Speight J.G. Natural Gas: A Basic Handbook
Подождите немного. Документ загружается.

66
Chapter
3
Composition and Properties
Table
3-2
Standard
Test
Methods for Natural
Gas
(ASTM,
2006)
(cont’d)
D4784-93(2003) Standard for LNG Density Calculation Models
D4810-88(1999) Standard Test Method for Hydrogen Sulfide in Natural
Gas Using Length-of-Stain Detector Tubes
D4888-88(1999) Standard Test Method for Water Vapor in Natural Gas
Using Length-of-Stain Detector Tubes
D4891-89(2001) Standard Test Method for Heating Value of Gases in
Natural Gas Range by Stoichiometric Combustion
D4984-89(1999) Standard Test Method for Carbon Dioxide
in
Natural Gas
Using Length-of-Stain Detector Tubes
D5287-97(2002) Standard Practice for Automatic Sampling of Gaseous
Fuels
D5454-93(1999) Standard Test Method for Water Vapor Content
of
Gaseous Fuels Using Electronic Moisture Analyzers
D5503-94(2003) Standard Practice for Natural Gas Sample-Handling and
Conditioning Systems for Pipeline Instrumentation
D5504-01
Standard Test Method for Determination of Sulfur
Compounds in Natural Gas and Gaseous Fuels by Gas
Chromatography and Chemiluminescence
D5954-98
Standard Test Method for Mercury Sampling and
Measurement in Natural Gas by Atomic Absorption
Spectroscopy
D6228-98(2003) Standard Test Method for Determination of Sulfur
Compounds in Natural Gas and Gaseous Fuels by Gas
Chromatography and Flame Photometric Detection
D6273-98(2003) Standard Test Methods for Natural Gas Odor Intensity
D6350-98(2003) Standard Test Method for Mercury Sampling and Analysis
in Natural Gas by Atomic Fluorescence Spectroscopy
significant amount
of
sulfur impurities, such as hydrogen sulfide, is
termed
sourgas
and often referred to as acid gas. Processed natural gas
that is available
to
end-users is tasteless and odorless, and,
in
itself,
is
harmless to the human body. However, natural gas is
a
simple asphyx-
iant and can kill
if
it sufficiently displaces life-supporting oxygen.

3.2
Properties
67
3.2
Properties
The properties of unrefined natural gas are variable because the com-
position
of
natural gas is never constant. Therefore, the properties
and behavior of natural gas are best understood by investigating the
properties and behavior of the constituents. Thus, assuming that the
natural gas has been cleaned (i.e., any constituents such as carbon
dioxide and hydrogen sulfide have been removed and the only con-
stituents remaining are hydrocarbons), the properties and behavior of
natural gas depend upon the properties and behavior of the relevant
hydrocarbons (see for example, Lange’s
Handbook
of
Chemistry,
2005).
