Speight J.G. Natural Gas: A Basic Handbook
Подождите немного. Документ загружается.


14
Chapter
1
History
and
Uses
a long, thin reservoir horizontally instead
of
vertically, enabling the oil
or gas from the reservoir to be recovered with fewer wells.
1.5
Conventional Gas
1
S.1
Associated
Gas
Associated
or
dissohed natural
gas
occurs either as free gas in a petro-
leum reservoir
or
as gas in solution in the petroleum. Gas that
occurs
as a solution in the petroleum is
dissolved
gas, whereas the gas that
exists in contact with the petroleum
(gas
cap)
is
associated
gas
(Figure
1-2).
Crude
oil
cannot be produced without producing some of its associ-
ated gas, which comes out of solution as the pressure is reduced
on
the way to and
on
the surface. Properly designed well completions
and reservoir management are used to minimize the production of
associated gas
so
as to retain the maximum energy in the reservoir
and thus increase ultimate recovery. Crude oil in the reservoir with
minimal or
no
dissolved associated gas is rare and as
dead crude
oil
is
often difficult to produce as there is little energy
to
drive it.
After the production fluids are brought to the surface, they are sepa-
rated at a tank battery at or near the production lease into a hydro-
carbon liquid stream (crude oil or
gas
condensate),
a produced water
stream (brine or salty water), and a gaseous stream. The gaseous stream
is traditionally very rich
(rich
gas)
in
natural
gas
Ziquids
(NGLs).
Natural
gas liquids include ethane, propane, butanes, and pentane
(C,H,,)
and
higher molecular weight hydrocarbons. The higher molecular weight
hydrocarbon product, which may also contain some pentane, is com-
monly referred to as
natural
gasoline.
Rich gas has a high heating value and a high hydrocarbon dew point.
However, the terms
rich
gas
and
lean
gas,
as used
in
the gas processing
industry, are
not
precise indicators
of
gas quality but only indicate the
relative amount
of
natural gas liquids in the gas stream. When refer-
ring to natural gas liquids in the natural gas stream, the term
gallons
per
thousand cubic
feet
of
gas is used as a measure of hydrocarbon rich-
ness. Thus,
in
the case
of
associated gas, crude oil may be assisted up
the wellbore by gas lift (Speight,
1993).
Gas is compressed into the
annulus
of
the well and then injected by means
of
a gas-lift valve near
the bottom
of
the well into the crude-oil
column
in the tubing. At the
top of the well the crude oil and gas mixture passes into
a
separation

1.5
Conventional
Gas
15
plant that drops the pressure down to nearly atmospheric
in
two
stages. The crude oil and water exits the bottom of the lower pressure
separator, from where it is pumped to tanks for separation
of
the
crude oil and water. The gas produced in the separators is recom-
pressed, and the gas that comes out
of
solution
with
the produced
crude
oil
(surplus gas) is then treated to separate out the natural gas
liquids
(NGLs)
that are treated
in
a gas plant to provide propane and
butane or a mixture
of
the
two
(liquefied petroleum gas,
LPG).
After
the propane and butane are removed, the higher boiling residue is
condensate, which is mixed with the crude oil or exported as a sepa-
rate product.
The gas itself is then
dry
and, after compression, is suitable to be
injected into the natural gas system where it substitutes for natural gas
from the non-associated gas reservoir. Pre-treated associated gas from
other fields enters the system at this stage (Manning and Thompson,
1991).
Another use for the gas is as fuel for the gas turbines
on
site.
This gas is treated
in
a fuel gas plant to ensure it is clean and at the cor-
rect pressure. The start-up fuel gas supply will be from the main gas
system, but facilities exist to collect and treat low-pressure gas from
the various other plants as a more economical fuel source.
1
S.2
Non-Associated
Gas
This gas (sometimes called
gas
well
gas)
is produced from geological
formations that typically do not contain much,
if
any, crude oil, or
higher boiling hydrocarbons
(gas
liquids)
than methane. However,
non-associated gas can contain non-hydrocarbon gases, such as
carbon dioxide and hydrogen sulfide.
