Speight J.G. Natural Gas: A Basic Handbook
Подождите немного. Документ загружается.


98
Chapter
4
Recovery, Storage, and Transportation
negative effects of possible terrorist activity, political changes, and
trade embargoes over long periods of time.
4.3.1
Pipelines
Pipelines are a very convenient transport method, but are not flexible
as
the gas will leave the source and arrive at its (one) destination
(Cranmore and Stanton,
2000).
Once the pipeline diameter is
decided, the quantities
of
gas that
can
be delivered is fixed by the
pressures, although an increase in the maximum quantity can be
achieved by adding compressors along the line, extra pipe
in
the form
of
loops, and/or by increasing the average pipeline pressure. However,
if
the pipeline must be shut down, the production and receiving
facilities, be it gas reservoir, processor or refinery, often also must be
shut down because gas cannot be readily stored, except perhaps by
increasing the pipeline pressure by some percentage.
Pipeline pressures (onshore) usually operate under pressure at
700
to
1,100
psi (although
4,000
psi lines are
in
operation), and offshore
pipelines operate under pressures on the order of
1,400
to
2,100
psi.
The pressure depends on the material of construction and the age of
the pipe, and the high operating pressure reduces the volume of the
natural gas being transported (by up to
600
times), as well as pro-
viding propellant force to move the natural gas through the pipeline.
Pipeline installation costs vary, but generally cost several million dol-
lars
(US)
per mile depending on the terrain plus compressor stations.
Subsea lines longer than
2,000
miles have, until recently, been
regarded as uneconomic, because of the subsea terrain making pipe-
line installation, maintenance expensive, and any recompression
along the route difficult, but changes are in the air!
In the United States, pipelines can be characterized as
interstate
or
intr-
astute.
Interstate pipelines carry natural gas across state boundaries, in
some cases clear across the country. Intrastate pipelines, on the other
hand, transport natural gas within a particular state.
In
addition, nat-
ural gas pipelines are subject to regulatory oversight, which in many
ways determines the manner in which pipeline companies must
operate. The interstate natural gas pipeline network transports pro-
cessed natural gas from processing plants
in
producing regions to
those
areas
throughout the
country
with high natural
gas
require-
ments, particularly large, populated urban areas.

4.3
Transportation
99
To ensure that the natural gas flowing through any one pipeline
remains pressurized, compression of this natural gas is required peri-
odically along the pipe. This is accomplished by compressor stations,
usually placed at 40-100-mile intervals along the pipeline. The natural
gas enters the compressor station, where it is compressed by either by
a turbine, a motor, or an engine.
Turbine compressors gain their energy by using up a small proportion
of
the natural gas that they compress.
The
turbine itself
serves
to
operate a centrifugal compressor, which contains a type of fan that
compresses and pumps the natural gas through the pipeline. Some
compressor stations are operated by using an electric motor to turn
the same type of centrifugal compressor. This type of compression
does not require the use of any of the natural gas from the pipe, but it
does require a reliable source of electricity nearby. Reciprocating nat-
ural gas engines are also used to power some compressor stations.
These engines resemble a very large automobile engine, and are pow-
ered by natural gas from the pipeline. The combustion
of
the gas
powers pistons
on
the outside of the engine, which serves to com-
press the natural gas.
In addition to compressing natural gas, compressor stations also usu-
ally contain some type of liquid separator, much like the ones used to
dehydrate natural gas during its processing. Usually, these separators
consist of scrubbers and filters that capture any liquids or other unde-
sirable particles from the natural gas in the pipeline. Although natural
gas
in
pipelines is considered to be
dry
gas,
it is not uncommon for a
certain amount of water and hydrocarbons to condense out of the gas
stream while
in
transit. The liquid separators at compressor stations
ensure that the natural gas in the pipeline is as pure as possible, and
usually filters the gas prior to compression.