Briefly, hydrocarbons are simple organic chemicals that contain only
carbon and hydrogen. When natural gas is refined and any remaining
hydrocarbons are removed, other than an odorizer, the gas that is
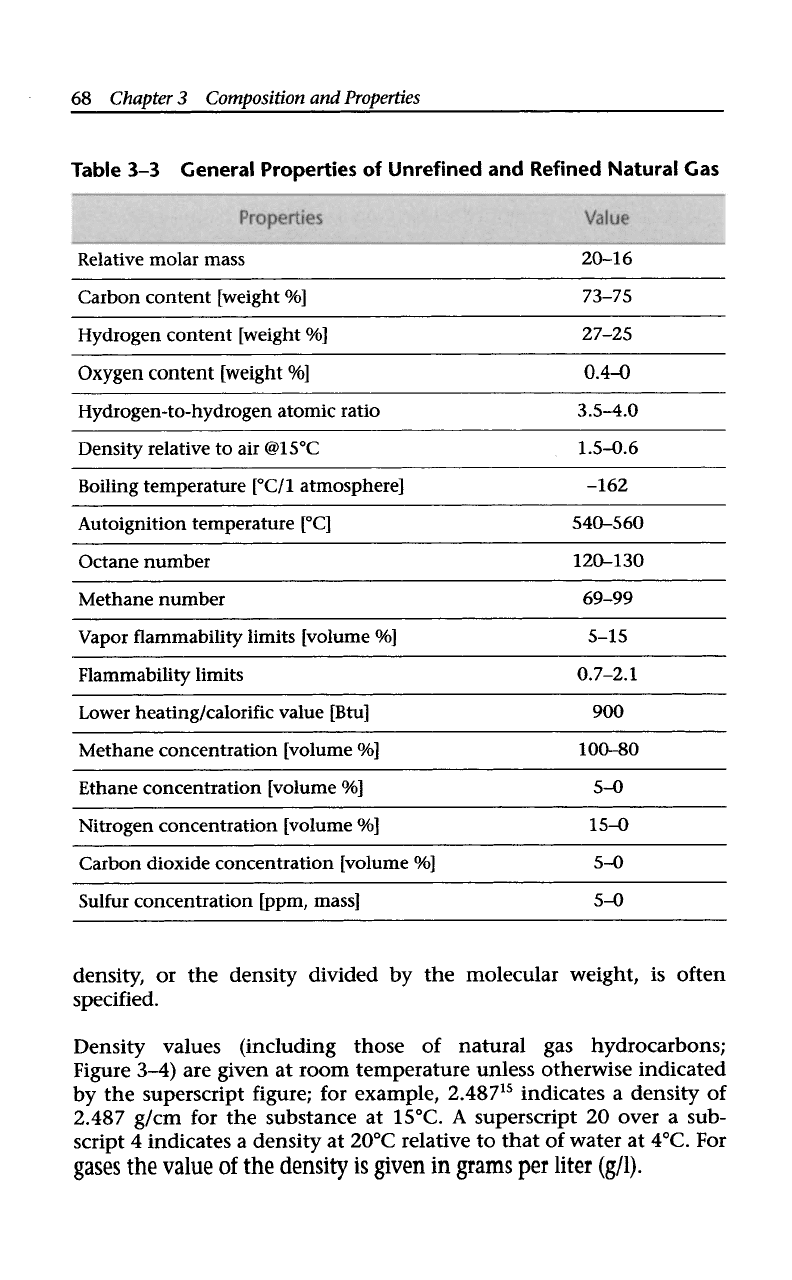
sold to the consumer is methane (CH,), and the properties are
constant (Table
3-3).
The composition of natural gas varies depending
on
the field, the for-
mation, and/or the reservoir from which it is extracted (Chapter
1
and Chapter
2).
The different hydrocarbons that form natural gas can
be separated using their different physical properties, such as weight,
boiling point, or vapor pressure (Chapter
6
and Chapter
7).