The non-associated gas recovery system is somewhat simpler than the
associated gas recovery system. The gas flows up the well under its
own energy, through the wellhead control valves, and along the flow
line to the treatment plant. Treatment requires the gas temperature to
be reduced to a point dependent upon the pressure
in
the pipeline
so
that all liquids that would exist at pipeline temperature and pressure
condense and are removed.
The water
in
the gas must also be dealt with to stop the formation
of
gas
hydrates
that may block the pipes. One method is to inject eth-
ylene glycol
(gZycoZ),
which combines with the water and is later
recovered
by
a glycol plant. The treated gas then
flows
from the top
of
the treatment vessel and into the pipeline. The water is treated
in
a

16
Chapter
1
History
and
Uses
glycol plant
to
recover the glycol. Any natural gas liquids are pumped
as additional feedstock to the liquefied petroleum gas plant.
1.6
Unconventional Gas
The boundary between conventional gas and unconventional gas
resources is not well defined, because it results from a continuum
of
geologic conditions. Coal-seam gas, more frequently called coal-bed
methane, is frequently referred to as unconventional gas. Tight shale
gas and gas hydrates are also placed into the category
of
unconven-
tional gas.
1.6.1
Coal-Bed Methane (CBM)
This is the generic term given to methane gas held in underground
coal seams and released or produced when the water pressure within
the seam is reduced by pumping from either vertical or inclined to
horizontal surface holes. The methane is predominantly formed
during the coalification process whereby organic matter is slowly
transformed into coal by increasing temperature and pressure as the
organic matter is buried deeper and deeper by additional deposits
of
organic and inorganic matter over long periods of geological time.
This is referred to as
thermogenic
coal-bed methane.
Alternatively, and more often (but
not
limited
to)
in
lower rank and
thermally immature coals, recent bacterial processes (involving natu-
rally-occurring bacteria associated with meteoric water recharge at
outcrop or sub-crop) can dominate the generation
of
coal-bed
methane. This is referred to as late stage
biogenic
coal-bed methane.
During the coalification process, a range of chemical reactions occur
that produce substantial quantities
of
gas. While much
of
this gas
escapes
into
the overlying or underlying rock, a large amount is
retained within the forming coal seams. However, unlike conventional
natural gas reservoirs, where gas is trapped in the pore or void spaces
of a rock, such as sandstone, methane formed and trapped in coal is
actually adsorbed onto the coal grain surfaces, or micropores, and held
in place by reservoir (water) pressure. Therefore, because the
micropore surface area
is
very large, coal can potentially hold signifi-
cantly more methane per unit volume than most sandstone reservoirs.
The
amount
of
methane stored in coal
is
closely related
to
the rank
and depth of the coal, the higher the coal rank and the deeper the

1.6
Unconventional Gas
17
coal seam is presently buried (causing pressure
on
coal) the greater its
capacity to produce and retain methane. Because coal has a very large
internal surface area
of
over one billion square feet per ton
of
coal, it
can hold
on
average three times as much gas
in
place as the same
volume
of
a conventional sandstone reservoir at equal depth and
pressure. To allow the “absorbed” gas to be released from the coal it is
often necessary to lower the pressure
on
the coal. This generally
involves removing the water contained
in
the coal bed. After the gas
is released from the internal surfaces of the coal,
it
moves through the
coal’s internal matrix until it reaches natural fracture networks in the
coal known as
cleats.
The gas then flows through these cleats, or frac-
tures, until it reaches the wellbore.
Gas derived from coal is generally pure and requires little
or
no pro-
cessing, because it is solely methane and not mixed with heavier
hydrocarbons, such as ethane, which are often present in conven-
tional natural gas. Coal-bed methane has a slightly higher energy
value than some natural gases. Coal-seam gas well productivity
depends mostly
on
reservoir pressure and water saturation.
