Vij D.R. Handbook of Applied Solid State Spectroscopy
Подождите немного. Документ загружается.

14.5 Applications
The calculations reproduce all of the spectral structures very well,
especially the enhancement of the peak at about 1.2 eV with increasing
excitation energies. The growth of the peak is due to enhanced transitions into
the
1
G
4
state. Changes in absolute intensities of inelastic scattering structures
corresponding to the transitions are reproduced on going from spectrum
a
to spectrum b. For spectra c and d, such changes in calculated intensities
are about three times higher as compared to those in the experiment.
The discrepancy may originate from the normalization procedure for the
experimental spectra to account for variations in the incident photon flux. The
intensity of the elastic peak was used as a reference in this procedure.
However, the elastic peak contains some contribution of diffuse scattering,
which may vary with varying excitation energies.
RIXS profiles, corresponding to the fof excitations, are found to be very
sensitive to the chemical state of U in different systems [114]. For example, it
is a matter of the presence or absence of these excitations when going from
U
4+
to U
6+
compounds. Therefore, RIXS measurements near the U 5d
threshold provide good fingerprints for the chemical state of U in different
systems in contrast to X-ray absorption spectra, which show only small
differences at the U 5d edge.
One interesting example concerns the chemical state of U in U
3
O
8
and
reduced UO
3
. In particular, for U
3
O
8
, it was discussed in the literature that the
chemical state of U is described as either U
VI
U
V,2
O
8
or U
VI,2
U
IV
O
8
. To our
knowledge there is no clear and convincing answer to this question and it is
still under debate. Thus, core level photoemission [115, 116, 117] and
electron spin resonance [118, 119] data have been interpreted in favor of
either situation by different groups, and therefore these techniques cannot
really provide an unambiguous answer. It turns out that the RIXS technique
can. RIXS spectra of fof excitations are a good indicator of whether uranium
is in the U(IV), U(V) or U(VI) state. The data are easy to interpret and not
very difficult to calculate. Establishing the real chemical state of U in oxides
is important for applied, environmental and fundamental science (e.g., a
development of the theory of nonstoichiometry is a fundamental problem).
Figure 14.33 displays RIXS spectra of transitions for a number of U
oxides. Close similarity of the transitions profile in U
3
O
8
to those in UO
2
and UF
4
, as well as to that calculated for the U(IV) ion (see Figure 14.32),
unambiguously indicates the presence of the U(IV) fraction in U
3
O
8
, thus
favoring the U
VI,2
U
IV
O
8
description of the uranium chemical state in this
oxide. Furthermore, a similar pattern is observed for reduced UO
3
, thus
indicating that oxygen deficiency leads to the creation of U(IV) species in the
compound.
643
fof
fof
fof
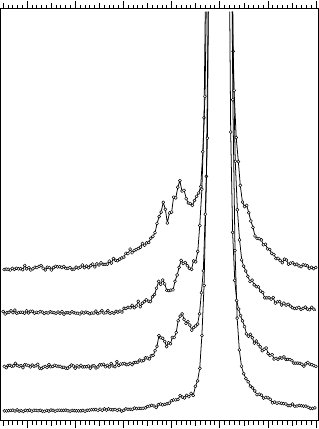
Figure 14.33 Resonant inelastic X-ray scattering spectra of U oxides recorded at the incident
photon energy of 99.9 eV.
Charge-transfer effects are expected to be significant in actinide
compounds as a result of metal 5f-ligand 2p hybridization. The analysis of our
data [114] obtained at the U 3d
5/2
threshold shows that the ligand 2p
o
U 5f
charge-transfer plays an important role in uranium compounds, such as UO
2
,
UO
2
(NO
3
)
2
u 6H
2
O, and even in UF
4
.
This is also supported by theoretical studies. Molecular orbital calculations
by several research groups [120, 121, 122, 123] gave values for the 5f
occupancy, which range from 2.3 to 2.9 electrons, while this occupancy was
estimated at about 2.3 electrons from the analysis of X-ray absorption and
photoemission data within an Anderson impurity model [124, 125]. These
results indicate a significant degree of covalency for U–O chemical bonds in
UO
2
. For UF
4
, a 5f contribution of a0.3 electron to the bonding orbitals was
also predicted from relativistic Dirac-Slater local density calculations [126].
