Spier C.A., Barros de Oliveira S.-M., Rosi?re C.A., Ardisson J.D. Mineralogy and trace-element geochemistry of the high-grade iron ores of the ?guas Claras Mine and comparison with the Cap?o Xavier and Tamandu? iron ore deposits, Quadril?tero Ferr?fe
Подождите немного. Документ загружается.

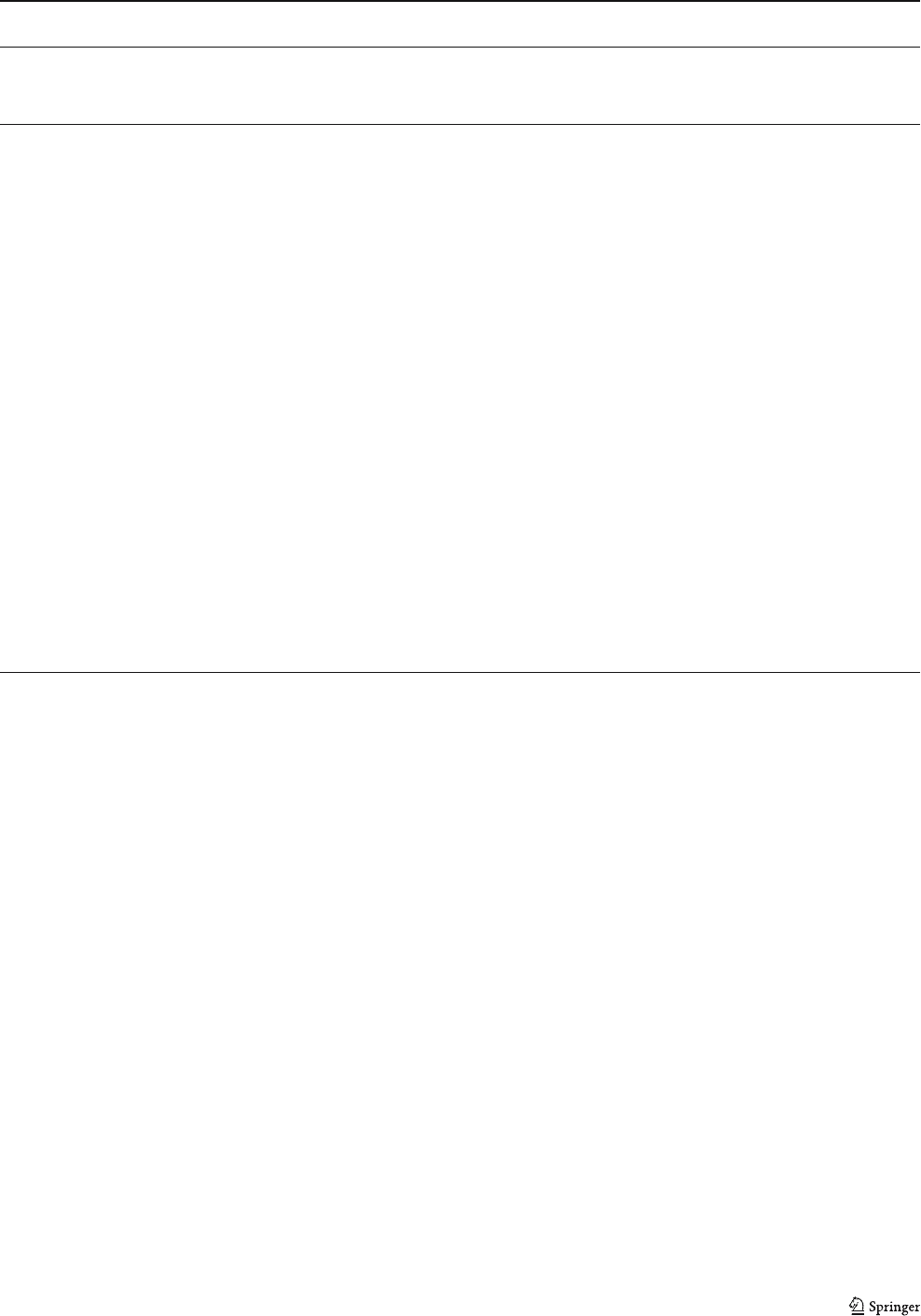
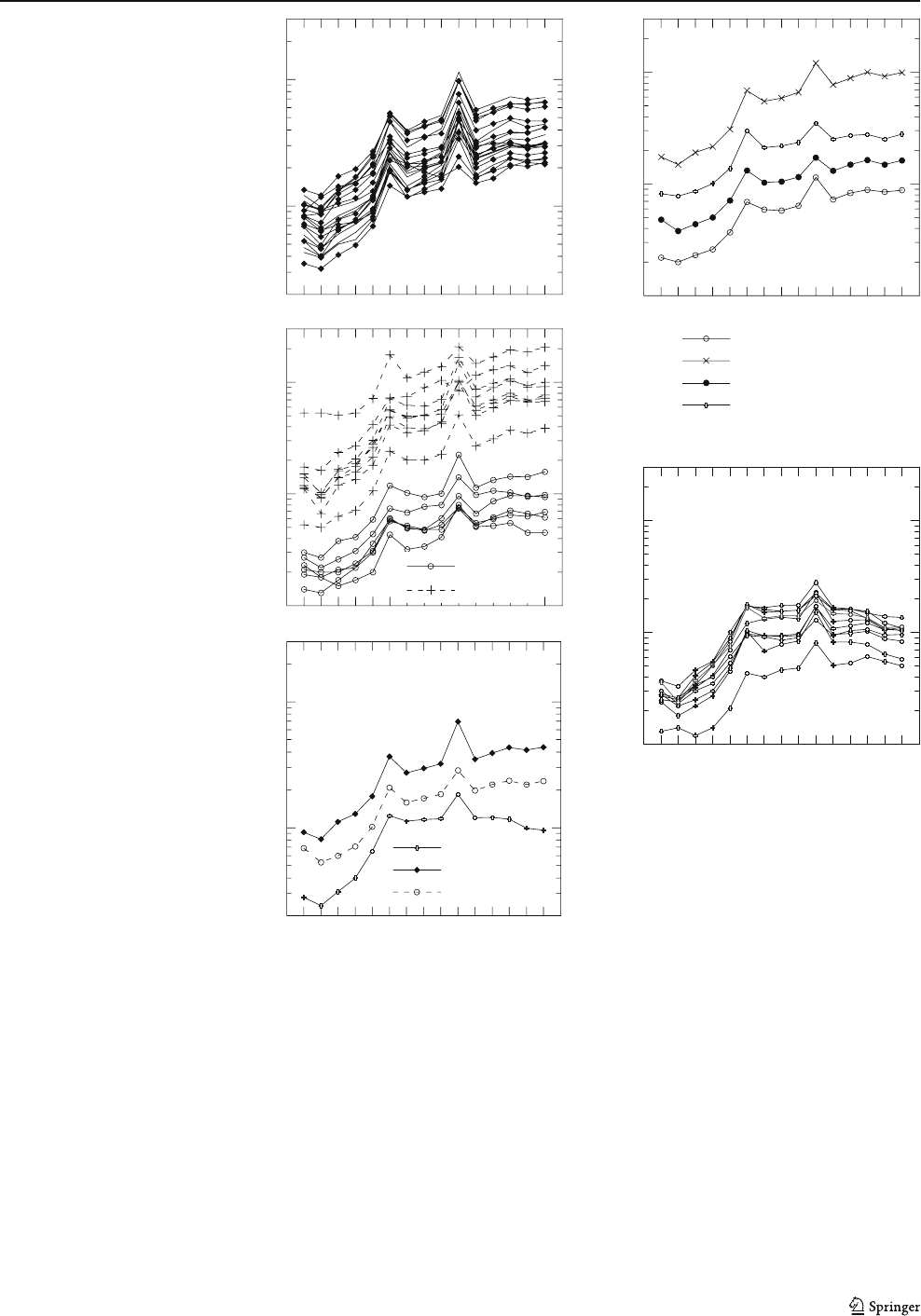
oxides (Figs. 8a–d and 9a). The soft ore is highly porous,
with intergranular porosity estimated in thin sections at 35–
45%. Hematite is the major iron oxide in both bands,
occurring as martite, anhedra l to granular/tabular hematite
and, locally, specularite. Magnet ite is locally observed as
relics within martite and granular hematite crystals, espe-
cially within thicker massive bands. Ferrihydrite (identified
by Mössbauer analyses; see text below) is more frequent
within the porous bands. Gangue minerals are rare,
increasing in amount in samp les obtained close to contacts
with both the protore and the phyllite. They are also more
frequent within the porous bands and consist of apatite,
talc, chlorite, and Mn oxides (Fig. 10a–f). Apatite is more
frequent in samples from the lower levels of the mine where
the pinnacles of dolomitic itabirite occur.