To recover coal-bed methane, multi-well patterns are necessary to
dewater the coal and to establish a favorable pressure gradient.
Because the gas is adsorbed
on
the surface
of
the coal and trapped by
reservoir pressure, initially there is low gas production and high water
production. Therefore, an additional expense relates to the disposal of
coal-bed water, which may be saline, acidic, or alkaline. As produc-
tion continues, water production declines and gas production
increases, before eventually beginning a long decline.
In
general,
however, coal-seam gas recovery rates have been low and unpredict-
able. Average per-well conventional gas production
in
a mature gas-
rich basin is about five times greater than average per-well coal-seam
gas production. Thus, several times as many wells must be drilled in
coal seams than in conventional gas accumulations to achieve similar
gas recovery levels.
1.6.2
Shale
Gas
Large continuous gas accumulations are sometimes present
in
low
permeability shale, (tight) sandstones, siltstones, sandy carbonates,
limestone, dolomite, and chalk. Such gas deposits are commonly clas-
sified as unconventional, because their reservoir characteristics differ
from conventional reservoirs, and they require stimulation to
be
pro-
duced economically.

18
Chapter
1
History and Uses
The tight gas is contained in lenticular or blanket reservoirs that are
relatively impermeable, occur downdip from water-saturated rocks,
and cut across lithologic boundaries. They often contain a large
amount
of
in-place gas, but exhibit low recovery rates. Gas can be
economically recovered from the better quality continuous tight res-
ervoirs by creating downhole fractures with explosives or hydraulic
pumping. The nearly vertical fractures provide a pressure sink and
channel for the gas, creating a larger collecting area
so
that the gas
recovery is faster. Sometimes, massive hydraulic fracturing
is
required,
using a half million gallons
of
gelled fluid and a million pounds
of
sand to keep the fractures open after the fluid has been drained away.
In
the United States, unconventional gas accumulations account for
about
2
trillion cubic feet (tcf)
of
gas production per year, some
10%
of
total gas output.
In
the rest
of
the world, however, gas is predomi-
nantly recovered from conventional accumulations.
1.6.3
Gas
Hydrates
A
gas
hydrate
is a molecule consisting
of
an ice lattice, or "cage,"
in
which low molecular weight hydrocarbon molecules, such as
methane, are embedded. The
two
major conditions that promote
hydrate formation are
(1)
high gas pressure and low gas temperature
and
(2)
the gas at or below its water dew point with free water present.
Gas hydrates are common constituents
of
the shallow marine geo-
sphere and occur both in deep sedimentary structures, and as out-
crops
on
the ocean floor. Methane hydrates are believed to form by
migration of gas from depth along geological faults, followed by pre-
cipitation, or crystallization,
on
contact
of
the rising gas stream with
cold sea water.
At high pressures methane hydrates remain stable at temperatures up
to
18"C,
and the typical methane hydrate contains one molecule of
methane for every six molecules
of
water that forms the ice cage, but
this ratio is dependent
on
the number
of
methane molecules that fit
into the various cage structures of the water lattice. One liter
of
solid
methane hydrate can contain up to
168
liters
of
methane gas.
Methane hydrates are restricted to the shallow lithosphere (i.e.,
<
2,000
meters depth). Furthermore, necessary conditions are found
only either in polar continental sedimentary rocks, where surface
temperatures are less than
O"C,
or
in oceanic sediment at water depths

1.7
Reserves
19
greater than
300
meters where the bottom water temperature is about
2°C
(35°F).
Continental deposits have been located
in
Siberia and
Alaska in sandstone and siltstone beds at less than
800
meters depth.
The methane in gas hydrates is dominantly generated by bacterial
degradation of organic matter
in
low-oxygen environments. Organic
matter in the uppermost few centimeters of sediments is first attacked
by aerobic bacteria, generating carbon dioxide, which escapes from
the sediments into the water column.