Depending
on
its content of higher molecular weight hydrocarbon
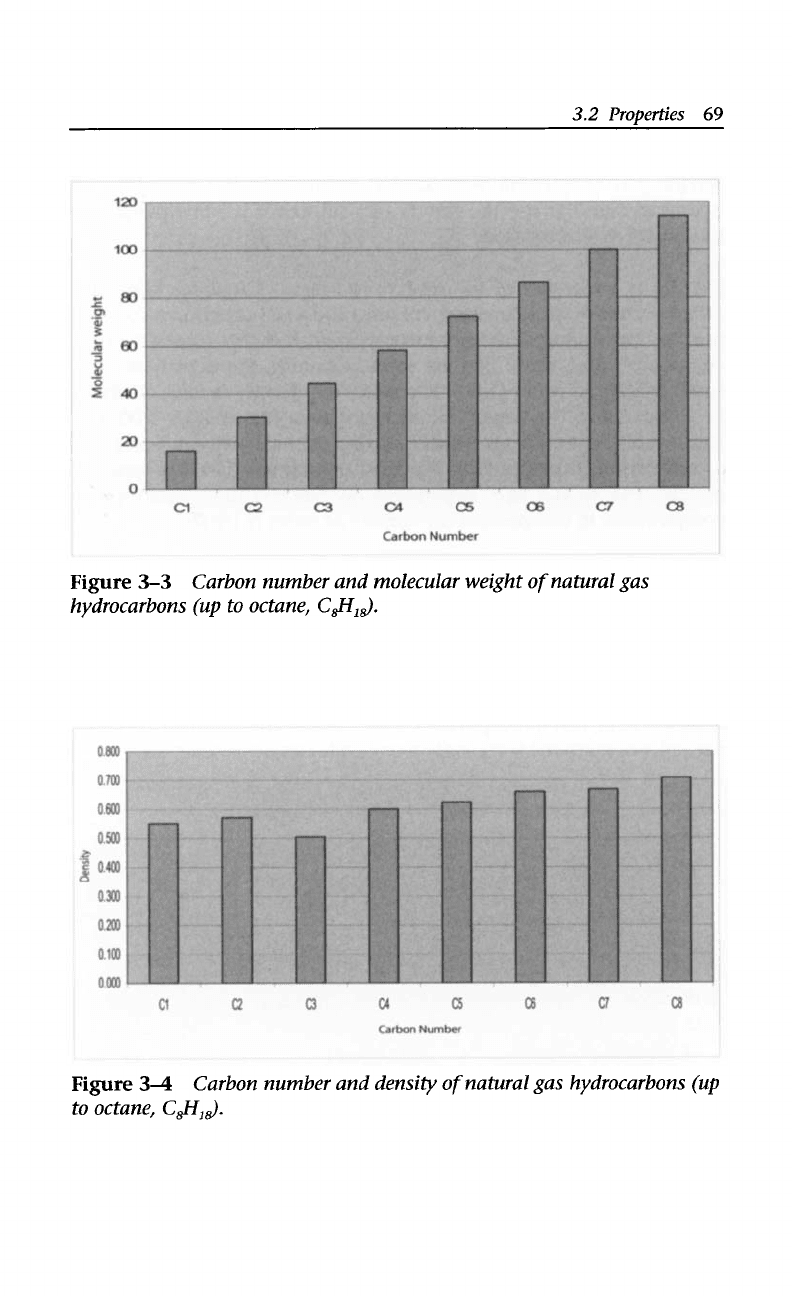
components (see Figure
3-3),
natural gas can be considered as rich
(five or six gallons or more of recoverable hydrocarbon components
per cubic foot) or lean (less than one gallon of recoverable hydro-
carbon components per cubic foot).
The following section presents a brief illustration of the properties
of
natural gas hydrocarbons from methane up to, and including n-octane
(C8H18). This will allow the reader to understand the folly of stating the
properties of natural gas as average properties rather than allowing for
the composition of the gas mixture and recognition of the properties of
the individual constituents.
3.2.1
Density
Density
is the mass of a substance contained in a unit volume (simply,
density is mass divided by volume).
In
the
SI
system of units, the ratio
of the density of a substance to the density of water at 15°C is known
as
the
specific gravily (relalive density).
Various units
of
density,
such
as
kg/m3, lb-mass/ft3, and g/cm3, are commonly used.
In
addition, molar
68
Chapter
3
Composition and Properties
Table
3-3
General Properties of Unrefined and Refined Natural Gas
Relative molar mass 20-16
Carbon content [weight
Yo]
73-75
Hydrogen content [weight
%]
27-25
Oxygen content [weight
%]
0.4-0
Hydrogen-to-hydrogen atomic ratio
3.54.0
Density relative to air @15”C
1.5-0.6
Boiling temperature [“C/1 atmosphere] -162
Autoignition temperature [“C]
540-560
Octane number 120-130
Methane number 69-99
Vapor flammability limits [volume Yo]
5-15
~~
Flammability limits 0.7-2.1
Lower heating/calorific value [Btu] 900
Methane concentration [volume
%]
100-80
Ethane concentration [volume Yo]
5-0
Nitrogen concentration [volume %]
15-0
~~
Carbon dioxide concentration [volume Yo]
5-0
Sulfur concentration [ppm, mass]
5-0
density, or the density divided by the molecular weight, is often
specified.
