Speight J.G. Natural Gas: A Basic Handbook
Подождите немного. Документ загружается.


7.5
Carbonate Washing
and
Water-Washing Processes
179
process include water washing the hydrocarbon liquid from the low
temperature separator to enhance the methanol recovery. The
IFPEXOL-2 process for acid gas removal is very similar to an amine
type process except for the operating temperatures. The absorber
operates below -20°F to minimize methanol losses, and the regener-
ator operates at about
90
psi. Cooling is required
on
the regenerator
condenser
to
recover the methanol. This process usually follows the
IFPEXOL-1 process,
so
excessive hydrocarbon absorption is not as
great
a
problem (Minkkinen and Jonchere,
1997).
7.5
Carbonate Washing and Water-Washing
Processes
Carbonate washing is a mild alkali process for emission control by the
removal of acid gases (such as carbon dioxide and hydrogen sulfide)
from gas streams (Speight, 1993). It uses the principle that the rate
of
absorption
of
carbon dioxide by potassium carbonate increases with
temperature. It has been demonstrated that the process works best
near the temperature
of
reversibility of the reactions:
K,CO,
+
CO,
+
H,O +2KHCO,
K,CO,
+
H,S
+KHS
+
KHCO,
Water washing, in terms
of
the outcome, is analogous
to
washing
with potassium carbonate (Kohl and Riesenfeld, 1985), and it is also
possible to carry out the desorption step by pressure reduction. The
absorption is purely physical, and there is also a relatively high
absorption of hydrocarbons, which are liberated at the same time as
the acid gases.
The process using
potassium phosphate
is known as phosphate desul-
phurization, and it is used
in
the same way as the Girbotol process to
remove acid gases from liquid hydrocarbons as well as from gas
streams. The treatment solution is a water solution of tripotassium
phosphate
(K,PO,),
which is circulated through an absorber tower and