Even massive bands of iron oxide are porous, but they
have less than 30% of pore space, and individual pores are
isolated in massive hematite. Generally massive bands are
broken and disrupted, whereas porous bands show individ-
ual aggregates of hematite between pores (Fig. 8c,e).
The massive bands of the soft ore generally show a
granoblastic fabric and consist of fine granular and tabular
hematite (5–20 μm), forming a dense package of hematite
(Fig. 8g). The porous bands have variable porosity, visually
estimated at 30–60%. They consist of aggregates ranging
from 50 μm to 10 mm of granular and tabular hematite
crystals (up to 60%) and/or of individual crystals of tabular
hematite ranging from 10 to 30 μm (Fig. 8e,f ). Tabular and
granular hematite overgrows coarser martite crystals
(∼50 μm). Disaggregation of these coarse martite crystals
releases fine tabular hematite crystals (Fig. 8h). Hematite
crystals are surro unded by a brownish residue of poorly
crystalline ferrihydrite (Fig. 9a–d).
The SEM observations of the <0.15-m m size fraction of
some samples (Electronic supplementary material
Appendix A) of the soft ore detected a limited amount of
small particles of Mn oxides besides hematite (Fig. 10d).
The TEM analyses also confirmed the presence of these Mn
oxides within the porous bands. They occur as small
particles (∼100 nm) mixed with the iron oxides.
In samples collected from the contact zone with the
protore, remnants of dolomite intergrown with hematite and
gangue are observed. Apatite is well preserved in this zone,
forming thin laminae parallel to the bandin g or occurring as
Table 2 Mössbauer hyperfine parameters of iron ore and dolomitic itabirite samples
Sample
code
Sample
description
Temperature
(K)
Subspectrum Isomer shift
(±0.05)
(mm·s
−1
)
Quadrupole
splitting (±0.05)
(mm·s
−1
)
Hyperfine
field (±0.6)
(T)
Line Width
(±0.5)
(mm·s
−1
)
Relative
abundance
(%)
Mac67 HO 300 Hematite 0.37 −0.16 51.9 0.34 100
Mac68 HO 300 Hematite 0.37 −0.15 51.9 0.34 100
Mac97 HO 300 Hematite 0.37 −0.16 51.9 0.34 100
Mac97 HO 50 Hematite 0.48 0.38 55.8 0.37 100
Mac105 HO 300 Hematite 0.37 −0.15 51.9 0.35 100
Mac126A SO-massive 300 Hematite 0.36 −0.16 51.9 0.34 100
Mac127A SO-massive 300 Hematite 0.37 −0.16 51.9 0.34 100
50 Hematite 0.48 0.37 54.9 0.37 100
Mac128A SO-massive 300 Hematite 0.37 −0.17 51.9 0.34 100
Mac129A SO-massive 300 Hematite 0.37 −0.15 51.9 0.35 100
Mac126B SO-porous 300 Hematite 0.37 −0.13 51.9 0.34 98
Ferrihydrite 0.34 0.68 – 2
Mac127B SO-porous 300 Hematite 0.37 −0.13 51.9 0.34 98
Ferrihydrite 0.34 0.68 – 2
50 Hematite 0.48 0.32 54.9 0.39 97
Ferrihydrite 0.42 0.76 – 0.48 3
Mac128B SO-porous 300 Hematite 0.37 −0.13 51.9 0.34 98
Ferrihydrite 0.34 0.68 – 2
Mac129B SO-porous 300 Hematite 0.36 −0.13 51.9 0.34 98
Ferrihydrite 0.35 0.68 – 2
Mac96 Dol. itabirite 300 Hematite 0.37 −0.14 51.9 0.34 98
Siderite 1.20 1.60 – 2
Mac100 Dol. itabirite 300 Hematite 0.37 −0.13 51.9 0.34 90
Siderite 1.20 1.55 – 10
50 Hematite 0.48 0.39 54.8 0.34 90
Siderite 1.37 2.03 – 0.51 10
HO Hard ore, SO-massive massive band of the soft ore, SO-porous porous band of the soft ore, Dol. itabirite dolomitic itabirite
Miner Deposita (2008) 43:229–254 239
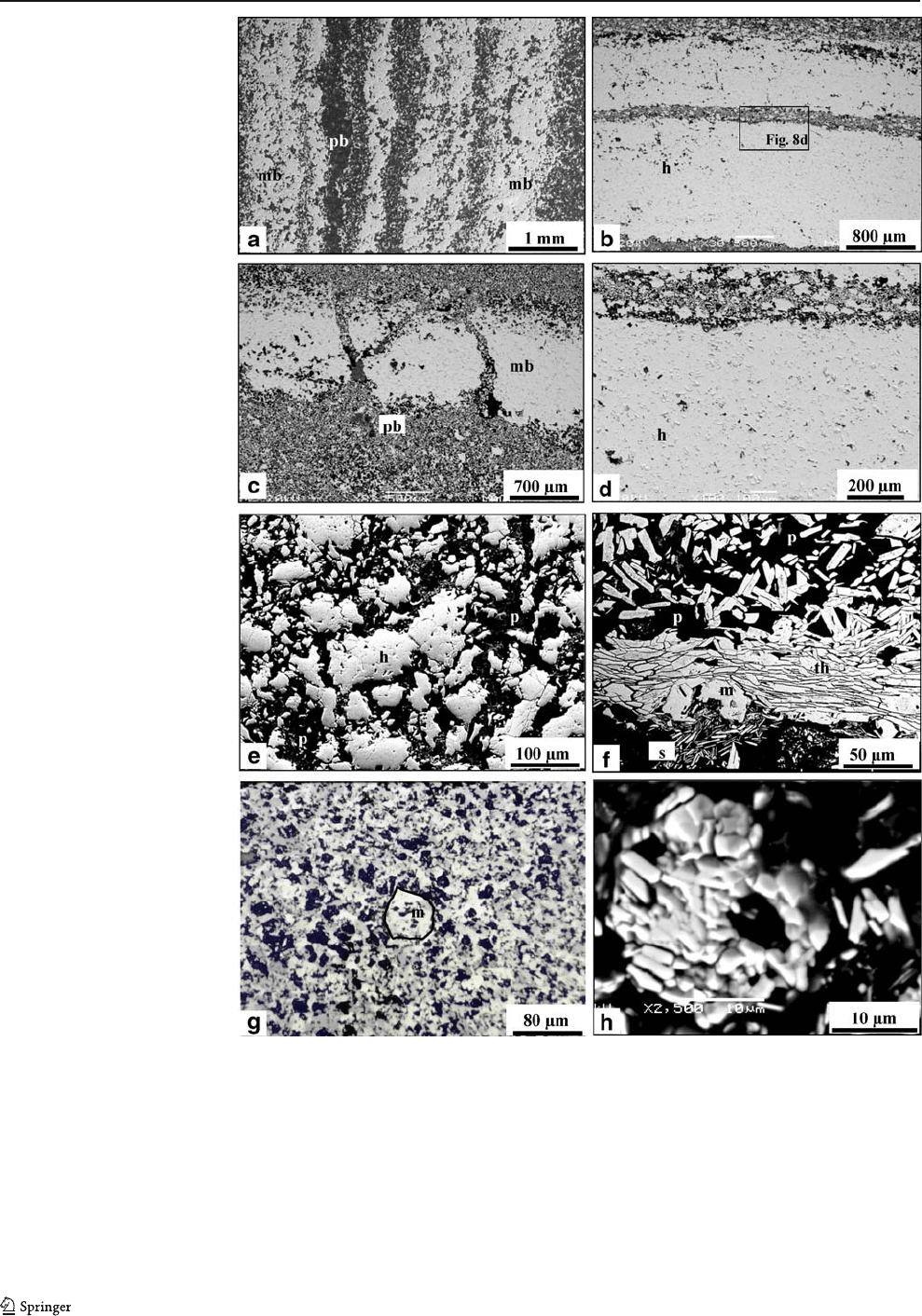
isolated euhedral crystals (Fig. 10a–c). Talc and chlorite
occur as isolated crystals or forming millimetric aggregates
(Fig. 10e,f).