Density values (including those of natural gas hydrocarbons;
Figure
3-4)
are given at room temperature unless otherwise indicated
by the superscript figure; for example,
2.48715
indicates a density
of
2.487
g/cm for the substance at
15°C.
A
superscript
20
over a sub-
script
4
indicates a density at
20°C
relative
to
that
of
water at
4°C.
For
gases
the value
of
the density
is
given in
grams
per
liter (g/l).
3.2
Properties
69
100
83
60
40
20
0
a
0
c3
a
c5
c6
c7
Carbon
Number
~~
Figure
3-3
Carbon number and molecular weight
of
natural gas
hydrocarbons (up to octane, C&I,$.
OW
If
I5
0400
n
OM
0200
0
100
c1
c2
w
c4
c5
cs
c7
Carbon
Number
CB
c8
Figure
34
to octane, C$i,$.
Carbon number and density
of
natural gas hydrocarbons (up
70
Chapter
3
Composition and Properties
Specific gravity
is commonly used is relation to the properties
of
hydrocarbons. The specific gravity
of
a substance is a comparison
of
its density to that of water.
Density is a measure of the relative
heaviness
of hydrocarbons and
other chemicals at a constant volume, and each constituent
of
nat-
ural gas has a unique density associated with
it.
For most chemical
compounds (i.e., those that are solid
or
liquid), the density is mea-
sured relative to water
(1.00).
For
gases, the density is more likely to
be compared to the density
of
air (also given the number
1.00,
but
this is arbitrary and bears
no
relationship
to
the density of water).
As
a
comparison, the density
of
liquefied natural gas
(LNG)
is approxi-
mately
0.41
to
0.5
kg/l, depending
on
temperature, pressure and
composition;
in
comparison the density
of
water is
1.0
kg/l.
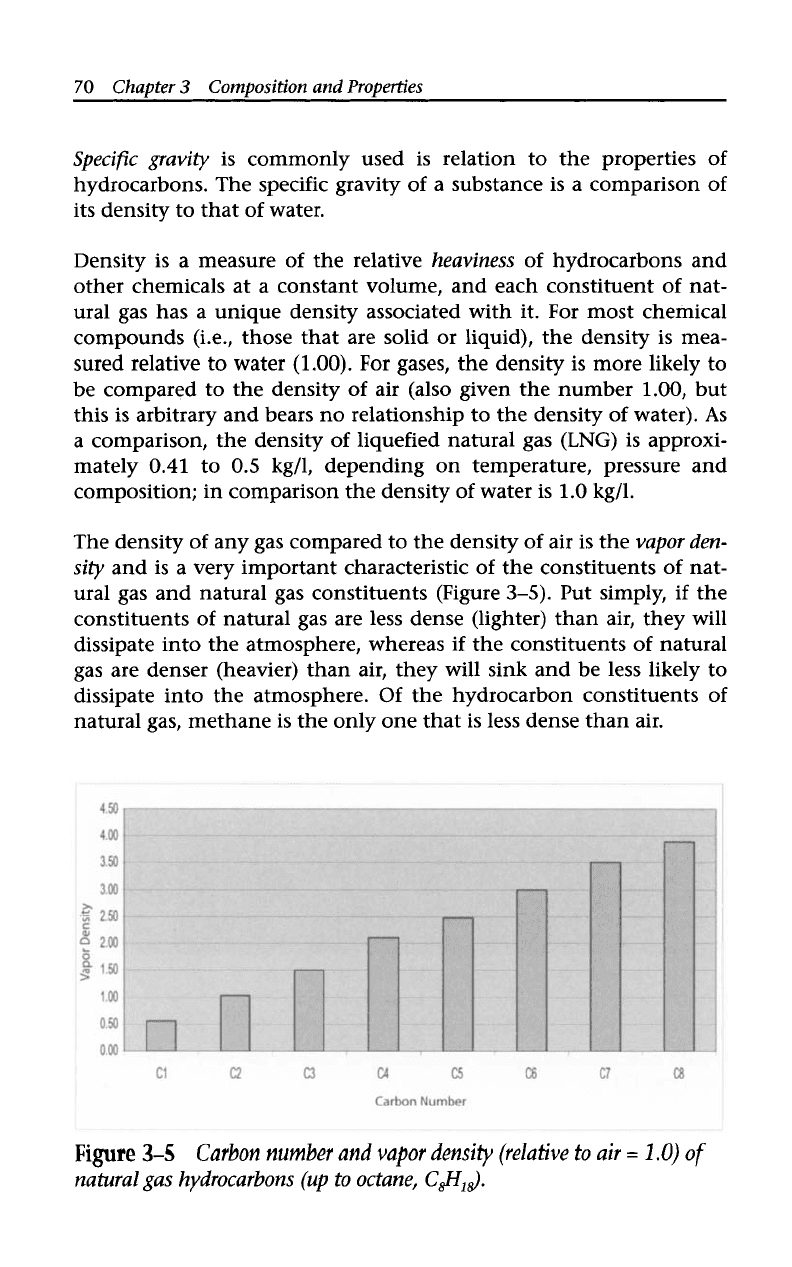
The density
of
any gas compared to the density
of
air is the
vapor den-
sity
and is a very important characteristic of the constituents
of
nat-
ural gas and natural gas constituents (Figure
3-5).
Put simply,
if
the
constituents of natural gas are less dense (lighter) than air, they will
dissipate into the atmosphere, whereas
if
the constituents
of
natural
gas are denser (heavier) than air, they will sink and be less likely to
