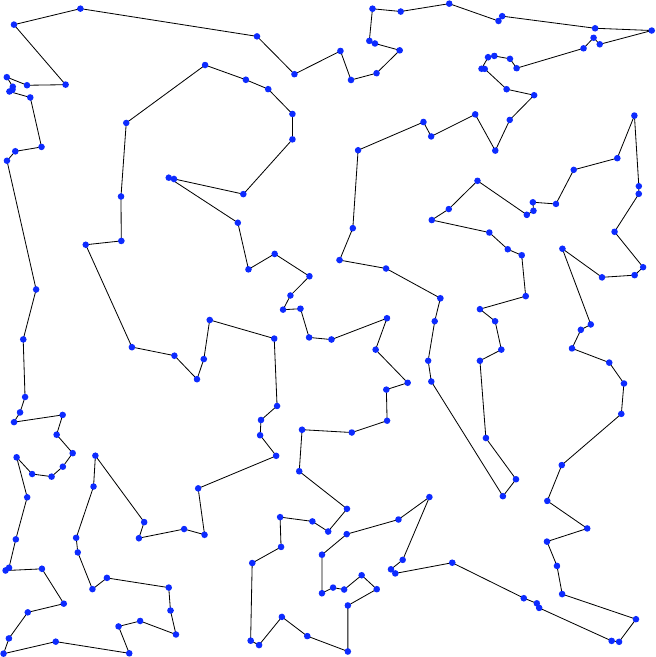
41 Simulated Annealing 399
Fig. 41.4. A tour that connects 200 cities
fitting of the pages’ edges thanks to a precise cutting machine. If you, how-
ever, flip quickly through the pages you might also notice that the edges of
the text are perfectly aligned. This alignment is due to the perfect adjust-
ment of the pages before binding them into a book. Technically, this is a
quite complex task: If you have a pile of loose pages (or cards from a deck),
it is quite hard to align their edges perfectly. Even brute force is not very
helpful.
The technical solution to this problem is a machine called a paper jog-
ger. It perfectly aligns the pages by using strong vibrations. Figure 41.5
shows a small paper jogger that can be operated manually and is used to
align exercise sheets coming freshly out of a printing machine. The control
dial is used to turn the vibration from strong to gentle: simulated anneal-
ing!