In
this region
of
aerobic bacte-
rial activity, sulfates are reduced to sulfides.
If
the sedimentation rate
is low
(4
cm per
1,000
years), the organic carbon content is low
(<lo/),
and oxygen
is
abundant, aerobic bacteria use up all the
organic matter
in
the sediments. But where sedimentation rates and
the organic carbon content are high, the pore waters
in
the sediments
are anoxic at depths of only a few centimeters, and methane is pro-
duced by anaerobic bacteria.
The presence
of
clathrates at a given site can often be determined by
observation
of
a
bottom
simulating
reflector
(BSR), which is a seismic
reflection at the
sediment-to-clathrate-stability-zone
interface caused
by the different density between normal sediments and sediments
laced with clathrates.
The size of the oceanic methane hydrate reservoir is not well defined
and estimates of its size have varied considerably over a wide range.
However, improvements in understanding the nature of the gas
hydrate resource have revealed that hydrates only form
in
a narrow
range
of
depths (such as in the area
of
continental shelves) and typi-
cally are found at low concentrations
(0.9-1.5%
by volume) at sites
where they do occur. Recent estimates constrained by direct sampling
suggest the global inventory lies between
1
x
lo”
to
5
x
lo1’
m3
of
gas.
1.7
Reserves
Reserves are the amount of a resource available for recovery and/or
production with the recoverable amount being usually tied to the
economic aspects of production. Natural gas is produced
on
all conti-
nents except Antarctica (BP,
2005).
The world’s largest producer
is
Russia. The United States, Canada, and the Netherlands are also
important producers. The proven reserves of natural gas are in excess
of
3,600
trillion cubic feet
(1
Tcf
=
1
x
lo”),
of which about
300
Tcf
that the total gas resource base (like any fossil fuel or mineral resource
exist
in
the United States and Canada.
It
should also be remembered

20
Chapter
1
History
and
Uses
base) is dictated by economics. Therefore, when resource data are
quoted, some attention must be given to the cost
of
recovering those
resources. Most important, the economics must also include a cost
factor that reflects the willingness to secure total, or a specific degree