a
reactivator tower
in
much the same
way
as
the ethanolamine
is
cir-
culated in the Girbotol process; the solution is regenerated thermally.

180
ChaDter
7
Processes
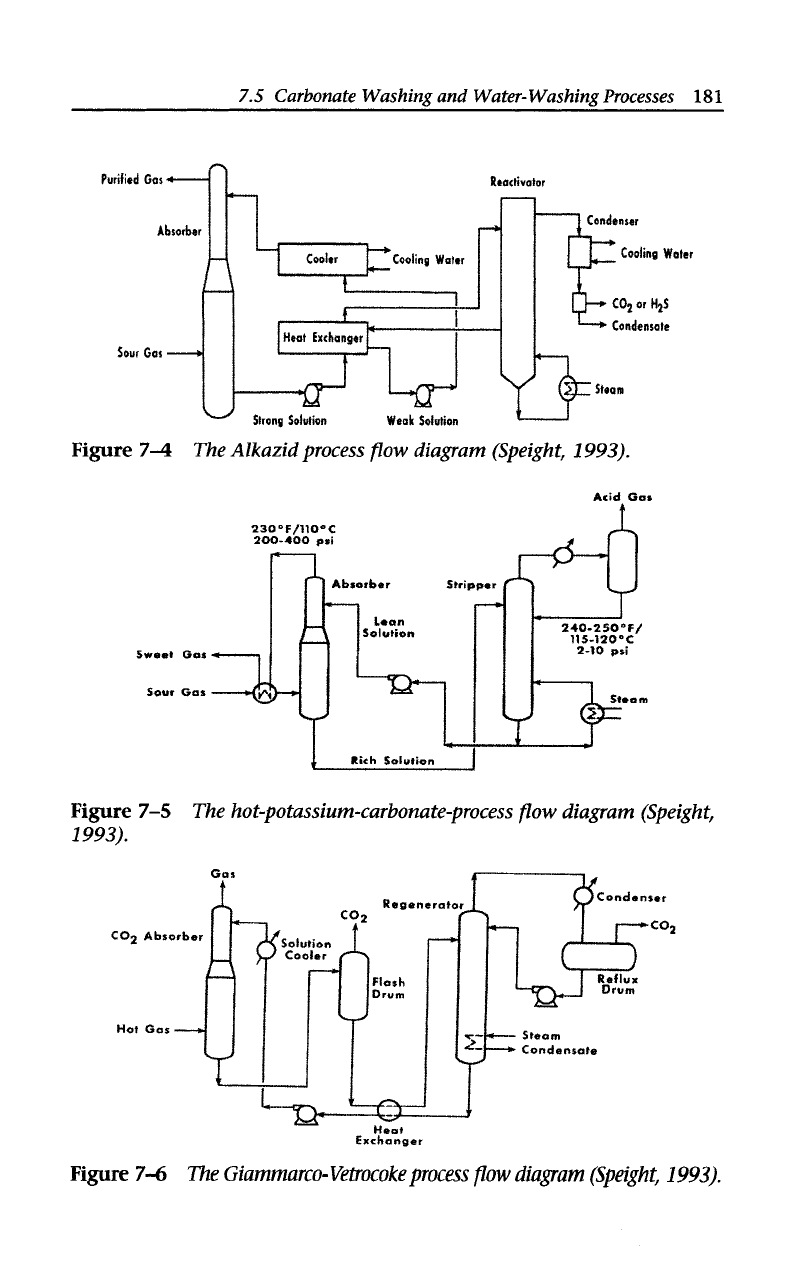
Other processes include the
Alkazid
process
(Figure
7-4),
which
removes hydrogen sulfide and carbon dioxide using concentrated
aqueous solutions
of
amino acids.
The
hot potassium carbonate process
(Figure 7-5) decreases the acid con-
tent
of
natural and refinery gas from as much as
50%
to
as low as
0.5% and operates in a unit similar
to
that used for amine treating.
The
Giammarco-
Vetrocoke process
is used for hydrogen sulfide and/or
carbon dioxide removal (Figure 7-6).
In
the hydrogen sulfide removal
section, the reagent consists
of
sodium or potassium carbonates con-
taining a mixture of arsenites and arsenates; the carbon dioxide
removal section uses
hot
aqueous alkali carbonate solution activated
by arsenic trioxide, selenous acid, or tellurous acid.
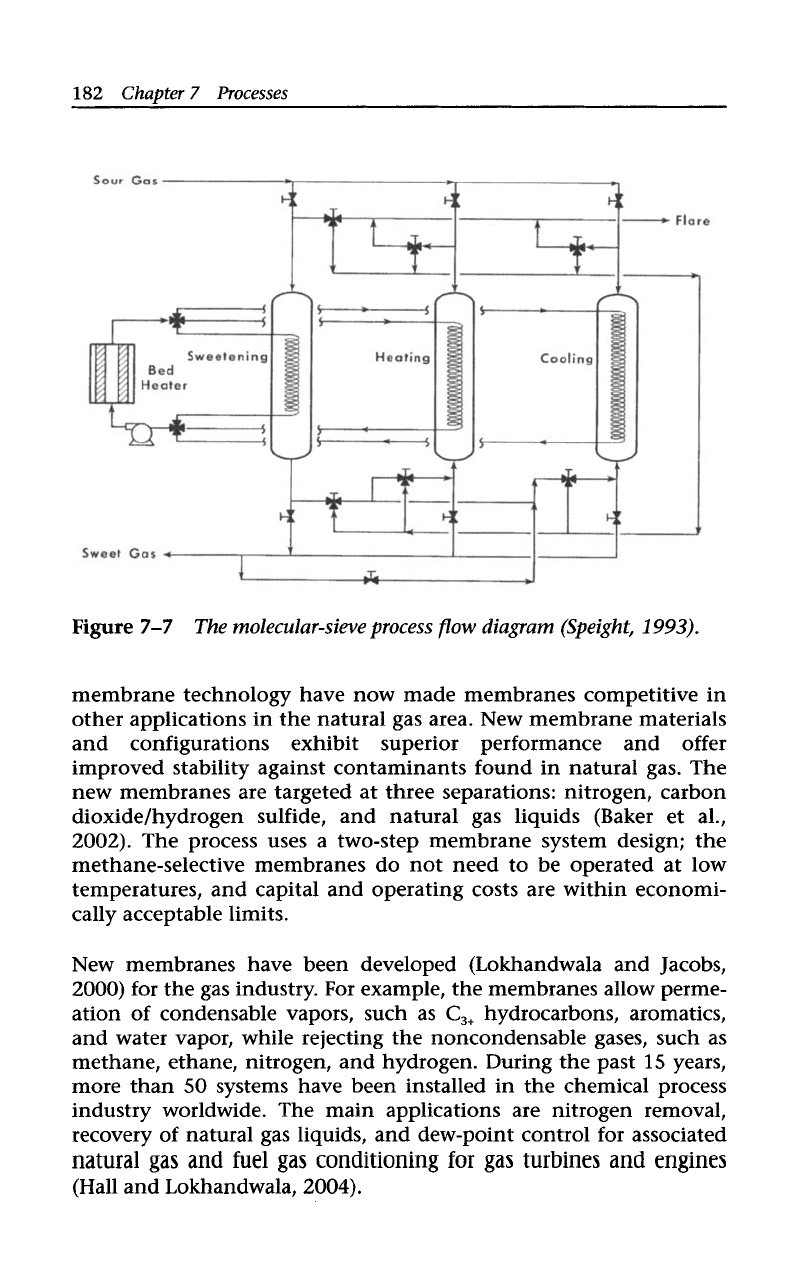
Molecular sieves are highly selective for the removal
of
hydrogen sul-
fide (as well as other sulfur compounds) from gas streams and over
continuously high absorption efficiency. They are also an effective
means
of
water removal, and thus offer a process for the simultaneous
dehydration and desulphurization of gas. Gas that has excessively
high water content may require upstream dehydration, however. The
molecular sieve process (Figure
7-7)
is similar
to
the iron-oxide pro-
cess. Regeneration
of
the bed is achieved by passing heated clean gas
over the bed.
As the temperature
of
the bed increases, it releases the adsorbed
hydrogen sulfide into the regeneration gas stream. The sour effluent
regeneration gas is sent to a flare stack, and up
to
2%
of
the gas seated
can be lost in the regeneration process.
A
portion
of
the natural gas
may also be lost by the adsorption
of