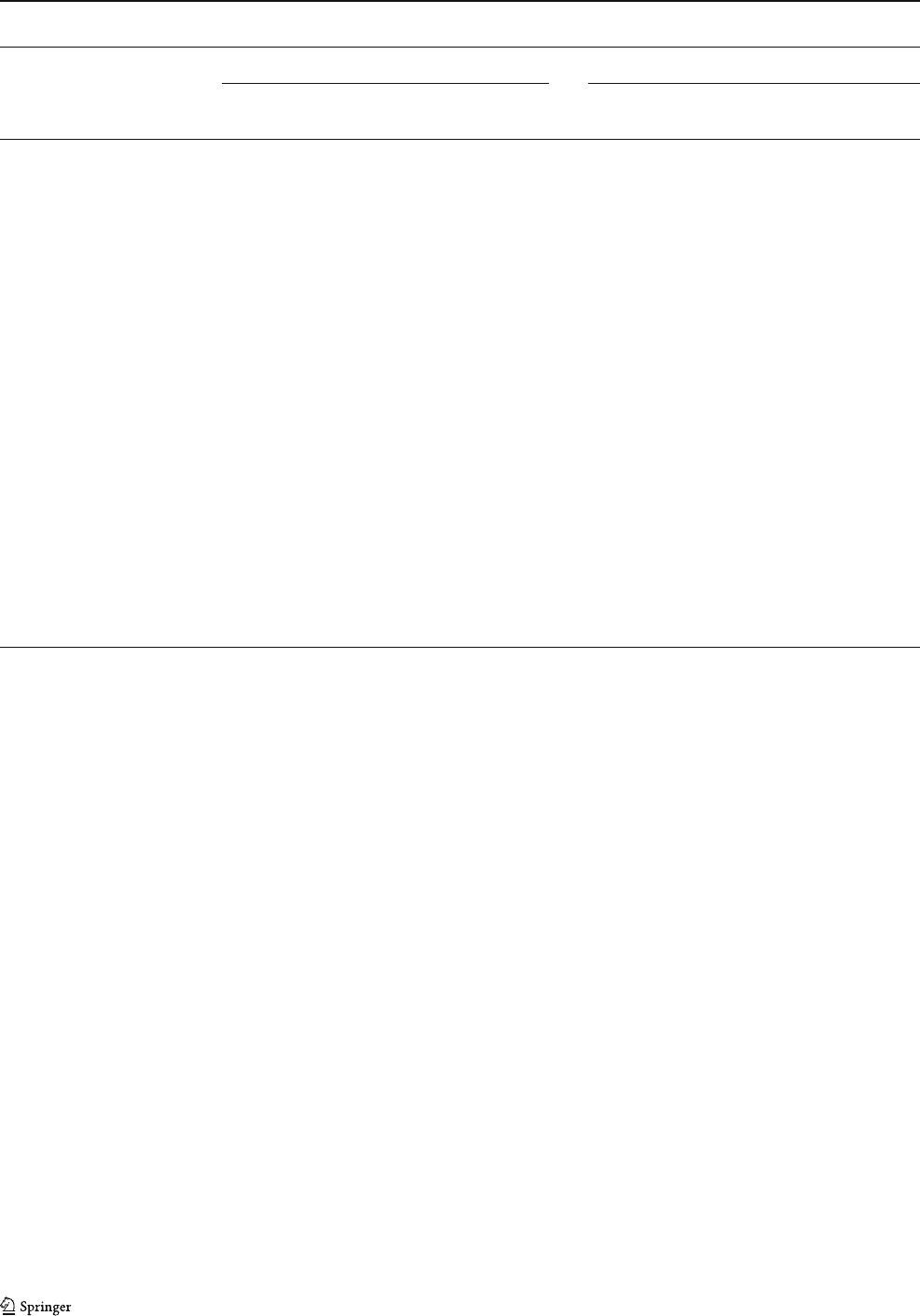
The 300 K Mössbauer spectra of the soft ore samples fit
perfectly with hematite (Table 2). MS also revealed the
presence of the Morin transition (a transition from an
antiferromagnetic to a weakly ferromagnetic state, at about
265 K). This transition is very sensitive to microcrystalline
effects, lattice imperfections, and impurities. Its record in
the studied samples also indicates a very pure composition
of hematite and crystallites larger than 20 nm, which was
also indicated by the unit cell parameters obtained for the
hematite in the sample MAC127A (Table 3), which are
similar to those of the reference cell of hematite.
A r epresentative Mössbauer spectrum at 300 and at
50 K of the porous bands from sample MAC127B is
Fig. 8 SEM backscattered
images and photomicrographs
of the soft ore. a, b and c
Typical image of soft ore show-
ing massive (mb, white) and
porous (pb, black) bands locally
broken and disrupted (c). Note
the preservation of microband-
ing of the protore. d Detail of a
massive band showing the dense
packing of hematite (h), SEM
image. e Detail of a porous band
showing aggregates and indi-
vidual crystals of hematite (h)
between pores (black). f Coarse
crystals of tabular hematite (th)
in the massive and porous
bands, surrounding a porphyro-
blast of martite (m) in the for-
mer. Note the small crystals of
specularite (s) in the lower part
of the picture. g Massive band
showing granoblastic fabric of
granular/tabular hematite grow-
ing over martite (m), reflected
light, PPL. h Small crystals of
tabular hematite developed
within ex-martite grain, SEM
image
240 Miner Deposita (2008) 43:229–254
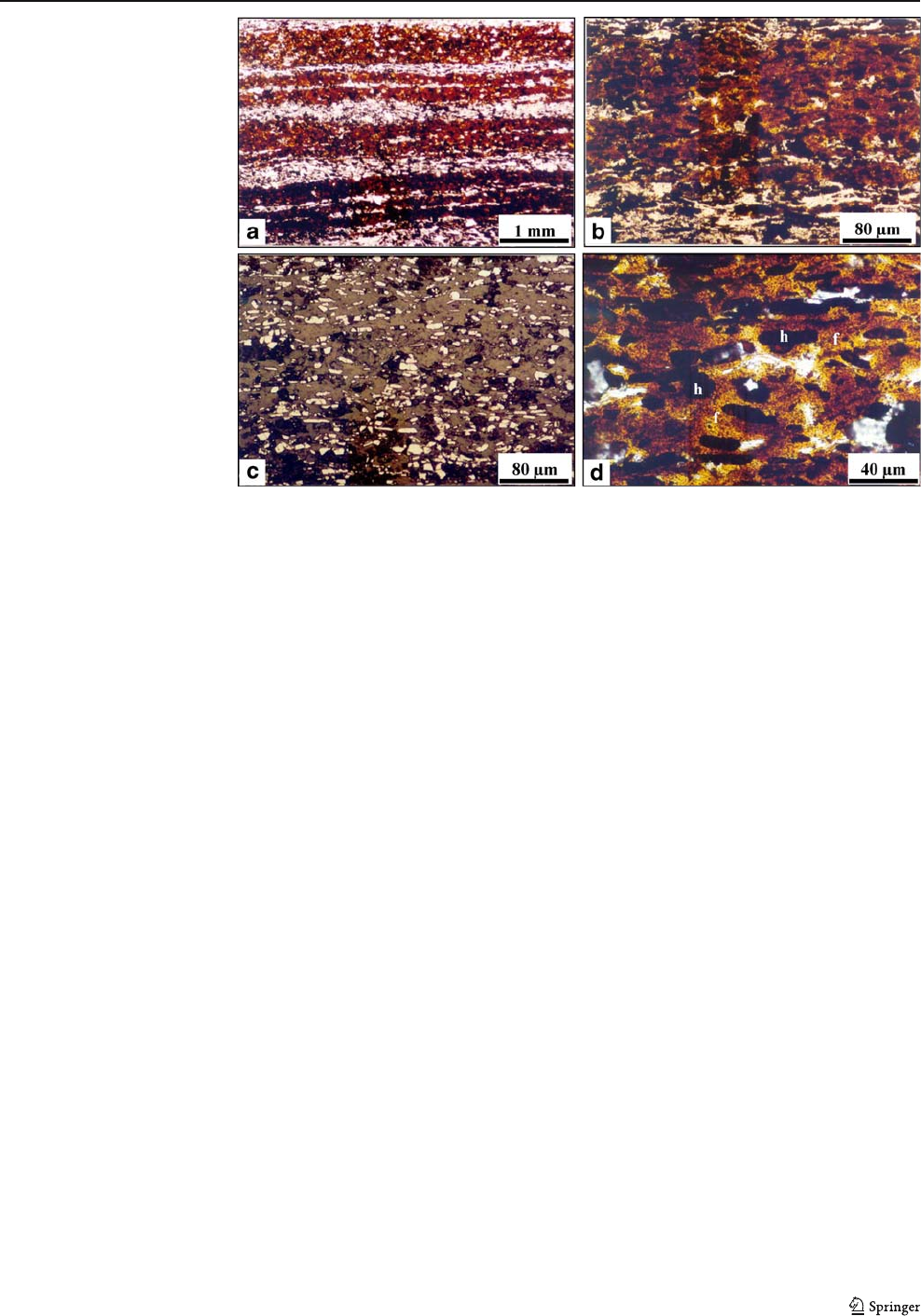
depicted in Fig. 11c,d. Besides the major sextet that f itted
perfectly with hematite, an ill-defined doublet super-
imposed to the hematite sextet is present in all spectra of
the studied samples (Fig. 11e). This doublet can be
ascribed to the amorphous limonitic brownish material
which is the residue of the dissolution of dolomite. The
isomer shift (0.34–0.35 mm/s) and the quadrupole split-
ting (0.68 mm/s) at 300 K of these samples match well
with those of ferrihydrite (Cornell and Schwertmann 1996,
p 149). In the MS at 50 K, the doublet is still present, with
an isomer shift of 0.42 mm/s and a quadrupole splitting of
0.76 mm/s. These values are compatible with those found
by Childs and Baker-Sherman (1984) for synthetic
ferrihydrite at 77 K. The absence of a sextet at 50 K
indicates the poor cryst allinity of ferrihydrite, given th at
magnetic ordering is strongly dependent on crystallinity
(Cornell and Schwertmann 1996). The low crystallinit y of
ferrihydrite, on the other hand, explains its absence from
the XRD patterns. Mössbauer spectroscopy confirms,
therefore, that the brownish mate ri al obser v e d by op ti c al
petrography in the residue of the dolomite dissolution is
ferrihydrite. Based on the values of the relative subspect ral
areas, t he abundances of hematite and ferrihydrite were
estimated. It is seen from Table 2 that ferrihydrite is a
subordinate component in the soft ore.
Geochemistry
Analytical results for the 48 samples analyzed for major,
trace, and REE of the soft and hard ores are presented in
Electronic supplementary material Appendices B and C.
Average compositions and standard deviations of popula-
tions for the soft and hard iron ores are given in Tables 4
and 5. Statistics for variables below or very close to the
detection limit were not calculated. When the results of just
a few samples were below this limit, statistics were
calculated using half the detection limit. This procedure
was chosen to establish a distinction for those values, which