of, energy independence.
1.8
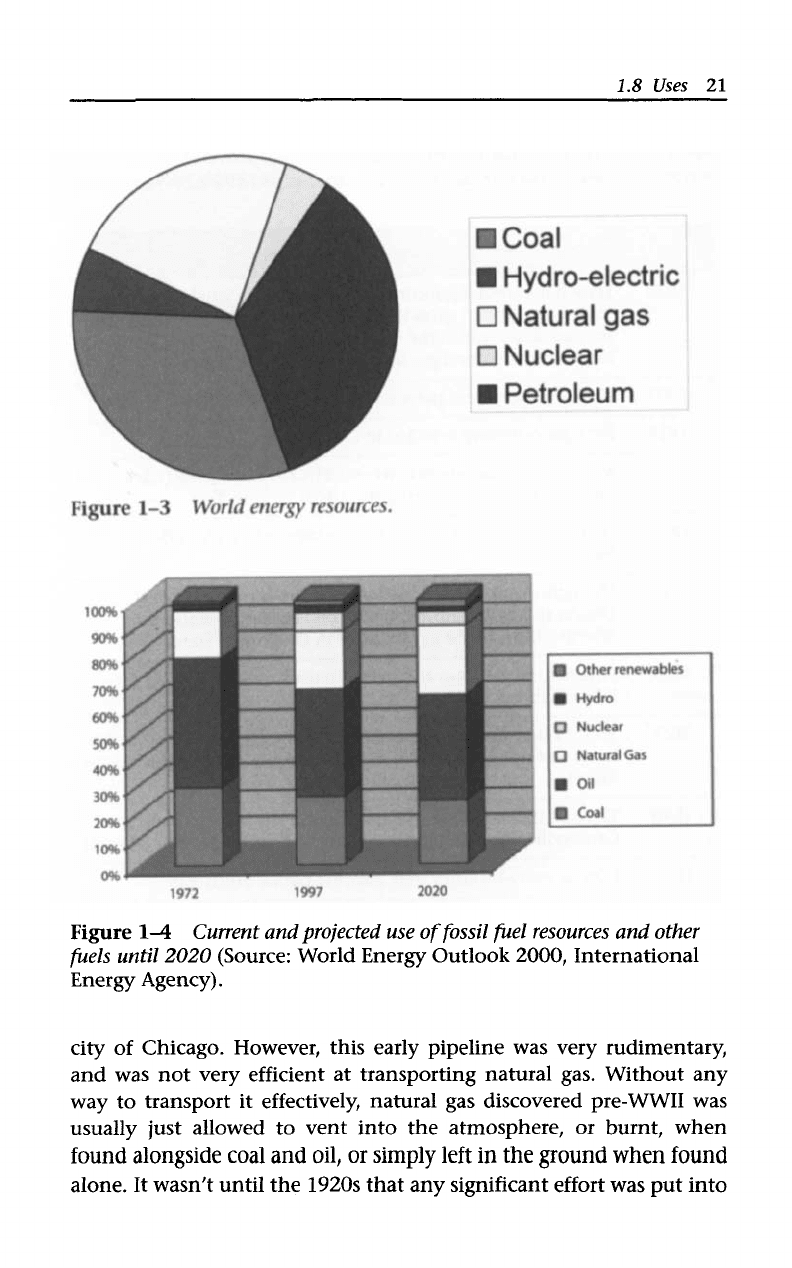
Uses
Currently, natural
gas
is
about
one
quarter
of
the energy
resources
of
the world (Figure
1-3)
with use projected to increase over the next
two
decades (Figure
1-4).
However, to understand the use of natural gas,
it
is
necessary to review the history
of
natural gas over the past
two
thou-
sand years and the use of natural gas
in
the United States (Table
1-2).
After the discovery by the Chinese more than
two
thousand years ago
that the energy
in
natural gas could be harnessed and used as a heat
source, the use of natural gas has grown. As noted, the American nat-
ural gas industry got its beginnings
in
the mid-nineteenth century,
and most in gas industry observers characterize the “Drake well”
(q.v.,
above) as beginning of the natural gas industry in America.
During most
of
the 19th century, natural gas was used almost exclu-
sively as a source
of
light. Without a pipeline infrastructure,
it
was dif-
ficult to transport the gas very far, or into homes to be used for
heating or cooking. Most
of
the natural gas produced in this era was
manufactured from coal, rather than from a well. Near the end
of
the
19th century, with the rise
of
electricity, natural gas lights were con-
verted to electric lights. This led producers of natural gas
to
look for
new uses for their product.
In 1885, Robert Bunsen invented what is now known as the
Bunsen
burner
(Figure
1-5),
which has long been a common tool
in
most
chemical laboratories throughout the world. Bunsen created the
device that mixed natural gas with air in the right proportions, cre-
ating a flame that could be safely used for cooking and heating. The
invention
of
the Bunsen burner opened up new opportunities for the
use of natural gas
in
America, and throughout the world. The inven-
tion
of
temperature-regulating thermostatic devices allowed for better
use of the heating potential
of
natural gas, allowing the temperature
of the flame to be adjusted and monitored.
One
of
the
first
lengthy pipelines
was
constructed in
1891.
It
was
120
miles long, and carried natural gas from wells in central Indiana to the
1.8
Uses
21
Figure
1-3
World energy resources.