In addition to compressing natural gas to reduce its volume and push
it through the pipe, metering stations are placed periodically along
interstate natural gas pipelines. These stations allow pipeline compa-
nies to monitor and manage the natural gas in their pipes. The
metering stations measure the flow of gas along the pipeline, and
allow pipeline companies to monitor the natural gas as
it
flows along
the pipeline. These metering stations employ specialized meters to
measure the natural gas as it flows through the pipeline, without
impeding its movement.

100
Chapter
4
Recovery, Storage, and Transportation
In
the United States, there are essentially three major types
of
pipe-
lines along the transportation route:
1.
The gathering system
2.
The interstate pipeline
3.
The distribution system
The gathering system consists
of
low-pressure, low-diameter pipelines
that transport raw natural gas from the wellhead
to
the processing
plant.
If
natural gas from a particular well has a high sulfur content
and a high carbon dioxide content (sour gas), a specialized sour gas
gathering pipe must be installed. Sour gas is extremely corrosive and
dangerous, thus its transportation from the wellhead to the sweet-
ening plant must be done carefully.
4.3.2
Liquefied Natural Gas
Natural gas liquefies at about
-162"
and has a volume about
1/600
that
of
gas at room temperature. However, facilities for liquefymg
natural gas require complex machinery with moving parts and special
refrigerated ships for transporting the liquefied natural gas to market.
The costs
of
building liquefied natural gas plant have lowered over
the past
25
years because
of
greatly improved thermodynamic effi-
ciencies
so
that liquefied natural gas is becoming a major gas export
method worldwide and many plants being extended, or new ones
built
in
the world.
Large cryogenic tanks are needed to store the liquefied natural gas;
typically these may be
70
m in diameter,
45
m high, and hold more
than
100,000
m3
of
liquefied natural gas.
At
the consumer end, an
infrastructure for handling the reprocessing
of
vast quantities
of
nat-
ural gas from liquefied natural gas is required, which is also expensive
and vulnerable to sabotage.
The current largest specially-built refrigerated tankers can carry
135,000
m3 liquefied natural gas, equivalent to
4.8
million scf of gas,
but are very expensive. This makes it difficult for liquefied natural gas
to use smaller isolated (offshore) reserves and
to
serve small markets
commercially because it is this large capacity, continuous running
that keeps thermodynamic efficiency and costs
to
a minimum. Thus,
small
volumes
of
intermittent
gas
are
not
economically attractive
to
the major gas sellers for liquefied natural gas facilities. However, small

4.3
Transportation
101
well-insulated liquefied natural gas container trade is being investi-
gated, and
if
successful, small quantities
of
liquefied natural gas may
be able
to
be delivered from the liquefied natural gas storage, just like
the gasoline tankers of today. Even
so,
the liquefied natural gas must
be stored for periods
of
time (months) without significant boil-off
losses, which is difficult.
4.3.3
Liquefied Petroleum
Gas
Liquefied petroleum gas (LPG)
is
the term applied to certain specific
hydrocarbons and their mixtures, which exist in the gaseous state
under atmospheric ambient conditions but can
be
converted
to
the
liquid state under conditions
of
moderate pressure at ambient
temperature.
