Vij D.R. Handbook of Applied Solid State Spectroscopy
Подождите немного. Документ загружается.

12.2 Spontaneous Emission, Absorption, and Induced Emission
511
It is rare the case when one can insert in a scientific article a literary citation.
This happens to be the case here. In The Sorrows of Young Werther of Goethe,
the protagonist is unable to see the woman he loves because of an engagement
he cannot refuse, and sends a servant to her, “only so that I might have someone
near me who had been in her presence…” This is then his reaction when the
servant comes back:
It is said that the Bologna stone, when placed in the sun,
absorbs the sun’s rays and is luminous for a while in the dark. I
felt the same with the boy. The consciousness that her eyes had
rested on his face, his cheeks, the buttons of his jacket and the
collar of his overcoat, made all these sacred and precious to me.
At that moment I would not have parted with him for a
thousand taler. I felt so happy in his presence [1].
The second important investigation on luminescence is due to Stokes and
dates to the year 1852. Stokes observed that the mineral fluorspar (or fluorite)
when illuminated by blue light gave out yellow light. Fluorite (CaF
2
) is colorless
in its purest form, but it absorbs and emits light when it contains such impurities
as Mn, Ce, Er, etc. The term “fluorescence” was coined by Stokes and has
continued to be used to indicate short-lived luminescence. A Stokes’ law has
been formulated according to which the wavelengths of the emitted light are
always longer than the wavelength of the absorbed light.
12.2 SPONTANEOUS EMISSION, ABSORPTION, AND
INDUCED EMISSION
12.2.1 Classical Bound, Radiating Electron
Let us consider first the equation of motion of a classical bound electron that we
assume to be nonradiating:
2
2
dx
Fkxm
dt
(12.1)
or
0Ȧ
2
0
2
2
x
d
t
xd
(12.2)
where
0
Ȧ
k
m
. (12.3)
The energy is given by
12. Luminescence Spectroscopy
512
22
11
const
22
EKEPE mv kx
. (12.4)
We take as solution to the equation of motion (12.1),
00
cos(Ȧ ).xx t
(12.5)
This means that at time t = 0, x = x
0
, and x = v = 0; the energy is then given by
222
000
11
Ȧ
22
Ekx mx
(12.6)
A radiating electron will produce radiation due to the vibrating dipole
0
Ȧ
00 0
cos(Ȧ )Re
it
dexex t exe (12.7)
The average energy radiated in the unit time is given by
2
42
2
00
33
2
Ȧ
.
33
d
ex
S
cc
(12.8)
The radiated energy is given out at the expense of the internal energy of the
electron:
22
00
1
Ȧ
2
E
SEmx
t
w
§·
¨¸
©¹
w
(12.9)
or
42
2
2
00 0
00
3
Ȧ
Ȧ
3
ex x
mx
ct
w
w
2
2
0
00
3
Ȧ
3
e
xx
c
(12.10)
We can write
00
1
Ȗ
2
xx
(12.11)
where
22 22
2
2
00 00
0
32
2Ȧ 2Ȧ 2Ȧ 2Ȧ
Ȗ
IJ
33 33
e
e
r
mc c mc c
(12.12)
and
2
13
0
2
24
0
2.8 10
IJ 9.38 10 sec.
e
r
mc
r
c
u
u
cm,
12.2 Spontaneous Emission, Absorption, and Induced Emission
513
If O = 6000 Å, Z
o
= 3 u 10
15 8 –1
(12.11) is
1
Ȗt
2
00 0
, const.xXe X
(12.13)
Therefore,
2
1
Ȗt
22 2
2
00 0 0
22
Ȗt Ȗt
00 0
Ȧ
11
Ȧ
22
1
Ȧ
2
mEmx Xe
mXe Ee
§·
¨¸
©¹
(12.14)
where
22
000
1
Ȧ .
2
EmX
(12.15)
J is the rate of radiative decay for the classical electron.
The equation of motion, equation (12.1), needs a revision on account of the
presence of radiation. We proceed as follows:
1
Ȗ
2
00
cos(Ȧ )
t
xXe t
(12.16)
1
Ȗ
2
00 0
Ȗ
Ȧ sin(Ȧ )
2
t
xxXe t
(12.17)
and
2
1
Ȗ
2
00 0
ȖȖ
Ȧ sin(Ȧ )
22 2
t
xxXe t
§·
¨¸
©¹
J
(12.18)
1
Ȗ
2
2
00 0 0
ȖȖ
Ȧ sin(Ȧ ) Ȧ .