hydrocarbon components by
the sieve.
In
this process, unsaturated hydrocarbon components, such
as olefins and aromatics, tend
to
be strongly adsorbed by the molec-
ular sieves. The molecular sieves are susceptible to poisoning by such
chemicals as glycols and require thorough gas-cleaning methods
before the adsorption step. Alternatively, the sieve can
be
offered
some degree
of
protection by the use
of
guard
beds
in
which a less
expensive catalyst is placed
in
the gas stream before contact
of
the gas
with the sieve, thereby protecting the catalyst from poisoning. This
concept is analogous to the use of guard beds or attrition catalysts
in
the petroleum industry (Speight,
1993).
Until recently, the
use of
membranes
for gas separation
has
been
lim=
ited to carbon dioxide removal (Alderton,
1993).
Improvements in
7.5
Carbonate Washing and Water- Washing Processes
181
m
Purilied
Gos
-
ifcaclivakor
*
c_
Absorber
4
Cooling
Wotw
I
Sour
Gas
--+
-
"
Skrong
Solution
Weak
Solution
Figure
74
The Alkazid process
flow
diagram (Speight, 1993).
Acid
Go.
Rich
Solution
Figure
7-5
The hot-potassium-carbonate-process
flow
diagram (Speight,
1993).
Exchanger
Figure
7-6
The
Giammarco- Vetrocoke process
flow
diagram
(Speight,
1993).
182
ChaDter
7
Processes
Figure
7-7
The molecular-sieve process
flow
diagram (Speight,
1993).
membrane technology have now made membranes competitive in
other applications in the natural gas area. New membrane materials
and configurations exhibit superior performance and offer
improved stability against contaminants found
in
natural gas. The
new membranes are targeted at three separations: nitrogen, carbon
dioxide/hydrogen sulfide, and natural gas liquids (Baker et al.,
2002).
The process uses a two-step membrane system design; the
methane-selective membranes do not need to be operated at low
temperatures, and capital and operating costs are within economi-
cally acceptable limits.