Figure
1-4
Current and projected use
of
fossil
fuel resources and other
fuels until
2020
(Source: World Energy Outlook
2000,
International
Energy Agency).
city
of
Chicago. However, this early pipeline was very rudimentary,
and was not very efficient at transporting natural gas. Without any
way to transport it effectively, natural gas discovered pre-WWII was
usually just allowed
to
vent into the atmosphere, or burnt, when
found alongside coal and oil,
or
simply
left in the ground
when
found
alone. It wasn’t until the
1920s
that any significant effort was put into
22
Chavter
1
History
and
Uses
Table
1-2
Timeline
for
Use
of
Natural
Gas
(Adapted from
www. cityofmesa.
org/utilities/gas/historyofgas.
asp#
7
800%20-
%20
1
850)
1620 French missionaries recorded that Indians in what is now New
York state, ignited gases in the shallows
of
Lake Erie and in the
streams flowing into the lake. It was in this same area (Fredonia,
NY)
that the natural gas industry in America began.
~ ~~
1803 Gas lighting system patented in London by Frederick Winsor.
1812 First gas company founded in London.
1815 Metering for households, invented in 1815 by Samuel Clegg, and
put into general use during the 1840s.
~~ ~
1816
First
US
gas company (using manufactured gas) founded in
Baltimore.
1817 The lighting
of
the first gas lamp on the corner
of
Market and
Lemon streets in Baltimore, MD, on February
7
marks the
effective birth
of
the gas industry in the United States.
1821
First natural gas from the wellhead used in Fredonia, NY for
house lighting.
1826
World’s first gas cooker was devised in England by James Sharp,
but it was not until 1851 that such equipment came into use in
America.
1840
The first industrial use
of
natural gas in the
US
is recorded near
Centerville, PA, when gas is used to evaporate brine to make salt.
1850
Fifty or more
US
cities were burning public utility gas.
1859
Edwin
L.
Drake dug the first well and hit oil and natural gas near
Titusville, PA. An iron 2-in.-diameter gas pipeline was built,
running
5-1/2
miles from the well to Titusville proving that
natural gas could be brought safely from its underground source
to be used
for
practical purposes.
Standardized metering begins with the formation of the
American Meter Co. under a 50-year New York charter.
1863
1870
An attempt was made at Bloomfield to convey natural gas
through a 20-mile main but this failed, chiefly because
of
excessive leakage from the pinewood pipes used.
1.8
Uses
23
Table
1-2
Timeline
for
Use of
Natural Gas
(Adapted
from
www.cityofmesa.org/utilities/gas/historyofgas.
asp#
7
800%20-%20
7
850)
(con
t'd)
1870
Pre-payment meters, patented in
1870
by T.S. Lacey, were
introduced in Great Britain, and helped to spread the use of gas
to the poorer sections of the community.
The first long-distance natural gas pipeline in the US is
completed in Pennsylvania.
1872
1880
Thomas Edison postulated replacing gas lighting by electric
lighting.
Manufacturers begin selling stoves fueled with gas.
The first gas circulating or tank water heater appears in the
US.
1880
1883
1885
Robert Bunsen invented the Bunsen burner (see Figure
1-5).
Carl
Auer von Welsbach in Germany developed a practical gas
mantle, which patented in
1885.
One of the first lengthy pipelines was constructed. This pipeline
was
120
miles long, and carried natural gas from wells in central
Indiana to the city of Chicago.
Internal combustion engine development allows the first
compressors to be installed to move gas farther distances.
1891
1899
1900
Natural gas had been discovered in
17
states. At this time, coal
was the nation's major energy source, accounting for
60%
of the
United States' energy needs. Wood provided
35%,
and oil and
natural gas together accounted for only
5%.
By
1910,
wood
almost completely disappeared as an energy source, and coal
continued to dominate usage until after World War
11.
~~
1904
Gas is used for the first time to power central heating and to
provide a large-scale supply of hot water in London. Also,
experiments
in
house central heating, using gas as a fuel, are
begun
in
St. Lois by Laclede Gas.
1907
The first gas well in
TX
was brought in from the Petrolia field.
Edwin Brown began pumping gas to nearby cities and by
1913
was serving Dallas, Fort Worth, and
21
other towns. Brown
formed the Lone Star Gas
in
1909.