22
t
xx Xe tx
(12.19)
Summing the above two relations we obtain
2
2
0
Ȗ
Ȗ
Ȧ 0,
2
xx x
ªº
§·
«»
¨¸
©¹
«»
¬¼
(12.20)
which represents the equation of motion of the radiating, bound electron.
12.2.2 Quantum Mechanical Radiative Decay
of the lower (upper) level are E
ab ab
wavefunction of the system, then
, andJ = 0.6 u 10 sec , the solution of
(E ) and ( ), respectively. If
is the
Consider a two-level quantum system, where the energy and the eigenfunction
ȥ
ȥȥ
12. Luminescence Spectroscopy
514
ȥ
ȥiH
t
w
w
= (12.21)
where
ȥȥ
.
ȥȥ
aaa
bbb
HE
HE
®
¯
(12.22)
In general, a quantum state of the system is given by
ȥȥ ȥ.
ab
ii
Et Et
ab
ae be
==
(12.23)
For a non-radiating system, H is independent of time:
0 const
0 const
aa
bb
o
®
o
¯
(12.24)
and
22
const.
ab
HaEbE¢² (12.25)
Let us now allow for dipolar radiation
00
Ȧ
0
ȦȦ
0
ȥȥ
ȥȥ ȥȥIJ
ȝȝ
Re
ab ab
it
EE EE
it it it it
ab ab
it it
ab ba
ex ex
ae be ex ae be d
abe abe
de
ªºªº
«»«»
¬¼¬¼
ªº
¬¼
³
== ==
(12.26)
where
0
Ȧ
ba
EE
=
(12.27)
ȝȥȥ
ab a b
ex (12.28)
ȝȥȥ
ba b a
ex (12.29)
0
2
ȝ
ab
dab
(12.30)
and where we have assumed
ȝȝ0.
aa bb
(12.31)
We have now from equation (12.8)
2
4
4
22
0
000
33
22
44
22 2
00
33
4Ȧȝ
Ȧ
33
4Ȧȝ 4 ȝ
1
33
ab
ab ab
dd
Sab
cc
bb b
cc
(12.32)
Ȧ
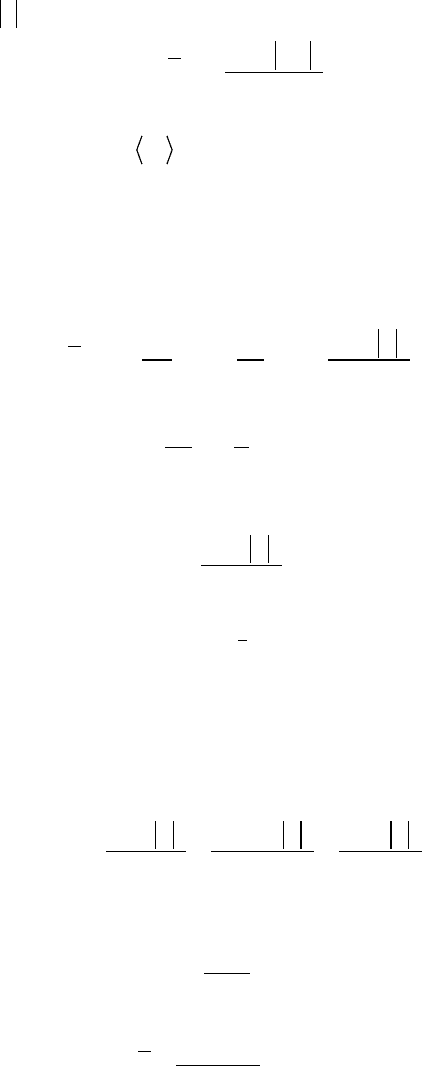
12.2 Spontaneous Emission, Absorption, and Induced Emission
515
assuming
2
<<1b . We can choose the phase of <
b
that makes b real:
2
4
0
2
3
4Ȧȝ
.