Liquefied petroleum gas is a hydrocarbon mixture containing pro-
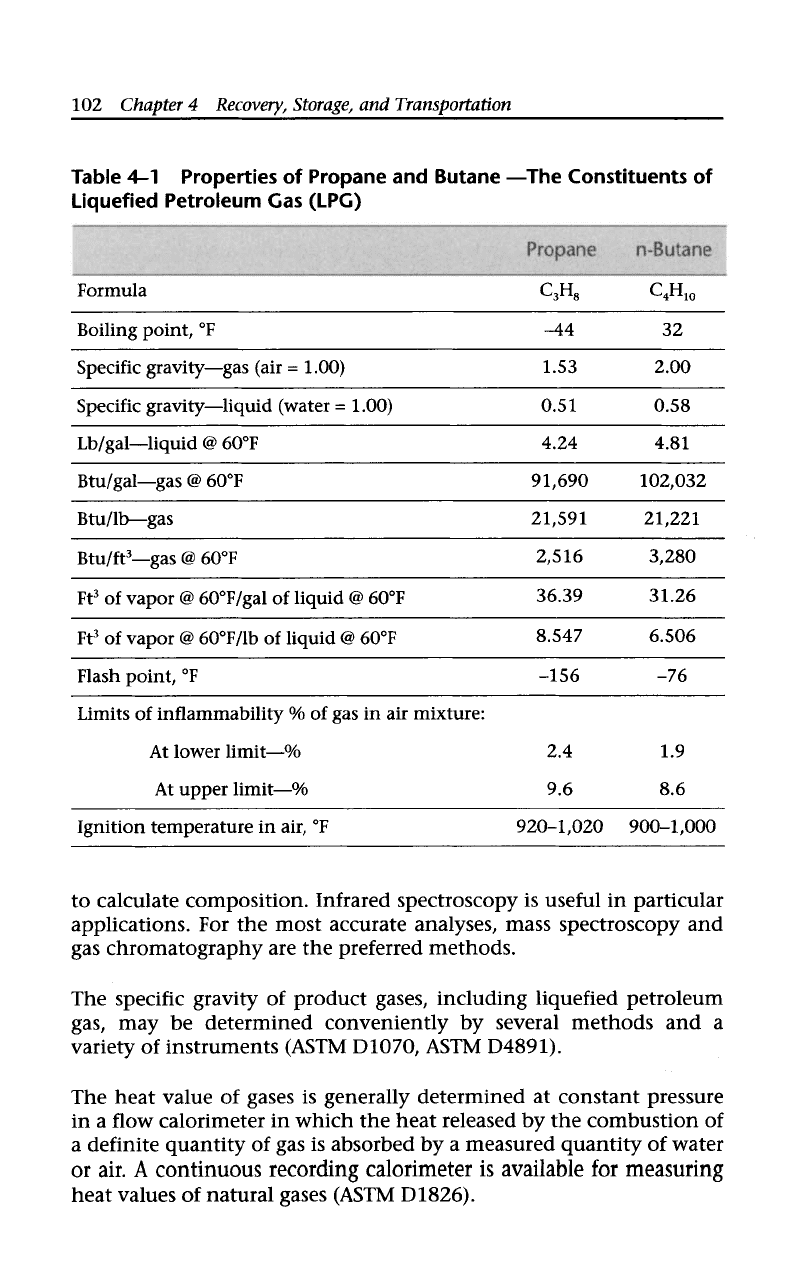
pane (CH,CH,CH,) and butane (CH,CH,CH,CH,) (Table
4-1).
To
a
lesser extent, iso-butane [CH,CH(CH,)CH,] may also be present. The
most common commercial products are propane, butane, or some
mixture
of
the
two
and are generally extracted from natural gas or
crude petroleum.
As already noted, the compositions
of
natural, manufactured, and
mixed gases can vary
so
widely,
no
single set
of
specifications could
cover all situations. The requirements are usually based
on
perfor-
mances
in
burners and equipment,
on
minimum heat content, and
on
maximum sulfur content. Gas utilities
in
most states come under
the supervision
of
state commissions or regulatory bodies, and the
utilities must provide a gas that is acceptable to all types
of
consumers
and that will give satisfactory performance
in
all kinds of consuming
equipment. However, there are specifications
for
liquefied petroleum
gas (ASTM D1835) that depend upon the required volatility.
The different methods for gas analysis (that may also be applied to
natural gas itself and other gases) include absorption, distillation,
combustion, mass spectroscopy, infrared spectroscopy, and gas chro-
matography (ASTM D2163, ASTM D2650, and ASTM
D4424).