For compounds containing U
6+
, the degree of covalency for metal-ligand
bonds is expected to be even higher than that for U
4+
systems. For example,
molecular orbital calculations yielded the 5f occupancy of a2.6 electrons for
the uranyl ion UO
2+,2
[127, 128]. Although the values for the 5f occupancy
obtained from molecular orbital calculations seem to be overestimated [129],
one cannot rule out the importance of the U 5f-ligand 2p hybridization even in
a compound with “ionic” bonds such as UF
4
.
644
14. Soft X-Ray Emission and Resonant Inelastic X-Ray Scattering Spectroscopy
Intensity (arb. units)
-4 -3 -2 -1
0 1 2
Energy loss (eV)
UO
3
UO
3-
δ
(reduced)
UO
2
U
3
O
8
U 5d RIXS
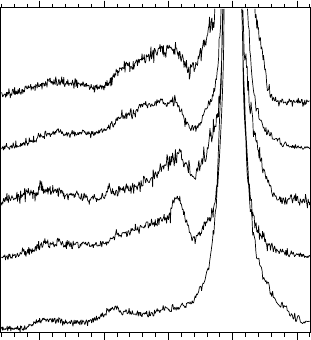
14.5 Applications
The consequence of high covalency and hybridization in the ground state
is an appearance of charge-transfer satellites in high energy spectroscopic
data. Figure 14.34 illustrates the dependence of the RIXS spectra on the
chemical environment of U atoms in various compounds.
Figure 14.34 Resonant inelastic X-ray scattering spectra of a number of U compounds
recorded at the incident photon energy of 115.0 eV.
The spectra were measured under conditions when the inelastic RIXS
cross-section is enhanced for the ligand 2p → U 5f charge-transfer transitions,
in particular, when the energy of the incident photon beam is tuned to the
main edge of the U 5d X-ray absorption spectrum (see Figure 14.31). At these
energies, the charge-transfer excitations dominate RIXS spectra, thus defining
the RIXS profile. The profiles can be clearly divided into three groups: (i)
UF
4
, (ii) UO
3
and UO
3–d
, (iii) U
3
O
8
and UO
2
. The differences between profiles
are determined by the character of the bonding and the local geometrical
arrangement of ligands, i.e., local crystal structure.
14.5.3.7 Molecular Systems and Liquids
Free Molecules: During the last decade, it became possible to measure sub-
keV X-ray emission of molecules in gas phase using excitation by
monochromatized synchrotron radiation. This was demonstrated by [130],
which presented resonant X-ray spectra of free molecular oxygen [130],
where the results gave conclusive proof that parity is conserved in the two-
photon, absorption-emission X-ray process. Cases where vibronic coupling
can lower the symmetry upon core excitation have been observed, notably
CO
2
. It has been demonstrated that by detuning the excitation energy down
Intensity (arb. units)
-15 -10
-5
0 5
Photon energy (eV)
UO
3
UO
2
UO
3-
δ
(reduced)
U
3
O
8
UF
4
U 5d RIXS
645
from resonance one effectively restores the symmetry by virtue of an effective
shortening of the X-ray scattering time [131]. Further, the symmetry-selective
character of this spectroscopy, spectator shifts, angular anisotropy, vibronic
coupling leading to symmetry breaking, and different types of interference
effects in the resonant and nonresonant spectra of molecular N
2
,O
2
, CO, CO
2
have been studied. Some of the molecular studies serve as illustrative
examples of the properties of resonant soft X-ray emission spectroscopy.
Figure 14.35 shows the nitrogen K emission spectra recorded at various
excitation energies, which resonantly promote a core electron to various
unoccupied levels of different symmetry. The top spectrum represents the
nonresonant case where continuum states above the ionization threshold are
excited. These are infinitely degenerate with respect to all symmetries. The
spectrum shows three bands, representing emission from the three outermost
valence orbitals of both gerade (g)andungerade (u) symmetry. When, on the
other hand, the excitation is to a level with a defined symmetry the emission
spectrum appears different, not showing emission from all three levels.
Instead, two, or even a single band, are observed in this case. When exciting
to a Rydberg state of ungerade symmetry, the 3S
u
, only emission from the
two ungerade levels are observed. The 3sσ
g
excitation leads to a single
emission band, which corresponds to the 3σ
g
state. This also KROGVIRUWKH
g
excitation where the 3σ
g
–1
g
1
g
final state is seen. In that spectrum, the high
energy band at ~400.5 eV is the so-called participator transition, where the
excited electron fills the core hole. One can note energy shifts of the 3σ
g
-
related emission in these spectra that are caused by the presence of the excited
electron in the intermediate and final state. The solid lines in Figure 14.35
represent simulations based on potential energy curves taken from the
literature and lifetime vibrational interference theory describing the
vibrational bands [132].