New membranes have been developed (Lokhandwala and Jacobs,
2000)
for
the gas industry. For example, the membranes allow perme-
ation
of
condensable vapors, such as
C,,
hydrocarbons, aromatics,
and water vapor, while rejecting the noncondensable gases, such as
methane, ethane, nitrogen, and hydrogen. During the past
15
years,
more than
50
systems have been installed in the chemical process
industry worldwide. The main applications are nitrogen removal,
recovery of natural gas liquids, and dew-point control for associated
natural gas and fuel gas conditioning for gas turbines and engines
(Hall and Lokhandwala,
2004).

7.6
Sulfur
Recovery Processes
183
In
another process (Lokhandwala,
2000),
a membrane-based process
for upgrading natural gas that contains C,, hydrocarbons and/or acid
gas is described. The conditioned natural gas can be used as fuel
for
gas-powered equipment, including compressors, in the gas field or
the processing plant. Optionally, the process can be used
to
produce
natural gas liquids.
7.6 Sulfur
Recovery
Processes
The side stream from acid-gas treating units consists mainly of
hydrogen sulfide/or carbon dioxide. Carbon dioxide is usually vented
to the atmosphere but sometimes is recovered for carbon dioxide
floods. Hydrogen sulfide could be routed
to
an incinerator or flare,
which would convert the hydrogen sulfide to sulfur dioxide. The
release
of
hydrogen sulfide to the atmosphere may be limited by envi-
ronmental regulations. There are many specific restrictions
on
these
limits, and the allowable limits are revised periodically.
In
any case,
environmental regulations severely restrict the amount
of
hydrogen
sulfide that can be vented or flared in the regeneration cycle.
Most sulfur recovery processes use chemical reactions to oxidize
hydrogen sulfide and produce elemental sulfur. These processes are
generally based either
on
the reaction
of
hydrogen sulfide and
oxygen or hydrogen sulfide and sulfur dioxide. Both reactions yield
water and elemental sulfur. These processes are licensed and involve
specialized catalysts and/or solvents. These processes can be used
directly on the produced gas stream. Where large flow rates are
encountered, it is more common to contact the produced gas stream
with a chemical or physical solvent and use a direct conversion
process
on
the acid gas liberated in the regeneration step.
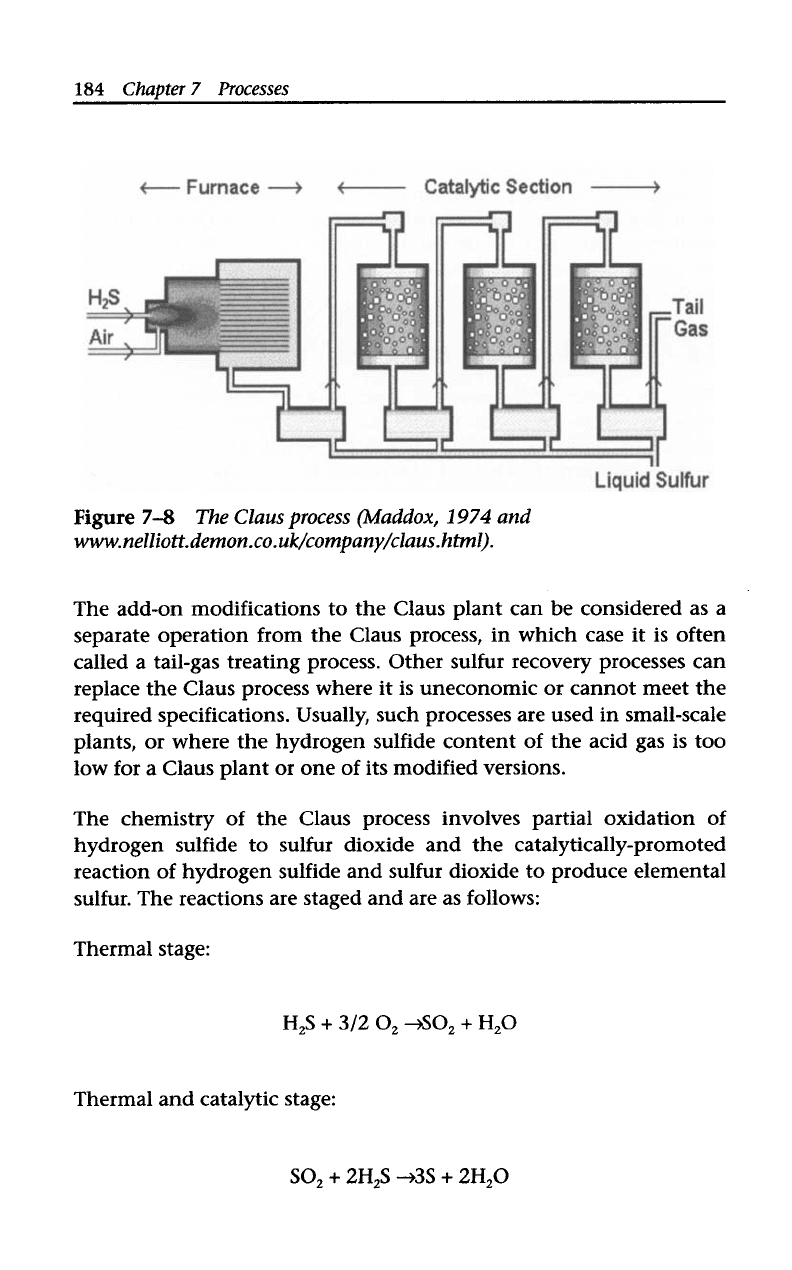
7.6.1
Claus
Process
Currently, the Claus sulfur recovery process (Figure
7-8)
is the most
widely used technology for recovering elemental sulfur from sour gas.