were slightly above the detection limit. The following
variables (with their respective detection limit) presented
analytical results below or very close to the detection limit:
Na
2
O (0.01%), K
2
O (0.01%), Sc (3 ppm), Be (3 ppm), Zn
(30 ppm), Ga (1 ppm), Rb (1 ppm), Nb (0.2 ppm), Mo
(2 ppm), In (0.1 ppm), Sn (1 ppm), Cs (0.1 ppm), Hf
(0.1 ppm), Tl (0.1 ppm), Pb (5 ppm), Bi (0.1 ppm), Au
(2 ppb), Br (0.5 ppm), Ir (5 ppm), and Se (3 ppm).
The soft iron ore consists almost entirely of Fe
2
O
3
(average 95.6 wt%), with FeO representing less than 0.5%
(average 0.1 wt%). The average values of major elements in
the massive and porous band of the soft ore were analyzed
separately to confirm the results of petrological analyses
and show that impurities are concentrated within the porous
bands (Table 4). These impurities consist almost entirely of
SiO
2
(average 1.7 wt%), MnO (average 1.7 wt%), and
Al
2
O
3
(average 0.8 wt%) and are mineralogically expressed
by chlorite, talc, and Mn oxides.
Trace element concentrations encountered in the soft ore
are very low (Table 4). Most elements show concentrations
of less than 10 ppm, except for Ba (33 ppm), V (60 ppm),
Cr (79 ppm), Y (19 ppm), Ni (24 ppm), Cu (79 ppm), and
∑REE (23 ppm). When compared to the average compo-
sition of dolomitic itabirite (Table 4), the soft ore is
Fig. 9 Photomicrographs of the
soft ore. a Microbanding of
massive and porous bands,
transmitted light. b, c Detail of a
porous band, transmitted light
(b), reflected light, PPL (c). d
Detail of the previous picture (b)
showing ferrihydrite (f)
surrounding aggregates and
individual crystals
of hematite
Miner Deposita (2008) 43:229–254 241

enriched in all trace elements, except for Sr and Nb. Trace
elements are concentrated in the porous bands of the soft
ore (Table 4). Only Ge, Cr, Sb, V, and Ni have similar
values in the porous and massive bands. Some elements,
such as Ba (10x), Y (10x), REE (9x), and Th (7x), are
highly enriched in the porous bands if compared to the
massive bands.
The average REE composition of the soft ore is presented
in Table 5.TheREEY
PAAS
patterns are shown in Fig. 12a,b.
These patterns show similar trends, characterized by a
pronounced depletion in the light REE relative to heavy
REE (average La/Yb
PAAS
=0.2). This depletion occurs either
in the LREE group (average La/Sm
PAAS
=0.5)aswellasin
the HREE group (average Dy/Yb
PAAS
=0.8). Most samples
display strong positive anomalies of Eu
PAAS
(Eu/Eu*=1.7)
and Y
PAAS
(Y/Y*=2.1), as well as negative anomalies of
Ce
PAAS
ranging from 0.7 to 1.0.
Mass balance calculation
The mass balance of the transition of dolomitic itabirite to
soft ore was calculated in accordance to the works of
Fig. 10 SE M b ackscattered
images of gangue minerals in
the iron ore. a Microbanding of
apatite-rich laminae (a)
between massive bands (mb)of
hematite. b Detail of the apatite-
rich laminae. c Porous band of
the soft ore showing apatite (a)
and aggregates of hematite (h)
between pores (p). d Detail of
the size fraction <0.15 mm of
the soft ore showing small crys-
tals of Mn oxides on the surface
of hematite aggregates, SEM
image, secondary electrons. e
Porous band of the soft ore
showing aggregates of talc (t)
and hematite (h) between pores.
f Detail of the previous
picture (e)
Table 3 Unit cell parameters of hematite in the iron ores of the Águas
Claras Mine
Sample Ore type aÅ cÅ
MAC67 Hard ore 5.0347(1) 13.7474(2)
MAC97 Hard ore 5.0355(1) 13.7485(2)
MAC127A Massive band
of the soft ore
5.0338(1) 13.7470(2)
MAC127B Porous band
of the soft ore
5.0339(1) 13.7468(2)
Reference cell
of hematite
5.0340 13.7520
Data for the refer ence cel l of hema tite a re from Cornell and
Schwertmann (1996, p 5).