Absorp-
tion methods involve absorbing individual constituents one at a time
in
suitable solvents and recording of contraction in volume mea-
sured. Distillation methods depend
on
the separation
of
constituents
by fractional distillation and measurement
of
the volumes distilled.
In combustion methods, certain combustible elements are caused to
burn
to
carbon dioxide and water, and the volume changes are used
102
ChaDter
4
Recovm, Storaae, and TransDortation
Table
4-1
Liquefied Petroleum Gas (LPC)
Properties of Propane and Butane -The Constituents
of
Formula
Boiling point,
OF
-44 32
Specific gravity-gas (air
=
1.00) 1.53 2.00
___~
Specific gravity-liquid (water
=
1.00) 0.51 0.58
Lb/gal-liquid
@
60°F 4.24 4.81
Btu/gal-gas
@
60°F 91,690 102,032
Btu/lb-gas 21,591 21,221
Btu/ft3-gas
@
60°F 2,516 3,280
Ft3 of vapor
@
60°F/gal
of
liquid
@
60°F
36.39 31.26
Ft3 of vapor
@
60"F/lb of liquid
@
60°F
8.547 6.506
Flash point, "F -156 -76
Limits of inflammability
Yo
of gas in air mixture:
At lower limit-Yo
2.4
1.9
At upper limit-Yo 9.6 8.6
Ignition temperature in air, "F 920-1,020 900-1,000
to calculate composition. Infrared spectroscopy is useful in particular
applications. For the most accurate analyses, mass spectroscopy and
gas chromatography are the preferred methods.
The specific gravity of product gases, including liquefied petroleum
gas, may be determined conveniently by several methods and a
variety
of
instruments (ASTM
D1070,
ASTM
D4891).
The heat value
of
gases is generally determined at constant pressure
in
a
flow calorimeter in which the heat released by the combustion of
a definite quantity
of
gas is absorbed by a measured quantity of water
or
air.
A
continuous recording calorimeter is available for measuring
heat values
of
natural gases
(ASTM
01826).

4.3
Transportation
103
The lower and upper limits of
flammability
indicate the percentage of
combustible gas in air below which and above which flame will not
propagate. When flame is initiated in mixtures having compositions
within these limits, it will propagate and therefore the mixtures are
flammable. Knowledge of flammable limits and their use in estab-
lishing safe practices in handling gaseous fuels is important, e.g.,
when purging equipment used in gas service, in controlling factory or
mine atmospheres, or in handling liquefied gases.
Many factors enter into the experimental determination of flammable
limits of gas mixtures, including the diameter and length of the tube
or vessel used for the test, the temperature and pressure of the gases,
and the direction of flame propagation-upward or downward. For
these and other reasons, great care must be used in the application of
the data.
In
monitoring closed spaces where small amounts of gases
enter the atmosphere, often the maximum concentration of the
combustible gas is limited to one fifth of the concentration of the gas
at the lower limit of flammability of the gas-air mixture.
4.3.4
Compressed
Natural
Gas
Gas can be transported in containers at high pressures, typically
1,800
psi for a rich gas (significant amounts of ethane, propane, etc.) to
roughly
3,600
psig for a lean gas (mainly methane). Gas at these pres-
sures
is
termed
compressed
nuturd
gas
(CNG). Compressed natural gas
is used in some countries for vehicular transport as an alternative to
conventional fuels (gasoline or diesel). The filling stations can be sup-
plied by pipeline gas, but the compressors needed to get the gas to
3,000
psi can be expensive to purchase, maintain, and operate.
An
alternative approach has dedicated transport ships carrying
straight, long, large-diameter pipes in an insulated cold storage cargo
package. The gas must be dried, compressed, and chilled for storage
onboard. By careful control of temperature, more gas can be trans-
ported in any ship of a given payload capacity, subject to volume lim-
itation and amount and weight of material of the pipe (pressure and
safety considerations). Suitable compressors and chillers are needed,
but would be much less expensive than a natural gas liquefier, and
would be standard,
so
that costs could be further minimized.