3
ab
Sb
c
(12.33)
The energy of the system is given by
2
22 2
2
1
bab a
ba a
EHbEaEbE bE
bE E E
(12.34)
If we set E
a
= 0, we obtain
2
0
,ȦEb = (12.35)
which can be contrasted with the classical formula (equation (12.6)). For non-
radiating systems, E remains constant. For radiating systems
2
4
0
2
0
3
4Ȧȝ
2 Ȧ
3
Eb
Sb b
tt c
ww
ww
=
(12.36)
where we have dropped the subscript ab from P. Then
1
Ȗ
2
b
b
t
w
w
(12.37)
where
2
3
0
3
4Ȧȝ
Ȗ
.
3c
=
(12.38)
The solution of equation (12.37) is
Ȗ
2
0
t
bbe
(12.39)
and
2
2
Ȗ
0
.
t
bbe
(12.40)
b
2
represents the probability of occupancy of the excited state. J is the quantum
mechanical rate of radiative decay or spontaneous emission. We may equate it
to the Einstein coefficient A:
222
3
43 4
0
333
4Ȧȝ 64ʌȝ 64ʌȝ
Ȗ
333Ȝ
A
chch
Q
=
(12.41)
In a nonmagnetic medium of index of refraction n an oscillating dipole emits
the power
3
2d
3
Sn
c
(12.42)
whose time average is
4
000
3
Ȧ
.
3
dd
Sn
c
(12.43)
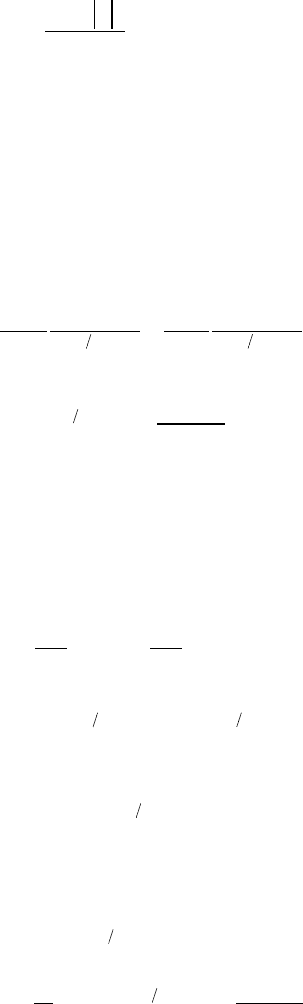
12. Luminescence Spectroscopy
516
Therefore, in this case,
2
3
0
3
4Ȧȝ
.
3
A
n
c
=
(12.44)
12.2.3 Absorption and Emission
Consider a cavity whose walls are at temperature T, and containing radiation and
an ensemble of atoms, and let each atom be represented by a two-level quantum
mechanical system with an energy level separation of
0
Ȧ= . In thermal
equilibrium, then, energy density per unit angular frequency range at Z
0
is given
by [2]:
0
00
32
3
3
000
Ȧ
ȦȦ
23 23
ȦȦȦ
ȡ
ʌ 1 ʌ 1
kT kT
n
n
ce ce
==
==
(12.45)
where
n index of refraction of the medium inside the cavity. We can write
0
0
3
3
Ȧ
0
Ȧ
23
Ȧ
ȡ 1.
ʌ
kT
n
e
c
=
=
(12.46)
In addition, because of detailed balance,
00
ee e
221Ȧ 212Ȧ 1
ȡȡ,
A
NB NB N (12.47)
where
e
1
N and
e
2
N are the equilibrium populations of atoms in the lower and
upper levels, respectively, and
0
21 Ȧ
ȡB and
0
12 Ȧ
ȡB are the probabilities per unit
time of induced downward and upward transitions, respectively. We can write
00
ee
22
21 Ȧ 12 Ȧ
ee
11
ȡȡ
NN
AB B
NN
(12.48)
and
00
00
ȦȦ
21 Ȧ 12 Ȧ
ȡȡ
kT kT
Ae B e B
==
(12.49)
or
0
0
Ȧ
Ȧ 12 21
ȡ .
kT
B
eBA
=
(12.50)
We set
12 21
.