242 Miner Deposita (2008) 43:229–254
Brimhall et al. (1991), Brimhall and Dietrich (1987 ), and
Chadwick et al. (1990) and is based on the assumption that
the transformation of a protolith into altered material takes
place together with conservation of some highly immobile
elements (e.g., Ti, Zr, etc.) and, at later stages, the collapse
of the original structures, generating a volume decrease.
The following equations were utilized for calculating
volume variation and mass balance:
"
i;w
¼ 100 ρ
p
C
i;p
.
ρ
w
C
i;w
hi
1
no
ð1Þ
τ
j;w
¼ 100 C
j;w
C
i;p
C
j;p
C
i;w
1
ð2Þ
where,
"
i,w
volume change during weathering (%) using
immobile element I
τ
j,w
gain (+) or loss (−) of the mobile element j during
weathering (%)
i subscript of the immobile element;
j subscript of the mobile element
C
i,p
chemical concentration of i in the dolomitic itabirite
(%=g/100 g)
-12 -9 -6 -3 0 3 6 9 12
0,8 5
0,9 0
0,9 5
1,0 0
MAC-127B
300K
Velocity (mm/s)
0,8 5
0,9 0
0,9 5
1,0 0
MAC-127A
300K
Relative Transmission (%)
-12 -9 -6 -3 0 3 6 9 12
0,93
0,96
0,99
MAC-127B
50 K
Velocity (mm/s)
0,92
0,96
1,00
MAC-127A
50K
b
Fig. 11E
a
d
c
-2 -1 0 1 2
0,94
0,96
0,98
1,00
Relative transmission
Velocit
y
(
mm/s
)
Ferrihydrite
e
Fig. 11 Mössbauer spectra at
300 and at 50 K of the massive
(a, b) and porous (c, d) bands of
the sample MAC127 (soft ore).
e Detail of the central part of the
spectra of the porous band at
300 K showing the doublet of
ferrihydrite
Miner Deposita (2008) 43:229–254 243

Table 4 Average major and trace element composition
Hard ore Soft ore Dolomitic
Itabirite
(n=11)
Soft ore Dolomitic itabirite
Massive band (n=6) Porous band (n=8) Iron-rich
band (n=6)
Dolomite-rich
band (n=6)
Wt% Avg Std Min Max n Avg Std Min Max n Avg Std Min Max Avg Std Min Max Avg Avg
SiO
2
0.66 0.33 0.24 1.32 12 1.06 0.63 0.18 3.57 62 1.01 0.35 0.13 0.15 0.51 1.69 1.48 0.38 4.74 0.91 0.73
Al
2
O
3
0.24 0.23 0.02 0.91 12 0.46 0.44 0.08 2.10 62 0.32 0.08 0.05 0.03 0.16 0.84 1.13 0.14 3.25 0.28 0.33
Fe
2
O
3
97.66 1.16 95.11 99.13 10 95.65 2.29 87.66 98.79 62 48.90 98.24 0.85 96.84 99.15 92.80 3.84 86.96 97.07 77.88 10.93
Fe
TOT
97.93 1.77 94.44 99.54 12 95.74 2.31 87.72 98.90 62 49.64 98.58 0.53 97.90 99.27 92.98 3.76 87.22 97.07 78.23 11.74
FeO 0.82 0.80 0.22 2.70 10 0.08 0.07 0.05 0.50 62 0.66 0.36 0.55 0.11 1.35 0.19 0.10 0.10 0.37 0.32 0.73
MnO 0.025 0.025 0.005 0.093 12 0.818 0.379 0.221 2.139 62 0.28 0.10 0.10 0.02 0.29 1.69 0.63 0.77 2.55 0.12 0.50
MgO 0.22 0.35 <0.03 1.20 12 0.36 0.32 0.03 2.04 62 10.33 0.08 0.03 0.02 0.11 0.54 0.73 0.07 2.29 4.11 18.50
CaO 0.33 0.42 0.02 1.41 12 0.19 0.22 <0.03 1.13 62 14.36 0.10 0.14 0.03 0.38 0.12 0.11 <0.03 0.31 5.45 26.19
Na
2
O <0.01 12 <0.01 36 <0.01 <0.01 <0.01 <0.01 0.05
K
2
O 0.044 0.041 <0.01 0.140 12 <0.01 36 0.03 <0.01 <0.01 0.02 0.06
TiO
2
0.018 0.009 0.006 0.033 12 0.031 0.035 0.005 0.156 62 0.021 0.006 0.003 <0.001 0.010 0.054 0.076 0.011 0.223 <0.001 <0.001
P
2
O
5
0.13 0.07 0.03 0.22 12 0.18 0.27 <0.03 1.58 62 0.12 0.06 0.10 <0.03 0.27 0.10 0.07 0.03 0.25 0.12 0.06
LOI 0.52 0.41 0.13 1.39 12 0.85 0.44 0.29 2.31 62 23.60 0.32 0.10 0.19 0.45 1.86 0.81 0.78 2.97 9.91 40.98
Ppm
a
Ba 13.1 10.6 <3.0 37.2 12 32.7 35.1 6.7 211.0 62 12.9 11.5 9.8 <3.0 29.4 116.2 127.2 20.7 405.2 11.17 20.67
Sr 6.2 3.2 <2.0 9.3 10 18.2 10.9 4.0 35.5 24 29.8 3.8 4.5 <2.0 12.4 17.4 12.9 2.3 42.8 12.83 46.17
Y 5.0 1.5 2.2 7.6 10 18.8 9.5 5.5 33.7 24 7.7 3.1 1.6 2.0 6.1 32.6 13.6 13.9 56.0 4.67 9.50
Sc <3.0 10 <3.0 24 <3.0 <3.0 <3.0 <3.0 <3.0
Zr 5.8 3.8 2.4 15.1 10 13.6 9.9 3.5 40.1 24 14.0 3.2 1.1 2.2 4.8 9.4 11.9 2.3 38.6 14.67 8.00