Compressed natural gas (CNG) transportation by ship is the answer to
providing access
to
the
world’s
stranded
natural
gas,
economically.
Because half of the world’s discovered gas is considered stranded and

104
Chapter
4
Recovery, Storage, and Transportation
over half
of
that gas is located offshore, a wealth
of
energy will soon
be accessible to many countries around the world.
A
new dynamic in
the global natural gas market is about
to
unfold. Transportation
of
compressed natural gas will not only provide energy for existing mar-
kets, but will also create many new markets. Island nations and
remote populations will now have economical access to natural gas
for power generation and economic development, Asia is poised to
benefit most from the safe and economical transportation of com-
pressed natural gas
by
ship.
4.3.5
Gas-to-Solid
Gas can be transported as a solid, with the solid being gas hydrate
(Berrrehaug and Gudmundsson,
1996;
Gudmundsson,
1996;
Gud-
mundsson, and Berrrehaug,
1996;
Gudmundsson et al.,
1997;
Gud-
mundsson et al.,
1995).
Natural gas hydrate (NGH) is the product of
mixing natural gas with liquid water
to
form a stable water crystalline
ice-like substance. Natural gas hydrate transport
is
believed to be a
viable alternative to liquefied natural gas or pipelines for the trans-
portation
of
natural gas from source
to
demand.
Natural gas hydrates are created when natural gas constituents
(methane, ethane, and propane) stabilize the physical structure of
water to form a three-dimensional cage-like structure with the gas
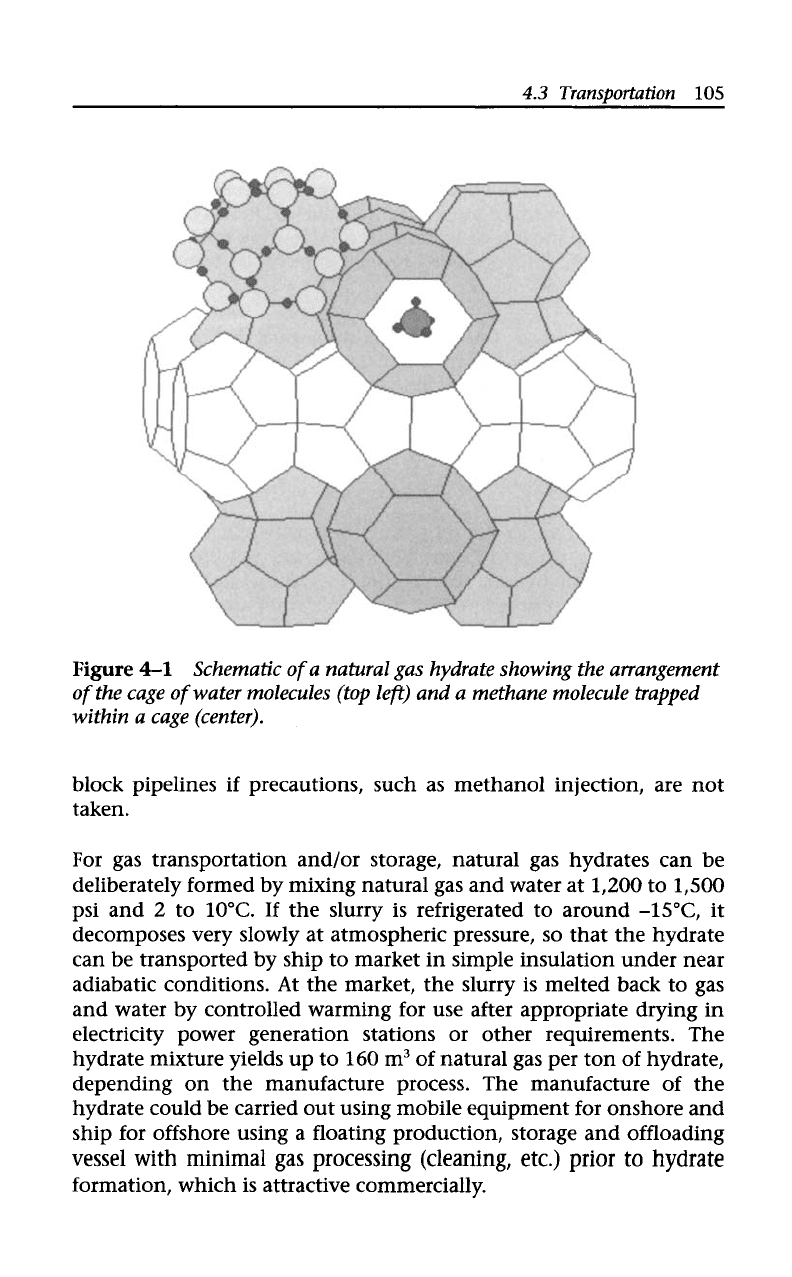
molecule trapped within the cages (Figure
4-1).