dissipate into the atmosphere. Of the hydrocarbon constituents
of
natural gas, methane is the only one that is less dense than air.
4.50
4.00
3.50
3.00
P
2.50
:
2.00
g
1.50
0
1.00
0.50
0.00
c1
c2
c3
cd
c5
c6
c7
ca
Carbon
Number
Figure
3-5
Carbon number and
vapor
density
(relative
to
air
=
1
.O)
of
natural gas hydrocarbons
(up
to octane,
C$i,$.

3.2
Proverties
71
The statement is often made that
natural
gas
is lighter than air.
This
statement often arises because
of
the continued insistence by engi-
neers and scientists that the properties of a mixture are determined by
the mathematical average of the properties of the individual constitu-
ents of the mixture. Such mathematical bravado and inconsistency of
thought is
detrimental to
safev
and must be qualified.
Relative to air, methane is less dense (Table
3-4),
but the other hydro-
carbon constituents of unrefined natural gas (i.e., ethane, propane,
butane, etc.) are denser than air (Figure
3-5).
Therefore, should a nat-
ural gas leak occur in field operations, especially where the natural
gas contains constituents other than methane, only methane dissi-
pates readily into the air whereas the other hydrocarbon constituents
that are heavier than air do not readily dissipate into the atmosphere.
This poses considerable risk if these constituents of natural gas accu-
mulate or pool at ground level, when it has been erroneously
assumed that natural gas is lighter than air.
3.2.2
Heat
of
Combustion (Energy Content)
The
heat
ofcornbustion
(energy content)
of natural gas is the amount of
energy that is obtained from the burning
of
a volume of natural gas
and is measured in British thermal units (Btu). The value of natural
gas is calculated by its Btu content. One Btu is the quantity of heat
required to raise the temperature of one pound of water
1
degree
Fahrenheit
(1°F)
at atmospheric pressure.
A
cubic foot
of
natural gas
has an energy content of about 1,031 Btu, but it can range between
500
and
1,500
Btu, depending upon the composition of the gas.