B
BB
(12.51)
Then
0
0
Ȧ
Ȧ
ȡ 1
kT
eBA
=
(12.52)
0
0
3
3
Ȧ
0
Ȧ
23
Ȧ
ȡ 1
ʌ
kT
n
A
e
B
c
=
=
(12.53)
and

12.2 Spontaneous Emission, Absorption, and Induced Emission
517
2
3
23 23 2
2
0
33
322
33
00
4 Ȧȝ
ʌʌ 4ʌ
ȝ
33
ȦȦ
n
cc
BA
cn
nn
==
==
(12.54)
or
3
2
1
0
3
23
2
22 2
4 Ȧ
ȝ sec
3
4ʌ cm
ȝ
3erg-sec
n
A
c
B
n
°
°
®
§·
°
¨¸
°
©¹
¯
=
=
(12.55)
3
3
0
23 3
Ȧ
erg-sec
.
ʌ cm
n
A
Bc
§·
¨¸
©¹
=
(12.56)
Let us now consider the more realistic situation in which the energies of the
two atomic levels are not sharply defined, but have a certain width'Zsuch that
0
Ȧ <<Ȧ' . We can set the following:
2 Ȧ
ȦNAd
= number of atoms that per unit time that decay by spontaneous
emission, giving out a photon with angular frequency in (Z, Z +
dZ);
2 Ȧ
ȦNBd
transition by induced emission,
g
ivin
g
out a photon with an
g
ula
r
1 Ȧ
ȡȦNB d
= number of atoms that in the unit time under
g
o an upward transition
by the absorption of a photon with an
g
ular frequenc
y
in
(Z, Z + dZ).
If we put a filter between the atoms and the walls that allows only the
radiation in the narrow band dZ to interact with the atoms we have
ee e
2 Ȧ 2 ȦȦ 1 ȦȦ
ȦȡȦ ȡȦNAd NB d NB d (12.57)
or
ee
22
ȦȦȦ ȦȦ
ee
11
ȡȡ
NN
AB B
NN
. (12.58)
Then
00
ȦȦ
ȦȦȦ ȦȦ
ȡȡ
kT kT
Ae B e B
==
(12.59)
0
Ȧ
ȦȦȦ
ȡ 1
kT
eBA
=
(12.60)
and
= number of atoms that in the unit time undergo a downward
frequency in (
Z, Z + dZ); and
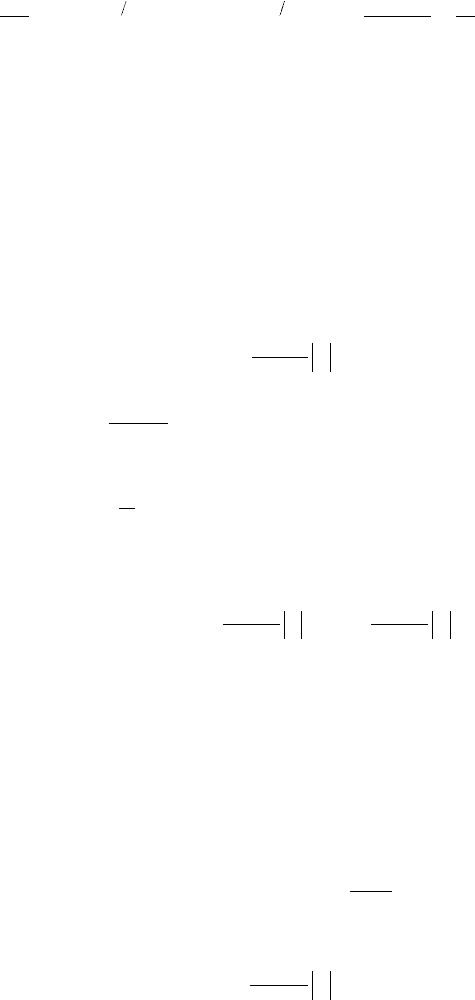
12. Luminescence Spectroscopy
518
00
0
3
3
ȦȦ
Ȧ 0
ȦȦ
23
Ȧ
Ȧ
ȡ 1 ȡ 1.