For molecules having inversion symmetry experimental spectra obey a g
→
g selection rule with respect to unoccupied (respectively, occupied) orbitals
[130, 132, 133, 134, 135, 136]. In a molecular orbital picture describing also
the core orbitals this comes out naturally because the dipole nature of the
transitions implies that the parity has to change at the absorption or the
emission of a photon, e.g., g
→
u or u
→
g, as described earlier. In the full
absorption-emission transition we have a g
→
u
→
g or u
→
g
→
u rule, which
explains the observations for N
2
. For polyatomic molecules, where vibrations
of non-total symmetry are possible, selection rules are observed to relax
because of dynamical (vibronic) symmetry breaking in the intermediate core
state. This effect can be observed in one of the simplest molecules of this
kind, CO
2
, where only two non-totally symmetric vibrations are present [131].
646
14. Soft X-Ray Emission and Resonant Inelastic X-Ray Scattering Spectroscopy
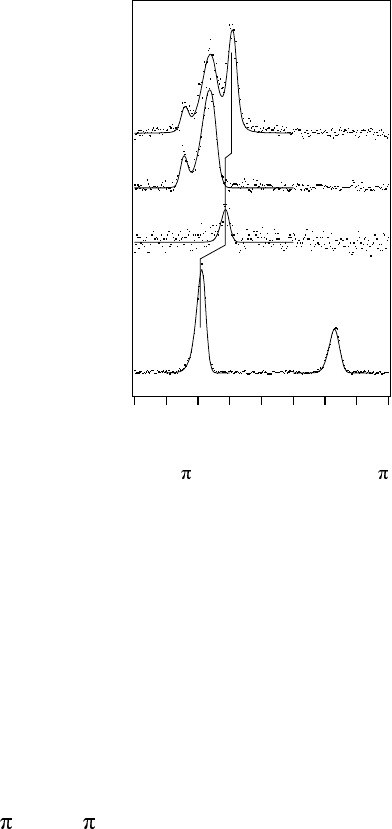
14.5 Applications
Figure 14.35 X-ray emission spectra of molecular N
2
. From the top, nonresonant spectrum,
resonantly excited to the Rydberg 3S
u
level, 3sσ
g
andtothe1
g
valence level, respectively.
Note the g-g selection rule, which holds for the resonant spectra.
The strict symmetry selection rules appropriate for a particular molecule
as described above can in some cases be broken [137]. For larger molecules
symmetry-forbidden transitions have been observed [131, 138]. As these
molecules possess a vast number of vibrations, the results are difficult to
describe in detail. Just as N
2
or O
2
are good cases for demonstrating that the
parity selection rule is valid, CO
2
is as good a candidate to study the effect of
symmetry breaking. The central carbon atom between the two oxygen atoms
adds an antisymmetric stretch mode that can couple the gerade and ungerade
oxygen core excited states and therefore break the parity selection rule [137].
of CO
2
and O
2
. The resonant excitations are to the first empty molecular
orbitals, 2
u
and 1
g
respectively. In the O
2
case only symmetry-allowed
transitions are observed [130], in a similar way as was described for N
2
[132],
i.e., only transitions that give gerade final states. The resonant O
2
spectrum
consists of only two bands, a spectator and a single participator. Especially
the absence of the
3
Σ
u
–
final state proves the validity of the selection rules.