A
cage is made up
of
several water molecules held together by hydrogen bonds. The
hydrates are formed from natural gas in the presence of liquid water
provided the pressure is above and the temperature is below specified
limits. The solids have a snow-like appearance and properties that
make them useful in many kinds
of
applications, including transpor-
tation and storage. Gas hydrates contain about
15%
by weight and
85%
by weight water and can contain up to
160
m3 of gas per m3
of
solid hydrate. The exact numbers depend
on
the gas composition and
the formation (production) pressure and temperature (Berecz, and
Balla-Achs,
1983;
Cox,
1983).
Vast quantities
of
gas hydrate have been found in permafrost and on
the seabed in depths below
500
m
(1,500
ft),
and
if
properly exploited
could become a major source
of
natural gas (therefore, a major energy
source)
in
the next
50
years. On the other hand, natural gas hydrates
are a pipeline nuisance and safety hazard, and require considerable
care by the operators to ensure that they do not form as they can
4.3
TransDortation
105
Figure
4-1
Schematic
of
a
natural gas hydrate showing the arrangement
of
the cage
of
water molecules (top lefr) and a methane molecule trapped
within a cage (center).
block pipelines if precautions, such as methanol injection, are not
taken.
For gas transportation and/or storage, natural gas hydrates can be
deliberately formed by mixing natural gas and water at
1,200
to
1,500
psi and
2
to
10°C.
If
the slurry is refrigerated to around
-15"C,
it
decomposes very slowly at atmospheric pressure,
so
that the hydrate
can be transported by ship to market in simple insulation under near
adiabatic conditions. At the market, the slurry is melted back to gas
and water by controlled warming for use after appropriate drying in
electricity power generation stations or other requirements. The
hydrate mixture yields up to
160
m3
of
natural gas per ton
of
hydrate,
depending
on
the manufacture process. The manufacture
of
the
hydrate could be carried out using mobile equipment
for
onshore and
ship for offshore using a floating production, storage and offloading
vessel with minimal gas processing (cleaning, etc.) prior to hydrate
formation, which is attractive commercially.

106
Chapter
4
Recovery, Storage, and Transportation
The water can be used at the destination
if
there is water shortage, or
returned as ballast to the hydrate generator and, because it
is
saturated
with gas, it will not take more gas into solution. Process operability
of
continuous production of hydrate
in
a large-scale reactor, long-term
hydrate storage, and controlled regeneration of gas from storage has
been demonstrated.
The hydrate mixture can be stored at normal temperatures
(0
to
-10°C)
and pressures
(10
to
1
atmosphere) where
1
m3 of hydrate
should contain about
160
m3 gas per m3 of water. This concentration
of gas is attractive because it is easier to produce, and safer and cheaper
to store, compared to the
200
m3 gas per
1
m3 of compressed gas (high
pressure,
3,000
psig) or the
637
m3 gas per
1
m3
of
liquefied natural gas
(at low temperatures of
-161°C).