Be <3.0 10 <3.0 24 <3.0 <3.0 <3.0 <3.0 <3.0
V 35.6 35.6 10.0 132.0 10 59.9 36.0 9.1 138.4 62 25.6 27.2 25.1 9.2 77.0 44.5 21.0 23.0 75.0 36.33 10.25
Ni <20.0 10 23.9 21.1 <20.0 96.0 62 10.0 <20.0 30.5 18.6 <20.0 63.6 15.13 7.31
Cu 10.0 6.0 <5.0 22.0 10 10.2 7.3 <5.0 45.3 62 7.0 <5.0 31.9 42.0 <5.0 115.3 0.50 5.19
Zn <30.0 10 <30.0 62 13.5 <30.0 101.2 128.1 <30.0 407.1 12.95 7.38
Ga <1.0 10 <1.0 24 <1.0 <1.0 2.1 1.7 <1.0 5.9 0.79 <1.0
Ge 4.3 1.1 2.8 6.0 10 3.0 1.1 0.7 5.0 24 1.8 3.8 0.8 3.1 5.3 4.0 1.3 2.1 6.0 3.6 <0.5
Rb <1.0 10 <1.0 24 <1.0 <1.0 <1.0 1.24 <1.0
Nb 0.4 0.4 <0.2 1.0 10 <0.2 24 0.9 0.3 0.3 <0.2 0.5 0.8 0.80 <0.2 2.4 0.36 0.88
Mo <2.0 10 <2.0 24 <2.0 <2.0 <2.0 1.49 <2.0
Ag <0.5 10 0.3 0.4 <0.5 1.3 24 <0.5 <0.5 <0.5 0.41 <0.3
In <0.1 10 <0.1 24 <0.1 <0.1 <0.1 0.05 <0.1
Sn <1.0 10 <1.0 24 <1.0 <1.0 <1.0 0.64 <1.0
Cs <0.1 10 <0.1 24 <0.1 <0.1 <0.1 0.21 <0.1
ΣREE 7.0 1.9 3.2 10.0 10 22.7 10.1 9.3 46.0 24 13.9 5.0 1.5 3.6 7.6 42.9 31.8 14.4 116.9 9.63 18.76
Hf <0.1 10 <0.1 24 0.1 <0.1 <0.1 <0.1 <0.1
Tl <0.1 10 <0.1 24 <0.1 <0.1 <0.1 <0.1 <0.1
Pb <5.0 10 <5.0 24 5.1 <5.0 13.7 23.2 2.5 70.5 1.5 7.01
244 Miner Deposita (2008) 43:229–254
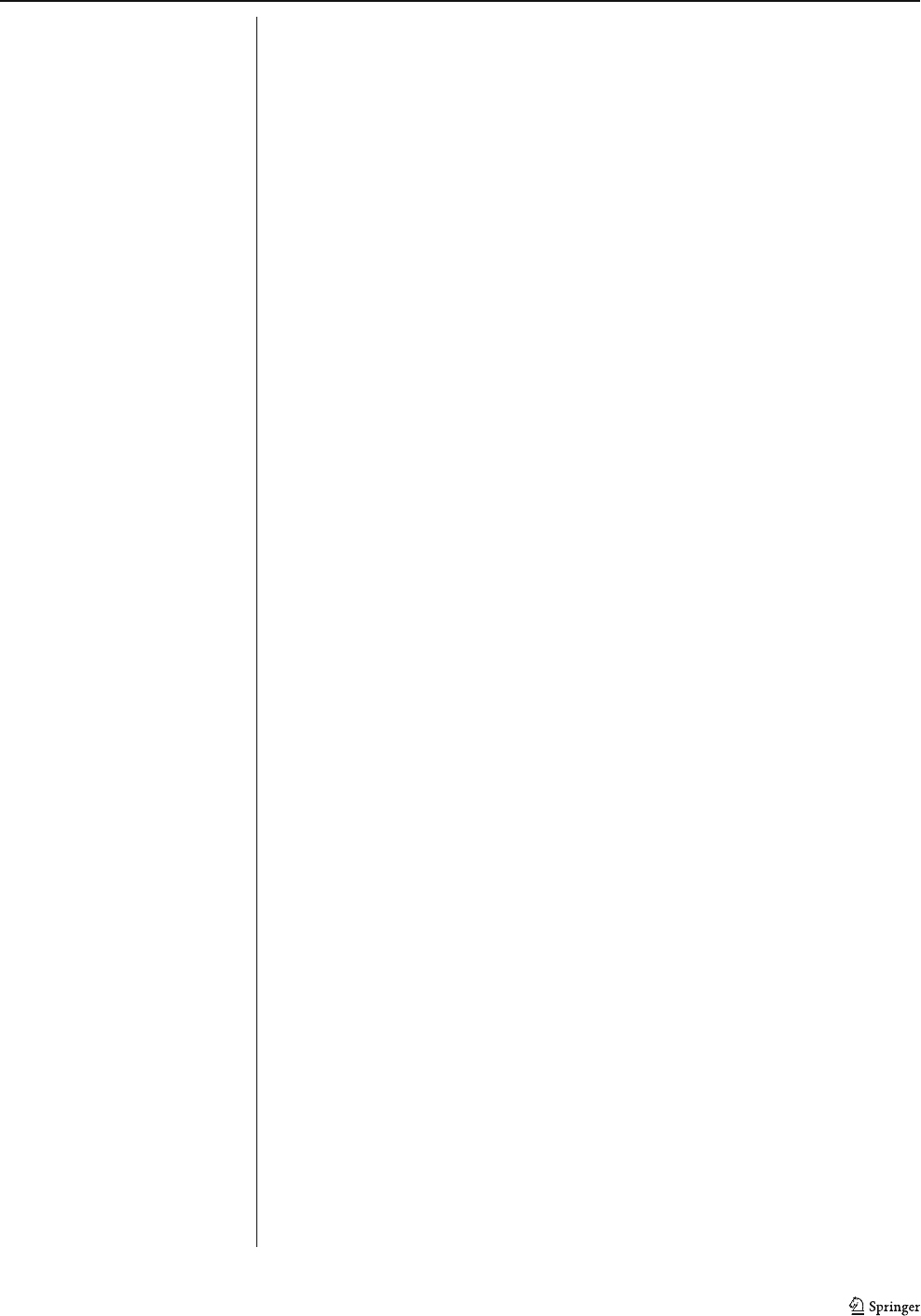
C
i,w
chemical concentration of i in the soft ore
(%=g/100 g)
C
j,p
chemical concentration of j in the dolomitic itabirite
(%=g/100 g)
C
j,w
chemical concentration of j in the soft ore
(%=g/100 g)
ρ
p
dry bulk density of the dolomitic itabirite (g/cm
3
);
ρ
w
dry bulk density of the soft ore (g/cm
3
).
A positive volume ("
i,w
) variation represents dilation,
and a negative one, collapse. The collapse is indicated by
Eq. 1 when an increase of the concentration of the
immobile element (C
i,w
) in the soft ore, caused by loss of
the mobile components of the dolomitic itabirite, is not
fully compensated by an inversely proportional decrease of
the bulk density (ρ
w
) due to a porosity increase so that the
product of C
i,w
by ρ
w
remains constant.
The mass transportation function (τ
j,w
) represents a
percentage of the mass of element j added to or removed
from the system, in relation to the mass originally present in
the dolomitic itabirite, regardless of any volumetric change.
Thus, τ
j,w
<0 values represent losses of the element j in the
soft ore in relation to dolomitic itabirite, τ
j,w
=0 values
represent conservation, and τ
j,w
>0 represent gains, which are
absolute. The expression relative gain is used when an
element, although present at a higher concentration in the
altered material than in the protolith, shows τ
j,w
<0 or τ
j,w
=0
(Brimhall et al. 1985).
The averages of chemical composition and density of
nine samples of the protore and 37 samples of the soft ore
(Table 6) were used for the calculations. Titanium was the
element considered as immobile. The results of loss and
gain calculations in the protore–ore transition (Table 6)
show that during soft ore formation there is an almost
complete loss of Ca, Mg, and LOI (close to 100%). The
oxides SiO
2
and P
2
O
5
have been also, to a lesser extent,
leached, but P shows a slight relative increase, and MnO
2
shows 49% absolute gain. With respect to Fe, calculations
show that the gains are just relative, as there is a strict mass
conservation, i.e., this element shows the same behavior
that is assumed for Ti. Unfortunately, our analyses of most
trace elements in dolomitic itabirite or in soft ore are at or
near detection limits so that it is difficult to assess their gain
and losses during dissolution of dolomitic itabirite and
formation of the soft ore.