647
Figure 14.36 shows resonant and nonresonant oxygen K emission spectra
Intensity (arb. units)
404
402
400
398
396
394
392
390
388
Emission ener
gy
(eV)
N1s -3s
σ
g
Non-resonant
N1s -1
π
g
(v=0)
Resonant
N1s -3p
π
u
3
σ
g
-1
3
σ
g
-1
1
π
g
1
π
u
-1
2
σ
u
-1
N
2
N-K emission
(spectator)
(participator)
X
1
Σ
g
+
a
1
Π
g
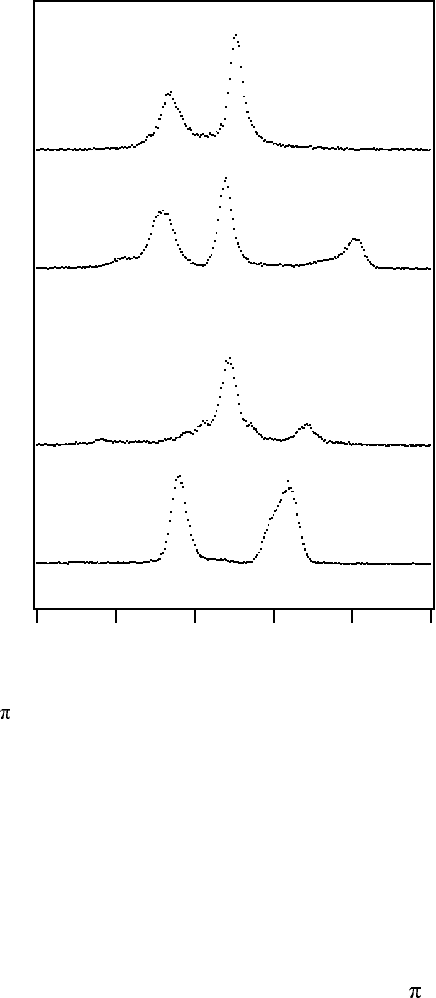
Figure 14.36
-excited and nonresonant oxygen K emission spectra of CO
2
and O
2
illustrating
the effect of symmetry-breaking in the intermediate core excited states of CO
2
. Symmetry-
forbidden transitions in ground state symmetry are given intensity by non-totally symmetric
vibrational excitations lowering the symmetry of the intermediate state. The resonant O
2
spectrum only shows intensity from symmetry-allowed transitions similar to the case of N
2
.
In contrast to how different the nonresonant and resonant spectra of O
2
are
the two CO
2
spectra are rather similar. Here, intensity from all valence
orbitals is observed in both spectra, despite the symmetry-selective excitation
to an ungerade state, providing proof that the symmetry of the intermediate,
core-excited state is broken. In particular, the appearance of the high energy
spectator transition involving the outer valence orbital, 1
g
, would be strictly
forbidden if the inversion symmetry would be retained in the core-excited
state. The relative intensities between the high and low energy bands are not
648
14. Soft X-Ray Emission and Resonant Inelastic X-Ray Scattering Spectroscopy
Intensity (arb. units)
540535530525520515
Ener
gy
(eV)
CO
2
Nonresonant
Resonant
O1s
- 1
π
g
O
2
Resonant
O1s
- 2
π
u
1
π
g
-1
1
π
u
-1
3
σ
g
-1
1
π
g
-1
1
π
u
-1
3
σ
u
-1
4
σ
g
-1
2
σ
u
-1
(participator)
(participator)
(spectator)
(spectator)
Nonresonant
1
π
g
-1
2
π
u
3
σ
g
-1
1
π
g
3
Π
g
14.5 Applications
the same in the nonresonant and resonant spectra. The deviation can give
information about the strength of the vibronic coupling and hence how strong
the symmetry breaking is [131]. The fact that some effect of the selection rule
pertains can be used in the determination of the parity of specific empty states
probed by resonant excitation [138]. The effect of symmetry-breaking is very
clear for CO
2
but observations of “forbidden” lines in other molecular systems
have been made, like in C
6
H
6
[136], and C
60
[133], which can also be
explained by the vibronic coupling.
It has been observed that the symmetry selection rule in polyatomic
molecules is more strict when the narrow bandpass excitation is detuned
below the first resonance [135, 136]. This is discussed in more detail for the
CO
2
molecule [131] where the above discussed forbidden high energy band
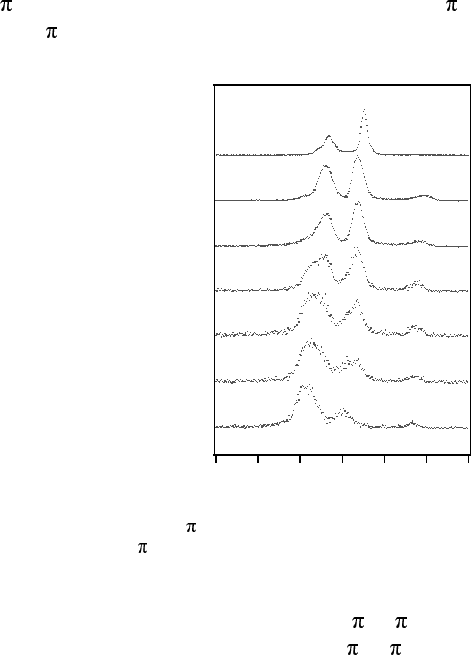
decreases monotonically with the detuning energy. Figure 14.37 shows the
detuning spectra referring to resonant excitation to the first unoccupied level,
u
,ofCO
2
giving the forbidden |g 〉 =1σ
u
–1
2
u
1
g
andallowed|g〉 =
1σ
g
–1
2
u
1
u
core-excited states.