Most
of
the world’s sulfur is produced from the acid gases coming
from gas treating.
Conventional three-stage Claus plants can approach
98%
sulfur
recovery efficiency. However, because environmental regulations
have become stricter, sulfur recovery plants are required to recover
sulfur
with
over
99.8%
efficiency.
To
meet these stricter regulations,
the Claus process underwent various modifications and add-ons.
184
ChaDter
7
Processes
Figure
7-8
The
Claus
process
(Idaddox,
1974
and
www.nelliott.demon.co.uk/company/claus.html).
The add-on modifications
to
the Claus plant can be considered as a
separate operation from the Claus process, in which case
it
is often
called a tail-gas treating process. Other sulfur recovery processes can
replace the Claus process where it is uneconomic or cannot meet the
required specifications. Usually, such processes are used in small-scale
plants, or where the hydrogen sulfide content
of
the acid gas is too
low for a Claus plant
or
one
of
its modified versions.
The chemistry
of
the Claus process involves partial oxidation of
hydrogen sulfide to sulfur dioxide and the catalytically-promoted
reaction
of
hydrogen sulfide and sulfur dioxide to produce elemental
sulfur. The reactions are staged and are as follows:
Thermal stage:
H,S
+
3/2
0,+!30,
+
H,O
Thermal and catalytic stage:
SO,
+
2H,S
+3S
+
2H,O

7.6
Sulfur Recovery Processes
185
The first stage
of
the process converts hydrogen sulfide
to
sulfur
dioxide and to sulfur by burning the acid-gas stream with air
in
the
reaction furnace. This stage provides sulfur dioxide for the next cata-
lytic phase
of
the reaction. Multiple catalytic stages are provided to
achieve a more complete conversion
of
the hydrogen sulfide. Each
catalytic stage consists
of
a gas reheater, a reactor, and a condenser.
Condensers are provided after each stage to condense the sulfur vapor
and separate it from the main stream. Conversion efficiencies
of
94-95%
can be attained with two catalytic stages, while up to
97%
conversion can be attained with three catalytic stages. The effluent
gas is incinerated or sent to another treating unit for
tail-gas
treating
before it is exhausted to atmosphere.
The sulfur recovery depends upon such things as feed composition,
age
of
the catalyst and number
of
reactor stages. Typical sulfur
recovery efficiencies for Claus plants are
90%
to
96%
for a two-stage
plant and
95%
to
98%
for a three-stage plant. Because
of
equilibrium
limitations and other sulfur losses, overall sulfur recovery efficiency
in
a Claus unit usually does not exceed
98%.