ʌ
kT kT
An
A
ee
B
cB
|
==
=
(12.61)
If g(Z) indicates the spectral lineshape:
ȦȦ1gd
³
(12.62)
we can write
ȦȦ
ȦȦ
Ȧ ; Ȧ
Ȧ ; Ȧ
A
A
g
Ad A
B
B
g
Bd B
°
®
°
¯
³
³
(12.63)
If we call w(Z)dZ the probability per unit time that an atom undergoes an
induced transition by absorbing or emitting a photon with angular frequency in
(Z,Z + d), we find
2
2
ȦȦ
22
2
2
4ʌ
ȦȦ ȦȡȦ ȝȡ ȦȦ
3
4ʌ
ȦȦȦ
3
wd Bg d gd
n
Igd
nc
=
=
(12.64)
where
Ȧ
ȦȦȡ Ȧ
c
Id d
n
= intensity of radiation with angular
frequency in (Z,Z + dZ). (12.65)
We can write
0
22
22
Ȧ 0
22 2
4ʌ 4ʌ
ȦȦ ȝȡ ȝ Ȧ
33
wd I
nnc
³
==
(12.66)
12.2.4 Absorption Coefficient and Absorption Cross-Section
Let us assume that a plane wave goes through a certain medium in the x
direction. Let the medium consist of atoms that have two possible energy levels
and let N
1
(N
2
) be the concentration of atoms in the lower (higher) energy level.
The energy intensity per unit angular frequency range I(Z), when the wave
travels through the medium distance dx, undergoes a change given by
12
2
er
g
ȦȦ Ȧ
cm
dI w N N dx
§·
¨¸
©¹
=
(12.67)
But, from equation (12.64):
2
2
2
4ʌ
ȦȝȦȦ.
3
wIg
nc
=
(12.68)
Then
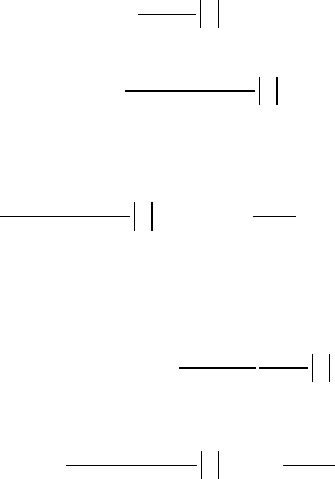
12.3 Measurements and Techniques
519
2
2
12
2
2
2
12
4ʌ
ȦȝȦȦȦ
3
4ʌ
ȝȦ Ȧ Ȧ Ȧ Ȧ
3
dI I g N N dx
nc
NN
gIdx Idx
nc
ªº
«»
¬¼
ªº
«»
¬¼
=
=
=
(12.69)
where the absorption coefficient D(Z) is given by
2
2
12
1
12
4ʌ
Ȧ
ĮȦ
ȝ
ȦȦ Ȧ cm
3
NN
n
gBNN
nc c
=
=
(12.70)
The solution of equation (12.69) is
Į
Ȧ; Ȧ;0
x
IxIx e
(12.71)
We define the
absorption cros-section of a radiative transition as follows
2
2
2
12
ĮȦ
4ʌ
ıȦ ȝ Ȧ Ȧ cm
3
g
NNnc
=
(12.72)
Note the following
2
2
12
11
0
012
4ʌ
Ȧ
ĮȦ Ȧ
ȝ
Ȧ cm sec
3
NN
n
dBNN
nc c
³
=
=
(12.73)
In Table 12.1, we have summarized the results obtained in the previous sections.
Luminescence spectroscopy relies on four fundamental measurements: (1)
absorption spectra, (2) luminescence spectra, (3) excitation spectra, and (4)
response to pulsed excitation. Technical improvements or breakthroughs have
not and probably will not produce any conceptually new additions to these four
basic measurements. The data that they make available can be considered
“scarce” when one contrasts them with the complexity of the processes.
However, an attentive and appropriate usage of the different techniques
generally yields a satisfactory model for the system under investigation.
Į
12.3 MEASUREMENTS AND TECHNIQUES
12.3.1 Absorption Spectra
The main purpose of absorption measurements is to set the energy level scheme
and to identify particular levels that may be convenient to “pump” the system for
the purpose of obtaining luminescence or laser emission. If the concentration of
the optically active centers is known, the ratio of the absorption coefficient and
of the concentration gives the
absorption cross-section.