The calculated volume variation ("
i,w
)is−40.7%, which
means that the initial volume of the dolomitic itabirite
generates a volume of soft ore that corresponds to just
59.3% of this ore. The residual porosity of the ore is
calculated by means of the formula:
Porosity ¼ 1 ρ
w
ðÞ
=
ρ
calculated
ÞðÞ*100;ð
Bi <0.1 10 <0.1 24 1.2 <0.1 <0.1 2.2 <0.1
Th 0.2 0.1 0.1 0.5 10 0.4 0.4 0.1 1.5 24 0.5 0.1 0.1 <0.05 0.2 0.8 1.3 0.1 3.8 0.25 0.84
U 4.5 3.4 0.6 12.3 10 3.9 1.8 1.0 7.2 24 2.9 1.7 1.2 0.7 4.1 4.7 2.5 1.5 8.4 2.07 1.67
Au <2.0 8 <2.0 24 na <2.0 9.4 12.5 <2.0 35.0 na na
As 12.4 6.7 <5.0 23.1 10 13.6 6.6 3.9 28.4 24 9.3 7.4 1.6 5.5 9.6 16.0 6.4 9.2 26.3 6.55 4.80
Br <0.5 8 <0.5 24 na <0.5 <0.5 na na
Cr 65.3 59.5 <20.0 202.0 12 78.8 73.0 16.0 429.0 62 52.9 54.5 62.1 23.0 181.0 68.7 59.8 26.0 206.0 21.84 23.50
Ir <5.0 8 <5.0 24 na <5.0 <5.0 na na
Sb 4.0 1.7 2.0 6.9 10 3.2 1.3 1.4 6.1 24 1.6 5.5 4.2 2.9 14.0 4.9 3.8 1.8 14.0 1.35 1.52
Sc 0.5 0.3 0.1 1.0 8 1.1 0.9 0.4 3.5 24 na 0.2 0.1 <0.1 0.3 1.7 2.7 0.3 7.7 na na
Se <3.0 8 <3.0 24 na <3.0 0.0 1.5 1.5 <3.0 na na
Fe# 0.988 0.009 0.970 0.997 9 0.999 0.001 0.994 0.999 61 0.980 0.996 0.006 0.985 0.999 0.998 0.001 0.996 0.999 1.000 0.910
Fe
TOT
expressed as Fe
2
O
3
. Fe# = (Fe
3+
/(Fe
3+
+Fe
2+
). Data of the dolomitic itabirite are from Spier et al. (2006).
a
Au in ppb
na Not analyzed
Miner Deposita (2008) 43:229–254 245
where ρ
calculated
is the density that would be assigned to the
material if porosity were zero, which is estimated by the
weighted average of the density of its compo nents:
D
calc
¼ C
Fe
þ C
other
ðÞ
C
Fe
5:26 þ C
Si;Al
2:65
þC
Mn
=
4:7 þ C
Ca; Mg; LOI
2:9
where:
C
Fe
, C
Si
, C
Al
, C
Mn
, C
Ca
, C
Mg
,=chemical composition of
the element subscribed; C
other
=sum of the contents of the
other chemical components in the soft; C
LOI
=loss on
ignition of the soft ore and the numbers are values for
density for hematite, quartz, pyrolusite and dolomite,
respectively.
Electronic supplementary material Appendix D shows
bulk density, calculated density, and residual porosity values
for the soft ore samples. The bulk density varies from 2.46
to 3.66, averaging 3.05 g/cm
3
; the calculated density varies
between 5.05 and 5.20, averaging 5.13 g/cm
3
; and the
residual porosity, between 28.2 and 51.5%, averaging
40.5%.
Hard iron ores
Mineralogy and petrology
Petrographic and SEM observations of the massive,
banded, and schistose hard iron ores show a simple
mineralogical composition. The iron oxide mineralogy is
similar to that of the soft ore. Gangue minerals are very
rare, consisting of dolomite and, locally, apatite and talc. In
weathered hard ores, gangue minerals are generally absent,
creating a macroporosity defined by the pore spaces
between hematite crystals. In highly fractured orebodies,
goethite and kaolinite occur as thin films on the surface of
the ore. There is no difference in the mineralogical
composition of the three major subtypes of hard ore, except
in fabric and porosity.
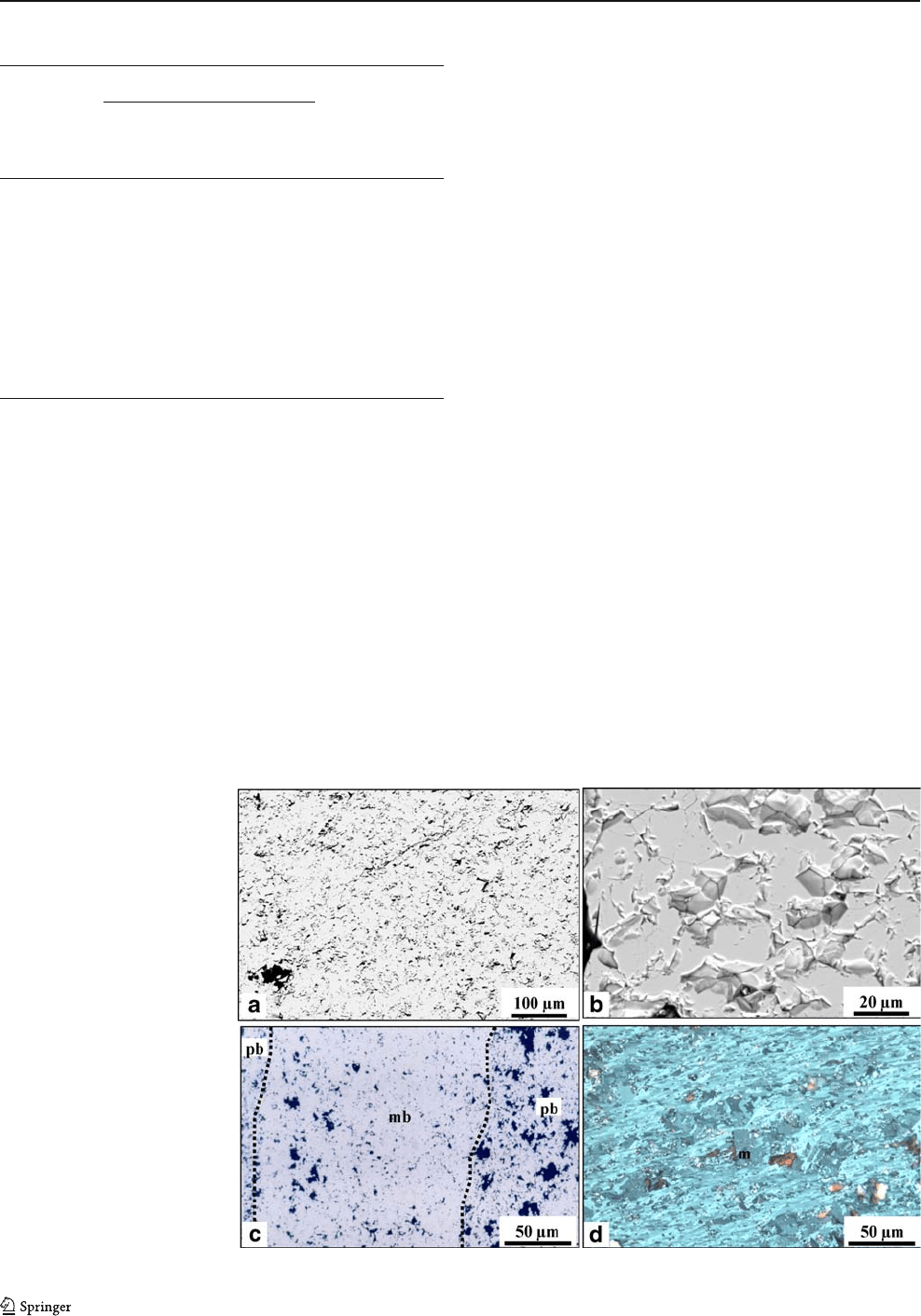
Massive hard ore consists of tabular and granular
hematite (up to 80%) ranging in size from 5 to 30 μm
(average 20 μm), forming a granoblastic fabric (Fig. 13a,b).