The off-gas leaving a Claus plant is referred to as tail gas and in the
past was burned
to
convert the unreacted hydrogen sulfide
to
sulfur
dioxide, before discharge to the atmosphere, which has a much
higher toxic limit. However, the increasing standards
of
efficiency
required by the pressure from environmental protection has led
to
the development
of
many Claus tail-gas clean-up units, based
on
dif-
ferent concepts, to remove the last remaining sulfur species (Gall and
Gadelle,
2003).
The oxygen-blown Claus process was originally developed to increase
capacity at existing conventional Claus plants and to increase flame
temperatures
of
gases having low hydrogen sulfide content. The pro-
cess has also been used to provide the capacity and operating flexi-
bility for sulfur plants where the feed gas is variable
in
flow and
composition such as often found in refineries.
7.6.2
Redox
Process
Liquid redox sulfur recovery processes are liquid-phase oxidation pro-
cesses which use a dilute aqueous solution
of
iron or vanadium to
remove hydrogen sulfide selectively by chemical absorption from
sour gas streams. These processes
can
be used
on
relatively small
or
dilute hydrogen sulfide stream to recover sulfur from the acid gas

186
Chapter
7
Processes
stream or, in some cases, they can be used in place
of
an acid-gas
removal process. The mildly alkaline lean liquid scrubs the hydrogen
sulfide from the inlet gas stream, and the catalyst oxidizes the
hydrogen sulfide to elemental sulfur. The reduced catalyst is regener-
ated by contact with air
in
the oxidizer(s). Sulfur is removed from the
solution by flotation or settling, depending
on
the process.
7.6.3 Wet Oxidation Processes
The wet oxidation processes are based
on
reduction-oxidation
(Redox) chemistry
to
oxidize the hydrogen sulfide
to
elemental sulfur
in
an alkaline solution containing an oxygen carrier. Vanadium and
iron are the
two
oxygen carriers.
The best example
of
a process using the vanadium carrier is the Stret-
ford process. The most prominent examples
of
the processes using
iron as a carrier are the LO-CAT process and the SulFerox process.
The Stretford process using vanadium finds little use now because
of
the toxic nature
of
the vanadium solution, and iron-based processes
are more common.
Both
the LO-CAT process and the SulFerox process are essentially the
same
in
principle. The SulFerox process differs from the LO-CAT in
that the oxidation and the regeneration steps are carried out
in
sepa-
rate vessels, and sulfur is recovered from the filters, melted, and sent
to sulfur storage. Also, the SulFerox process uses a higher concentra-
tion
of
iron chelates (about
2
to
4%
by weight vs.
0.025
to
0.3%
by
weight for the LO-CAT process).
Both processes are capable
of
up
to
99+%
sulfur recovery. However,
using the processes for Claus tail-gas treating requires hydrolysis
of
all
the sulfur dioxide in the tail gas to hydrogen sulfide, because the
sulfur dioxide will react with the buffering base potassium hydroxide
(KOH)
and form potassium sulfate
(K2S04),
which will consume the
buffering solution and quickly saturate it.
7.6.4 Tail-Gas Treating Processes
Tail-gas treating involves the removal
of
the remaining sulfur com-
pounds from gases remaining after sulfur recovery. Tail gas from a
typical Claw process, whether a conventional
Clam
or
one
of
the
extended versions
of
the process, usually contains small but varying

7.6
Sulfur
Recovery
Processes
187
quantities
of
carbonyl sulfide, carbon disulfide, hydrogen sulfide,
sulfur dioxide, and sulfur vapor.
In
addition, there may be hydrogen,
carbon monoxide, and carbon dioxide
in
the tail gas.
To remove the rest
of
the sulfur compounds from the tail gas, all of
the sulfur bearing species must first be converted to hydrogen sulfide,
which is then absorbed into a solvent and the clean gas vented
or
recycled for further processing.