Table
3-4
Boiling Point and Density
of
Methane Relative to Air and
Water
Property
value
Boiling point
(760
mm
Hg)
-161.5"C
(-258.7'
F)
Gas
specific
gravity
0.55-0.64
(air
=
1.00)
Specific
gravity
of liquefied
natural
gas
Gas
density
(varies
slightly)
0.42-0.46
(water
=
1.00)
0.0438
lbm/scf

72
Chapter
3
Composition and Properties
Thus, the energy content of natural gas is variable because natural gas
has variations in the amount and types of energy gases (methane,
ethane, propane, butane) it contains: the more non-combustible
gases in the natural gas, the lower the energy (Btu).
In
addition, the
volume mass of energy gases present in a natural gas accumulation
also influences the Btu value of natural gas. The more carbon atoms
in a hydrocarbon gas, the higher its Btu value. It is necessary to con-
duct the Btu analysis of natural gas at each stage of the supply chain.
Gas chromatographic process analyzers are used to conduct fractional
analysis of the natural gas streams, separating natural gas into identi-
fiable components. The components and their concentrations are
converted into a gross heating value in Btu-cubic foot.
In
the
USA,
at retail, natural gas is often sold in units of therms (th)
(1
therm
=
100,000
Btu). Wholesale transactions are generally done in
decatherms (Dth), in thousand decatherms (MDth), or in million
decatherms (MMDth).
A
million decatherms is roughly one billion
cubic feet of natural gas.
The gross heats of combustion of crude oil and its products are given
with fair accuracy by the equation:
Q
=
12,400
-
2,100d2
where d is the
60/60F
specific gravity. Deviation from the formula is
generally less than
1%.
3.2.3
Measurement
Before natural gas is sold to the consumer, it must be evaluated (tested
for favorable properties) and measured
so
that the consumer receives
the correct amount of gas. Natural gas can be measured in several
ways, but it is more often measured by volume at normal tempera-
tures and pressures, and the volume is commonly expressed in
cubic
feet
(ft3)
at a temperature of
60°F
and an atmospheric pressure of
14.7
pounds per square inch (psi). Thus, natural gas is measured (either at
the time of production or at the time of delivery to the consumer) in
thousands or millions of cubic feet (Mcf or Mscf and MMcf or MMscf,
where
scf
is standard cubic feet under prescribed conditions);
resources and reserves are calculated in trillions of cubic feet (Tcf).
Natural gas is sold
in
cubic feet (a container
10
feet deep,
10
feet long,

3.2
Properties
73
and
10
feet wide would hold one thousand cubic feet
of
natural gas)
or
in
British thermal units (Btu), which is a measure of the heat
content or burning properties
of
natural gas.
As noted, when natural gas is delivered to a residence,
it
is measured
by the gas utility
in
therms
for billing purposes and a
them
is equiva-
lent to
100,000
Btu, or just over
97
ft3
of
natural gas. On the other
hand, production and distribution companies commonly measure
natural gas
in
thousands of cubic feet (Mcf), millions
of
cubic feet
(MMcf),
or
trillions
of
cubic feet (Tcf).
The measurement of natural gas may not always be an understand-
able concept because natural gas can be measured in several different
ways (Table
3-5).
To place the measurement
in
context:
1,000
ft3
of
natural gas is about enough
to
meet the natural gas needs
of
an
average home (space-heating, water-heating, cooking, etc.) for four
days, and
5
trillion cubic feet
of
natural gas is enough
to
meet the
needs
of
5
million households (in the United States) for about
15
years. By comparison,
1,000
ft3
of
natural gas (about
1
million Btu or
10
therms) is equivalent to
90
pounds
of
coal,
125
pounds
of
oven-
dried wood, or to
8
gallons
of
(automobile) gasoline.
3.2.4
Volatility, Flammability, and Explosive Properties
The boiling point (boiling temperature)
of
a substance is the tempera-
ture at which the vapor pressure
of
the substance is equal to
atmospheric pressure.