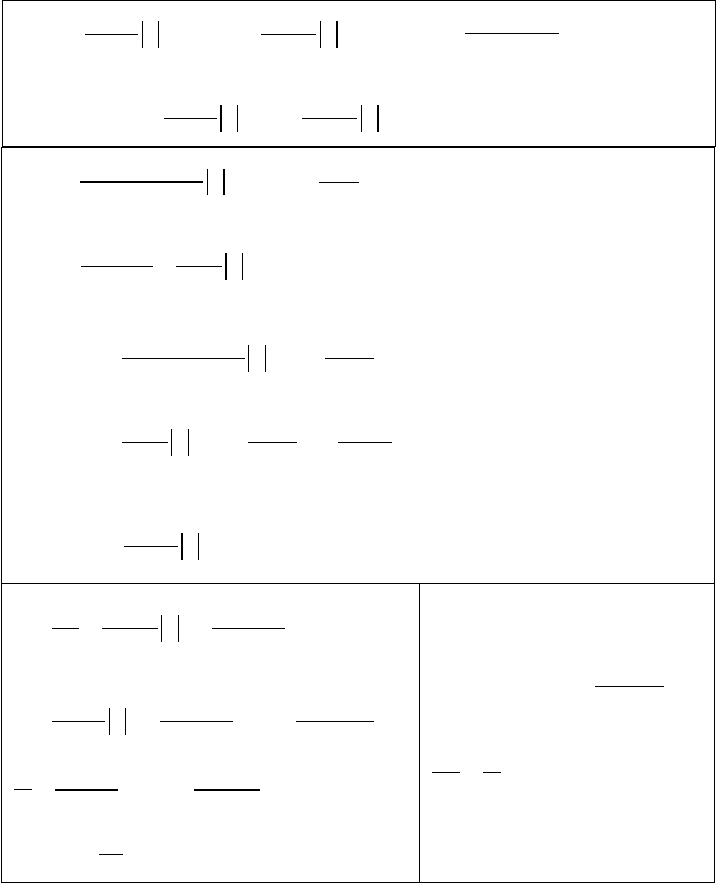
12. Luminescence Spectroscopy
520
2
2
12
1
Ȧ 12
4ʌ
Ȧ
ĮȦ ȝ Ȧ Ȧ cm
3
NN
n
gBNN
nc c
=
=
2
2
2
12
ĮȦ
4ʌ
ıȦ ȝ Ȧ Ȧ cm
3
g
NN nc
=
2
2
12
11
0
012
4ʌ
Ȧ
ĮȦ Ȧ
ȝ
Ȧ cm sec
3
NN
n
dBNN
nc c
³
=
=
222
2
21
0
0
Ȧ
4ʌ 2ʌ
ıȦ Ȧ ȝ Ȧ cm sec
3
n
e
dBf
nc c mcn
³
=
=
where the f-number is
2
0
2
2 Ȧ
ȝ
3
m
f
e
=
(pure number)
32
2
2
1
00
33
sp
4 Ȧ 2 Ȧ
1
ȝ sec
IJ 3
nen
Af
cmc
=
222 3
2
22 2 2
0
4ʌ 2ʌ cm
ȝ
3 Ȧ erg-sec
e
Bf
nmn
§·
¨¸
©¹
==
3
3
0
23 3
Ȧ
erg-sec
ʌ cm
n
A
Bc
§·
¨¸
©¹
=
2
sp
Ȝ
IJ 1.5f
n
Ȧ
= Ȧ pure numberAAg
2
Ȧ
cm
Ȧ
erg-sec
BBg
§·
¨¸
©¹
Ȧ
Ȧ
A
A
B
B
22
22
Ȧ
22 2
ȦȦ
4ʌ 4ʌ
ȦȝȡȦȝȦȦ
33 Ȧ
I
wgIg
nnc
V
== =
00
22
22
1
Ȧ 0 Ȧ
22 2
4ʌ 4ʌ
ȦȦ ȝȡ ȝ Ȧ ȡ sec
33
wwd I B
nnc
³
==
The conventional absorption measurements are related to transitions
originating in the ground state of the material. Transitions originating in an
excited state and ending up in still higher states may be observed if one is able to
populate the initial state. This procedure is called excited state absorption (ESA)
Table 12.1
Summary of Section 12.2 formulas.