Relics of martite (20 to 50 μm) with remnants of magnetite
occur between hematite crystals. Hematite overgrows
martite crystals. Porosity in hard massive ores varies from
Table 5 Average rare earth element composition
Hard ore
(n=10)
Soft ore Dolomitic itabirite
Bulk (n=24) Massive band
(n=6)
Porous band
(n=8)
Bulk (n=9) Iron-rich band
(n=6)
Dolomite-rich
band (n=6)
Ppm
La 1.06 3.50 0.85 6.65 2.65 1.85 3.13
Ce 1.88 6.42 1.58 11.95 4.21 3.00 6.24
Pr 0.28 0.98 0.20 1.70 0.53 0.39 0.76
Nd 1.34 4.36 0.90 7.35 2.40 1.71 3.41
Sm 0.36 0.98 0.20 1.72 0.57 0.39 0.77
Eu 0.14 0.40 0.07 0.74 0.22 0.14 0.32
Gd 0.53 1.27 0.28 2.56 0.74 0.48 0.99
Tb 0.09 0.23 0.04 0.46 0.13 0.08 0.17
Dy 0.55 1.51 0.30 3.10 0.86 0.54 1.11
Y 4.99 18.76 3.11 32.59 7.44 4.67 9.50
Ho 0.12 0.35 0.07 0.77 0.20 0.13 0.25
Er 0.34 1.12 0.24 2.53 0.63 0.43 0.78
Tm 0.05 0.18 0.04 0.41 0.10 0.07 0.11
Yb 0.28 1.17 0.24 2.59 0.62 0.42 0.71
Lu 0.04 0.19 0.04 0.43 0.10 0.07 0.12
ΣREE 6.96 22.66 5.04 42.94 13.86 9.63 18.76
La/Yb
PAAS
0.28 0.22 0.26 0.17 0.27 0.32 0.34
La/Sm
PAAS
0.46 0.52 0.61 0.53 0.61 0.69 0.60
Dy/Yb
PAAS
1.17 0.78 0.75 0.71 0.82 0.78 0.94
Ce :Ce
PAAS
0.82 0.80 1.04 0.76 0.86 0.82 0.89
Eu
Eu
PAAS
1.48 1.67 1.67 1.63 1.60 1.57 1.74
Pr
Pr
PAAS
1.02 1.09 1.01 1.12 1.00 1.01 1.00
Y
Y
PAAS
1.68 2.09 2.09 1.85 1.63 1.40 1.52
Eu
Eu
PAAS
¼ Eu
=
Eu
PAAS
ðÞ
=
Sm
=
Sm
PAAS
ðÞGd
=
Gd
PAAS
ðÞðÞ
1=2
, Ce
Ce
PAAS
, Pr
Pr
PAAS
and Y
Y
PAAS
calculated by similar way. Data of the
dolomitic itabirite are from Spier et al. (2006).
246 Miner Deposita (2008) 43:229–254
approximately 5 to 20%, and pore size from <1 to 50 μm.
Unweathered ores exhibit the lowest porosity of all hard
ores, around 3%, increasing up to 20% in weathered
samples.
Banded hard ore consists of alternating massive and
porous layers (Fig. 13c). Tabular and granular hematite are
the main iron oxides (up to 70%) with grain sizes ranging
from 5–20 μm. Hematite grows over martite or occur as
isolated crystals. Martite with relics of magnetite may occur
as individual crystals ranging from 30–50 μmorforming
dense aggregates, representing up to 5% of the volume.
Specularite (up to 5 vol.%) occurs locally, along shear planes
parallel to, or cutting, the banding and defining a discontin-
uously developed foliation. Porosity is highly variable in
banded iron ores, reaching up to 30% of the volume.
Schistose hard ore presents a lepidoblastic fabric which
is composed mainly of tabular hematite and specularite (up
to 70 vol.%) wrapping around porphyroclastic aggregates
of tabular and granular hematite (up to 50 vol.%, Fig. 13d).
Porosity in the schistose ore ranges from 5 to 20 vol.%.
Hematite was the only iron oxide identified by XRD in
samples submitted for MS analysis, with very sharp peaks.
These results are confirmed by the Mössbauer spectra
(Table 2 ). Sharp XRD peaks and Möss bauer parameters of
0.1
1
a
Sample / PAASSample / PAASSample / PAAS
Soft Ore
0.01
0.1
1
Massive band
Porous band
b
0.1
1
Hard ore
Soft ore
Dol. itabirite
La Ce Pr NdSmEuGd Tb D
y
YHoErTmYbLu
c
Average Ratios
0.01
0.1
1
La Ce Pr NdSmEuGd Tb Dy Y Ho Er TmYb Lu
Soft ore - massive band
Soft ore - porous band
Dol. itabirite - iron-rich band
Dol. itabirite - dolomite-rich band
Sample / PAAS
d
Average Ratios
0.01
0.1
1
La Ce Pr NdSmEuGd Tb Dy Y Ho Er TmYb Lu
Sample/ PAAS
e
Hard Ore
Fig. 12 PAAS-normalized
REEY data. a Soft ore. b
Massive and porous bands of the
soft ore. c Averaged iron ores
and dolomitic itabirite. d
Averaged massive and porous
bands of the soft ore and iron-
rich and dolomite-rich bands of
the dolomitic itabirite (protore of
the soft ore). Data for the
itabirites are from Spier
(2005). e Hard ore
Miner Deposita (2008) 43:229–254 247
hematite indicate a high degree of crystallinity, with little
heterogeneity. The unit c ell parameters of hematite in
samples MAC67 and MAC97, very similar to those of the
reference cell of hematite (Table 3), corroborate this
indication.
Geochemistry
The bulk geochemical composition of the hard ore is
simple, consisting almost entirely of Fe
2
O
3
(average
97.6 wt%; Table 4). The trace element composition of the
hard ore is also simple, with only Cr (average 65 ppm) and
V (average 35 ppm) showing average values higher than
30 ppm (Table 4).
The hard ore has low REE conten ts, with an average con-
tent of 7 ppm, ranging from 3 to 10 ppm. The REEY
PAAS
patterns are all similar (Fig. 12e), being characterized by a
strong depletion in light REE relative to heav y REE,
negative Ce
PAAS
anomalies (with the exception of sample
MAC68), marked positive Eu
PAAS
and Y
PAAS
anomalies,
and depletion of the heavy REE relative to the middle REE
(Dy/Yb
PAAS
>1).
Discussion
Comparison of the Águas Claras orebody with the Capão
Xavier and Tamanduá orebodies
Comparing the macroscopic features of the orebodies from
the Águas Claras, Capão Xavier and Tamanduá mines as
summarized in Table 7 (see Figs. 2aand5a,b), we notice that
the main differences between them are: (1) mineralogical
composition of the protore; (2) dominant types of ore, and
(3) importance of the structural control.
High-grade soft ores may form both from dolomitic
(Águas Claras and Capão Xavier) and siliceous itabirite
(Tamanduá). By comparing the average of the grades of all
samples collected during the operation of the Águas Claras
Mine (Spier et al. 2003) to the average of the grades from
Capão Xavier and Tamanduá (Table 1), we have verified
that although these ores formed from protore of different
mineralogical composition, the chemical composition of
soft ores is similar.
Hundreds of drill holes drilled in the Tamanduá Mine,
many of them passing through the orebody until they
Table 6 Mass balance calculations for the transition from dolomitic
itabirite to soft ore—Ti constant
Average composition Mass balance
(τ
j.w
)
Dolomitic itabirite
(n=9)
Soft ore
(n=37)
wt% wt% %
Fe
2
O
3
48.28 96.34 0
P
2
O
5
0.09 0.13 −28
Al
2
O
3
0.15 0.36 20
SiO
2
1.07 0.89 −58
MnO
2
0.34 1.01 49
CaO 16.17 0.21 −99
MgO 11.25 0.36 −98
TiO
2
0.01 0.02 0
LOI 22.95 0.82 −98
d (g/cm
3
) 3.62 3.05
Fig. 13 SE M b ackscattered
images and photomicrographs
of the hard ores. a, b Massive
hard ore, SEM image. c Banded
hard ore, reflected light, PPL.
Note alternation of highly
porous (pb) with less porous
bands (mb). d Schistose hard ore
showing porphyroclastic aggre-
gates of martite (with relics of
magnetite) and granular/tabular
hematite wrapped around by
porous and platy (specularite)
hematite, reflected light, PPL
248 Miner Deposita (2008) 43:229–254