At
the boiling point, a substance changes its state from liquid
to
gas.
A
stricter definition
of
boiling point is the temperature at which the
liquid and vapor (gas) phases of a substance can exist in equilibrium.
When heat is applied to a liquid, the temperature of the liquid rises
until the
vapor pressure
of
the liquid equals the pressure
of
the sur-
rounding atmosphere (gases). At this point there is no further rise in
temperature, and the additional heat energy supplied is absorbed as
latent heat
of
vaporization to transform the liquid
into
gas. This
transformation occurs
not
only at the surface
of
the liquid (as
in
the
case of
evaporation)
but also throughout the volume
of
the liquid,
where bubbles of gas are formed. The boiling point
of
a liquid is low-
ered
if
the pressure
of
the surrounding atmosphere (gases) is
decreased. On the other hand,
if
the pressure
of
the surrounding
reason,
it
is customary when the boiling point
of
a substance is given
atmosphere
(gases)
is increased, the boiling point is raised.
For
this
74
ChaDter
3
ComDosition
and
ProDerties
Table
3-5
Measurement Units Often Applied to Natural Gas
Unit
Equivalent
1
ft3
(cf
or
scf
standard
cubic
foot)
1,027
Btu
100
ft3
(scf)
=
1
therm (approximate)
1,000
ft3
(Mcf)
=
1,027,000
Btu
(1
MMBtu)
1,000
ft3
(Mcf)
=
1
dekatherm
(10
therms)
1
million (1,000,000)
ft3
(MMcf)
- -
1,027,000,000
Btu
1
billion
(1,000,000,000
ft3
(Bcf)
1.027
trillion
Btu
1
trillion
(1,000,000,000,000)
ft3
(Tcf)
=
1.027 quadrillion
Btu
to include the pressure at which it is observed,
if
that pressure is
other than standard, i.e.,
760
mm
of
mercury or
1
atmosphere (STP,
Standard Temperature and Pressure).
The boiling points
of
petroleum fractions are rarely,
if
ever, distinct
temperatures. It is, in fact, more correct to refer to the boiling ranges
of
the various fractions; the same is true
of
natural gas. To determine
these ranges, the material in question is tested
in
various methods of
distillation, either at atmospheric pressure
or
at reduced pressure.
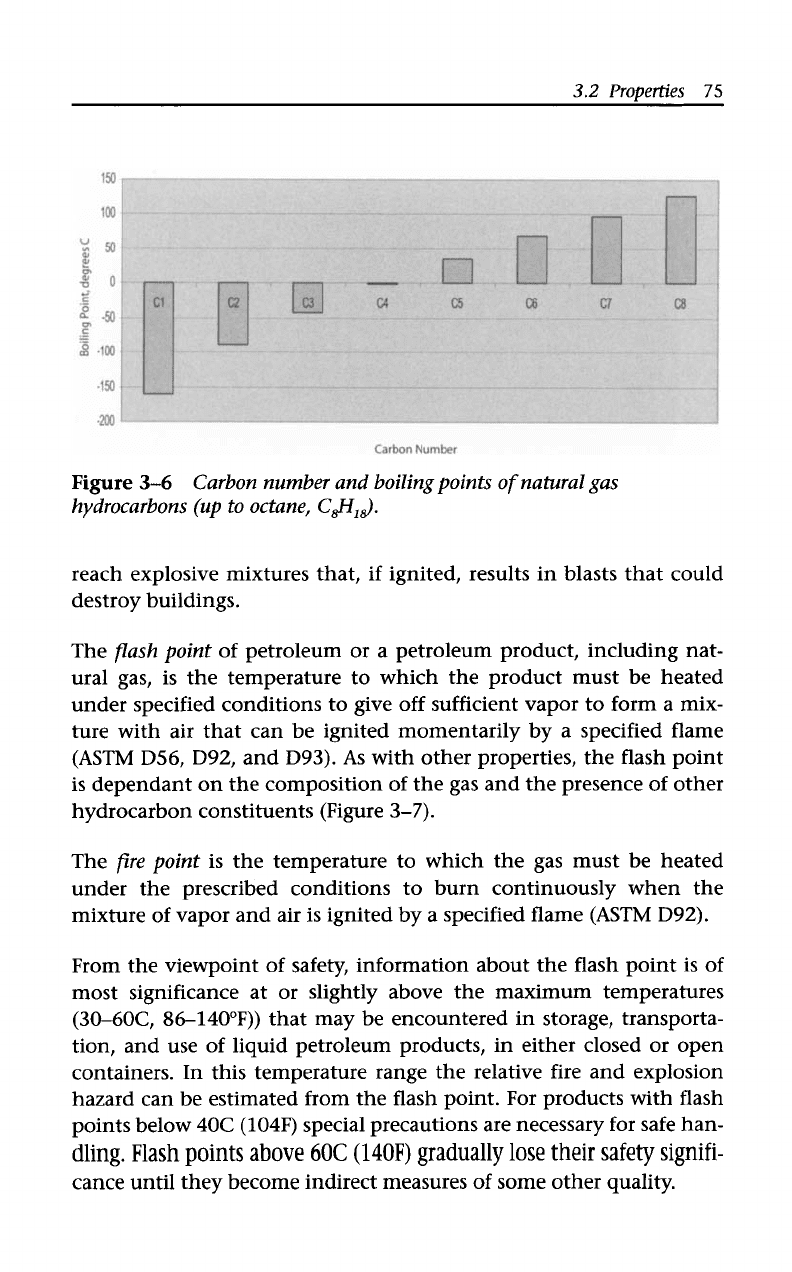
Thus, the boiling points
of
the hydrocarbon constituents
of
natural
gas increase with molecular weight and the initial boiling point
of
natural gas corresponds