Figure 14.37 Oxygen K 2
u
resonant X-ray emission spectra of CO
2
with different detuning
energies below the 2
u
resonance.
The spectra consist of two peaks; the high energy peak derives from
transitions to the forbidden |f(u) 〉 =|1
g
–1
2
u
〉
1
u
final state, and the low
energy peak to the allowed |f(g) 〉 =|1
u
–1
2
u
〉
1
g
final state (configuration
state splitting and contribution from the weak 4σ
g
emission can be neglected).
As seen in the figure, the high energy peak receives significant intensity in
649
Intensity (arb. units)
540535530525520515510
Energy (eV)
1
π
g
-1
1
π
u
-1
3
σ
u
-1
4
σ
g
-1
CO
2
O-K emission
resonant O1s-2
π
u
535.0 eV
-1.0 eV
-1.5 eV
-1.75 eV
-2.0 eV
-2.3 eV
Nonresonant
551 eV

violation of the selection rule and appears quite like the nonresonant X-ray
emission spectrum of CO
2
. Thus, the core-excited state cannot be described as
a pure |1
–1
2
u
〉
1
u
state, but must be mixed with the |1
u
–1
2
u
〉
1
g
state. By
detuning the energy it is clear that the spectrum becomes symmetry-purified.
In a time-dependent picture this can be seen as an effective shortening of the
scattering time, allowing sub-femtosecond study of dynamics.
Liquids: Our microscopic understanding of a liquid is very much based on the
study of spatial and spatio-temporal correlation functions. The study of
correlations allows us to appreciate the local organization of one molecule
surrounded by others, and to unravel the microscopic dynamics. Neutron
diffraction and scattering have played and continue to play a major role in
these studies. X-ray diffraction is important as well, and recently X-ray
inelastic scattering has become available. Using X-ray absorption and
selectively excited X-ray emission spectroscopy to probe unoccupied and
occupied electronic states, one can establish a firm interpretation for the
unusual thermodynamic properties of molecular liquids. Furthermore, one can
elucidate finer details of the structural properties of molecular liquids. XAS
and XES spectra reflect the local electronic structure of various
conformations; in this case, the oxygen X-ray absorption and emission line-
shapes are sensitive to the hydrogen bonding configurations.
Water is a very abundant substance on our planet, and it is the principal
constituent of all living organisms. Chemical reactions taking place in liquid
water are essential for many important processes in electrochemistry,
environmental science, pharmaceutical science, and biology in general. Many
models have been proposed to view the details of how liquid water is
geometrically organized by hydrogen bond networks. The hydrogen bond is
an attractive interaction forming a link of a hydrogen atom with a highly
electronegative and nonmetallic element that contains a lone pair of electrons.
Although H-bonds are much weaker than conventional chemical bonds, they
have important consequences on the properties of water. Diffraction of X-rays
[139] and neutrons [140] provide strong evidence that tetrahedral hydrogen-
bond order persists beyond the melting transition, but with substantial
disorder present [141]. Important questions remain about the precise nature of
the disorder and how it is spatially manifested.
The local structure of liquid water is still under debate. It has been
demonstrated that soft X-ray emission spectra, emanating from the radiative
decay, subsequent to core excitation, can be useful in assigning structures in
X-ray absorption spectra [138, 142, 143]. Especially, it has been shown that
the resonantly excited SXE measurements on liquid water are compatible with
the traditional view that three and four hydrogen bonds dominate the structure
[142].
650
14. Soft X-Ray Emission and Resonant Inelastic X-Ray Scattering Spectroscopy
g
14.5 Applications
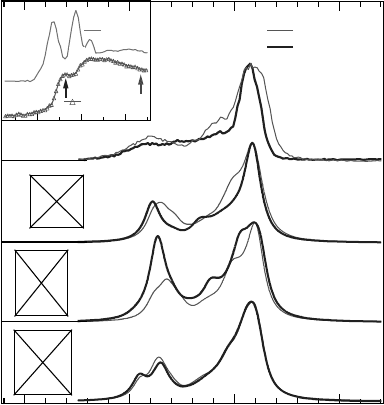
An X-ray absorption study of liquid water [144] suggests that the four
hydrogen-bonding networks mainly contribute to a single broad feature, while
a shoulder located at 534.7 eV suggests the presence of broken hydrogen
bonds. Indeed, recent theoretical simulations assign this pre-edge structure to
a particular three-hydrogen bond structure with one missing hydrogen bond at
the hydrogen site.