Gas storage in hydrate form becomes especially efficient at relatively
low pressures where substantially more gas per unit volume is
contained in the hydrate than in the free state or in the compressed
state when the pressure has dropped. When compared to the transpor-
tation of natural gas by pipeline or as liquefied natural gas, the hydrate
concept has lower capital and operating costs for the movement of
quantities of natural gas over adverse conditions. Thus, gas hydrate is
very effective for gas storage and transport because it eliminates low
temperatures and the necessity
of
compressing the gas to high pres-
sures. Dry hydrate pellets yield about
160
m3 of gas at standard condi-
tions from
1
m3 of hydrate, compared to the
637
m3 per
1
m3
of
liquefied natural gas. This is a considerable volume penalty (and hence
transport cost)
if
considered in isolation, but by using cheaper ships to
transport the hydrate, the process could be economical.
4.3.6
Gas-to-Power
Currently, much of the transported gas is used as fuel for electricity
generation. Electricity generation at or near the reservoir source and
transportation by cable to the destination(s) (gas-to-power, GTP) is
possible. Thus, for instance, offshore or isolated gas could be used to
fuel an offshore power plant (may be sited in less hostile waters),
which would generate electricity for sale onshore or to other
off-
shore customers. Unfortunately, installing high-power lines to reach
the shoreline appear to be almost as expensive as pipelines, so that
gas-to-power could be viewed as defeating the purpose of an alterna-
tive cheaper solution
for
transporting
gas.
There is significant
energy loss from the cables along the long-distance transmission

4.3
TransDortation
107
lines, more so if the power is AC rather than DC; additionally, losses
also occur when the power is converted
to
DC from AC and when it
converted from the high voltages used in the transmission to the
lower values needed by the consumers.
Some consider having the energy as gas at the consumers’ end gives
greater flexibility and better thermal efficiencies, because the waste
heat can be used for local heating and desalination. This view is
strengthened by the economics
as
power generation
uses
about
1
mil-
lion scf/day of gas for every
10
MW
of
power generated,
so
that even
large generation capacity would not consume much
of
the gas from
larger fields, and thus not generate large revenues
for
the gas pro-
ducers. Nevertheless, gas-to-power has been an option much consid-
ered in the
US
for
getting energy from the Alaskan gas and oil fields to
the populated areas.
There are other practical considerations to note, such as
if
the gas is
associated gas, then
if
there is generator shutdown and
no
other gas
outlet, the whole oil production facility might also have to be shut
down,
or
the gas released to flare. Also,
if
there are operational prob-
lems within the generation plant, the generators must be able to shut
down quickly (in around 60
s)
to keep a small incident from esca-
lating. Additionally, the shutdown system itself must be safe,
so
that
any plant that has complicated processes that require a purge cycle
or
a cool-down cycle before
it
can shut down is clearly unsuitable (Bal-
lard, 1965). Finally,
if
the plant cannot shut down easily and/or be
able
to
start up again quickly (perhaps in an hour), operators will be
hesitant to ever shut down the process, for fear
of
financial retribu-
tion from the power distributors.
4.3.7
Cas-to-Liquids
In
gas-to-liquids (GTL) transport processes, the natural gas is con-
verted
to
a liquid, such as syncrude methanol, ammonia, etc., and
transported as such (Knott, 1997; Skrebowski, 1998; Thomas, 1998;
Gaffney Cline and Associates, 2001).
Methane is first mixed with steam and converted
to
syngas (mixtures
of carbon monoxide and hydrogen, CO
+
H,)
by one of several routes
using suitable new catalyst technology. The syngas is then converted
into
a
liquid using
a
Fischer-Tropsch
process
(in
the
presence
of
a
cat-
alyst) or an oxygenation method (mixing syngas with oxygen in the