7.6.5
Hydrogenation and Hydrolysis Processes
The reduction
of
carbonyl sulfide, carbon disulfide, sulfur dioxide,
and sulfur vapor in Claus tail gas
to
hydrogen sulfide is necessary
when sulfur recovery
of
99.9+%
is required. Usually, the sulfur
recovery level is set by the allowable emissions
of
sulfur from the tail-
gas incinerator.
In
addition, the reduction
of
carbonyl sulfide is done
on raw synthesis gas, when the downstream acid-gas removal process
is unable to remove carbonyl sulfide to a sufficient extent to meet
sulfur emissions regulations from combustion
of
the cleaned fuel gas.
These sulfur compounds are reduced to hydrogen sulfide by hydroge-
nation or by hydrolysis, at a raised temperature, over a catalytic bed.
In
these processes, elemental sulfur and sulfur dioxide are reduced
mainly via hydrogenation, while carbonyl sulfide and carbon disul-
fide are mainly hydrolyzed
to
hydrogen sulfide. Sulfur and sulfured
dioxide are virtually completely converted to hydrogen sulfide when
an excess of hydrogen is present.
The hydrogen can be supplied from an outside source, can already be
in
the Claus tail gas, or can be obtained by partial oxidation of the
fuel gas
in
a furnace. The tail gas is preheated to the reactor tempera-
ture by an in-line burner that combusts fuel gas directly into the tail
gas. The same burner can also be used to supply the needed hydrogen
by partial combustion
of
the fuel gas.
When oxygen enrichment is used
in
the Claus plant, there is often
sufficient hydrogen in the tail gas
to
carry out the reduction without
an outside hydrogen source. There is usually sufficient water vapor
in
the Claus tail gas for the hydrolysis reactions.
The SCOT (Shell Claus Off-gas Treating) process was developed
in
the early
1970s
and
consists
of
a
combination
of
a
catalybc hydrogenation/hydrol-
ysis step and
an
amine scrubbing unit. The
hydrogenation/hydrolysis
of

188
Chapter
7
Processes
the
sulfur
compounds
in
the tail gases from the Claus unit has already
been covered.
The early SCOT units consisted
of
a
hydrogenation/hydrolysis
reactor
and a conventional amine unit (Figure
7-1).
The Claus tail gas, after
being reduced in the reactor, is cooled in a quench column and
scrubbed by a Sulfinol solution. The clean tail gas goes to a Claus
incinerator and the acid gas rich solution is regenerated
in
a stripping
column. The acid gas off the top
of
the stripper is recycled back to the
Claus plant for further conversion
of
the hydrogen sulfide. The
absorber is operated at near atmospheric pressure, and the amine sol-
vent is not highly loaded with acid gases. Because the solution is not
highly loaded, unlike high-pressure operation, there is
no
need for an
intermediate flash vessel, and the loaded solution goes directly
to
a
stripper.
Early SCOT units used diisopropanolamine in the Sulfinol (Sulfinol-
D)
solution. Methyl diethanolamine-based Sulfinol (Sulfinol-M) was
used later to enhance hydrogen sulfide removal and to allow for selec-
tive rejection
of
carbon dioxide in the absorber.
To achieve the lowest possible hydrogen sulfide content in the treated
gas, the Super-SCOT configuration was introduced.
In
this version, the
loaded Sulfinol-M solution is regenerated in
two
stages. The partially
stripped solvent goes to the middle
of
the absorber, while the fully
stripped solvent goes to the top
of
the absorber. The solvent going to
the top of the absorber is cooled below that used
in
the conventional
SCOT process.
The SCOT process can be configured
in
various ways. For example,
it
can be integrated with the upstream acid-gas removal unit, if the
same solvent is used in both units. Another configuration has been
used to cascade the upstream gas cleanup with the SCOT unit.
A
hydrogen sulfide-lean acid gas from the upstream gas treating unit is
sent to a SCOT process with
two
absorbers.
In
the first absorber, the
hydrogen sulfide-lean acid gas is enriched, while the second absorber
treats the Claus tail gas.
A
common stripper is used for both SCOT
absorbers and,
in
this latter configuration, different solvents could be
used in both the upstream and the SCOT units.