This assignment is fully confirmed by X-ray emission spectra, excited on
this particular pre-edge shoulder (Figure 14.38). Different local structures
provide different nonresonant spectra because of their different valence band
structures. The nonresonant spectrum should be a sum of the ones from all
different structures. However, since the SYM structure is the dominating
structure in the liquid water, its nonresonant spectrum is close to the
experimental one. Compared to the nonresonant spectrum we observe a
substantial narrowing of the 1b
1
peak at 526 eV, and a further attenuation of
the 3a
1
-associated structure at 524.5 eV. This is in excellent agreement with
the predictions for the DASYM configuration.
Figure 14.38 The sharp structures in the X-ray absorption spectrum of the water molecule
(inset) are smeared out in the liquid water due to the diffuse nature of the corresponding
molecular orbitals. A pre-peak is resolved at 534.7 eV in the spectrum of liquid water. The
arrows indicate the photon energies used for resonantly and normally excited X-ray emission
spectra. The resonantly excited spectrum is in good agreement with the prediction for the three-
bonded D-ASYM configuration.
Intensity (arb. units)
530525520515
Energy (eV)
Normal
Resonant
SYM
A-ASYM
D-ASYM
Experiment
540
536532
O 1s
gas water
2b
2
4a
1
liquid water
651
The early hypothesis of cyclic structures by Pauling [145] has been both
supported [146] and contested [147] by neutron diffraction analysis, the
competing interpretation being that the majority of liquid molecules are
ordered in chains with up to 10 members [147, 148] or linear trimer-tetramer
chains [149]. This uncertainty is accentuated when one considers alcohol-
water solutions. The observed entropy increase upon mixing alcohol and
water is much smaller than expected for an ideal solution. Early neutron
diffraction data provide structure information of water cages around
hydrophobic headgroups in solution [150, 151, 152, 153]. Recently, a new
neutron diffraction analysis demonstrated that incomplete mixing at the
molecular level is essential to understanding the smaller than expected
entropy increase [154]. This contrasts with the prevailing view that a
rearrangement of the water network around hydrophobic methyl groups of the
methanol clusters is the main feature behind the unusual thermodynamic
properties [155].
A comparison of the theoretical and experimental X-ray emission spectra
(in Figure 14.39) associated with possible molecular arrangements makes it
obvious that both rings and chains are present in liquid methanol. The maxima
at 526 eV and 527 eV can be assigned to ring and chain structures,
respectively. In addition, we find that the measured spectra are typical for
rings and chains consisting of 6 and 8 molecules. Configurations with fewer
than 6 molecules do not give representative predictions, and odd-numbered
configurations yield an unobserved high energy feature at 528 eV. Chains
with more than 10 molecules are unlikely in light of neutron diffraction data
[147, 148]. Therefore, the 6- and/or 8-membered rings and chains provide the
predominant structures in liquid methanol. From the excitation energy-
dependence we can state that the pre-shoulder in the XA spectrum is due to
ring structures (A); that the onset of absorption in the chains is located at the
main absorption band (B); from the emission spectrum corresponding to high
energy excitations (C) we estimate that the relative abundance of chains and
rings in liquid methanol is equal.
Details in the water-methanol interaction are revealed in the X-ray
emission spectra generated at excitation energies near threshold. The X-ray
emission spectra that are excited in this region (Figure 14.40) are significantly
different from the emission spectra of the pure liquids. The 0.8-eV linewidth
of the main peak is narrower than any X-ray emission feature of either liquid
phase: for water (1.0 eV), for methanol (1.9 eV). The main peak E
1
shifts
towards higher energy as the excitation energy is lowered from 532.5 eV to
531.5 eV and 530.6 eV. We also note that the spectrum excited at 530.6 eV
shows depletion of intensity in the region around 525 eV. One can conclude
that specific bonded structures involving water as well as methanol are
responsible for this spectral behavior.
652
14. Soft X-Ray Emission and Resonant Inelastic X-Ray Scattering Spectroscopy