to
the boiling point
of
the most volatile
constituents (i.e., methane) (Figure
3-6).
Purified natural gas is neither corrosive nor toxic, its ignition temper-
ature is high, and it has a narrow flammability range, making it an
apparently safe fossil fuel compared to other fuel sources.
In
addition,
purified natural gas (i.e., methane) having a specific gravity
(0.60)
lower than that of air
(1.00)
rises if escaping and dissipates from the
site
of
any leak.
However, methane is highly flammable, burns easily and almost
completely. Therefore, natural gas can also be hazardous to life and
property through an explosion. When natural gas
is
confined, such
as within a house or in a
coal
mine, concentration
of
the
gas
can
3.2
Properties
75
150
100
Carbon
Number
Figure
3-6
Carbon number and boilingpoints
of
natural gas
hydrocarbons
(up
to octane, C$l,&.
reach explosive mixtures that,
if
ignited, results
in
blasts that could
destroy buildings.
The
flash point
of petroleum or a petroleum product, including nat-
ural gas, is the temperature to which the product must be heated
under specified conditions to give
off
sufficient vapor
to
form a mix-
ture with air that can be ignited momentarily by a specified flame
(ASTM D56, D92, and D93). As with other properties, the flash point
is dependant
on
the composition
of
the gas and the presence
of
other
hydrocarbon constituents (Figure
3-7).
The
fire point
is the temperature to which the gas must be heated
under the prescribed conditions
to
burn continuously when the
mixture of vapor and air is ignited by a specified flame (ASTM D92).
From the viewpoint
of
safety, information about the flash point is
of
most significance at or slightly above the maximum temperatures
(30-60C, 86140°F)) that may be encountered in storage, transporta-
tion, and use of liquid petroleum products, in either closed or open
containers.
In
this temperature range the relative fire and explosion
hazard can be estimated from the flash point. For products with flash
points below 40C (104F) special precautions are necessary for safe han-
dling. Flash points above
60C
(140F) gradually lose their
safety
signifi-
cance until they become indirect measures
of
some other quality.
