Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Nitriding: modelling of hardness profiles and the kinetics 513
for the prediction of microhardness profiles with high accuracy within the
range of the data used for the models.
The influence of the nitriding processing parameters on the microhardness
profiles, calculated using both models, is explained from a metallurgical
point of view, showing good agreement with the fundamental diffusion theory
and the data from the literature.
The results obtained with the models are quite similar so it can be assumed
that they both may be used for simulations of microhardness profiles with
sufficient accuracy. The first model can potentially be used effectively for
optimisation of the processes of gas and plasma nitriding and it can predict
microhardness profiles of titanium alloys, nitrided at temperatures between
700 and 1100 °C for periods of time in the range of 1–100 hours. The second
model can be used for the same purpose but only for gas nitriding in the
same temperature and time ranges.
These models can be used by titanium users, saving them both experimental
time and cost. Similar models can be created for the simulation and prediction
of different materials’ properties and characteristics.
18.2 Kinetics of gas nitriding
In this section, the objective is to model the kinetics of formation and growth
of nitrided layers in commercial titanium alloys under different thermodynamic
conditions. The modelling can help in optimisation of the processing parameters
in order to achieve a desirable combination of microstructure and properties.
The gas nitriding of titanium and titanium alloys is a diffusion process.
Hence, the kinetics of formation and growth of the surface nitrided layer can
be modelled by appropriate application of the fundamental diffusion theory.
For this purpose, analytical and numerical solutions are introduced, based on
the physical model discussed below.
18.2.1 Phase transformations during the
process of nitriding
The formation of nitrided layers in titanium alloys is a complicated process
and involves several reactions taking place simultaneously at the boundary
between the gas atmosphere and the metal and within the substrate. The
kinetics of the diffusion process of nitriding have been studied in the past. A
simplified physical model for the formation and growth of nitrided layers
during gas nitriding in titanium is suggested and described by Malinov et al.
(2003). The model is based on reaction diffusion rules and is applicable for
nitriding temperatures below the β-transus. If the titanium material is in an
active nitrogen containing environment at high temperature, a nitrogen mass
transfer from the medium to the solid occurs. The nitrogen absorbed at the
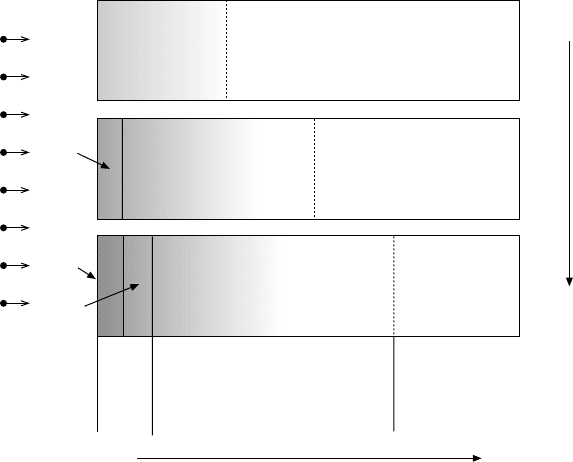
Titanium alloys: modelling of microstructure514
surface diffuses into the titanium, forming an interstitial solution of nitrogen
in the hcp α titanium phase (Fig. 18.11 top). The surface layer formed is
called the diffusion zone, α(N). This process can continue as long as the α
titanium matrix can dissolve nitrogen at the nitrogen medium–solid interface,
where the nitrogen concentration is the highest. If the concentration of nitrogen
at the interface becomes higher than the α phase is able to retain in interstitial
solution, a reaction at the interface occurs leading to the formation of a new
phase – Ti
2
N (Fig. 18.11 middle). There is a concentration jump of nitrogen
at the surface and, as a result, the total nitrided layer consists of a compound
layer (Ti
2
N) on the top and a diffusion zone underneath. Following the same
rules, when the concentration of nitrogen at the interface becomes higher
than the one acceptable in Ti
2
N, there is a phase transformation at the surface
and the Ti
2
N transforms to TiN (Fig. 18.11 bottom). The sub-layer with
titanium nitrides only (TiN and Ti
2
N) forms the compound layer, while α(N)
is the diffusion zone.
For real cases of nitriding of titanium alloys, the alloying elements present
can cause different deviations and modifications. The presence of alloying
elements in the general case might result in:
• simultaneous formation of two or more titanium nitrides;
N
2
Ti
2
N
TiN
Ti
2
N
Compound
layer
Diffusion zone
Increasing thickness
Reduced nitrogen concentration
Base
material
Time of nitriding
α(N)-Ti α-Ti
α-Ti
α-Ti
α(N)-Ti
α(N)-Ti
18.11
A schematic presentation of the kinetics of formation and
growth of surface layers during gas nitriding of titanium.
Nitriding: modelling of hardness profiles and the kinetics 515
• formation of complex stable or metastable nitrides of the alloying elements;
• change of the composition ranges of existence of the different phases.
Despite these possible influences, the alloying additions in the classical
titanium alloys are small in quantity and usually are dissolved in the hcp α-
titanium matrix by forming a substitutional solid solution. Hence, dramatic
changes to the kinetics of formation and the phase compositions of nitrided
layers in titanium alloys are not very likely. This has been confirmed by
experimental studies of the microstructure and the phase compositions of
nitrided layers in different titanium alloys (Lakshmi et al., 2002).
18.2.2 Physical model
In most of the cases, the main phases observed on the surface after nitriding
of different titanium alloys at different thermodynamic conditions are TiN
and Ti
2
N (Chapter 16) (Shashkov, 2001; Song et al., 2002; Spies et al.,
2001). Hence, the model suggested in Section 18.2.1 can be adopted with
sufficient accuracy to model the formation of nitrided layers in titanium
alloys. The development of the mathematical model based on this physical
model for solving the diffusion equation in appropriate conditions will allow
quantitative simulations of the kinetics of nitride layer formation under various
processing conditions. The mathematical models are discussed in the following
sections.
It should be admitted that the presence of TiO
2
is not taken into account
in the models. The oxygen may have significant influence on the kinetics of
formation and growth of nitrided layers and should be a subject for further
studies. At present, it is difficult to model simultaneous formation of titanium
nitrides and titanium oxides. The models will work with better accuracy
when there is no formation of oxides or the oxygen is a very small amount.
18.2.3 Diffusion coefficients
Precise data on the diffusion coefficients are necessary for accurate
mathematical modelling of any diffusion process. A literature survey on the
diffusion coefficients of nitrogen in α titanium, however, reveals some
discrepancies between diffusion coefficients given by different authors. Various
values of A and Q have been given, based on the general form of the equation,
Eq. [16.1].
Samsonova (1976) gave parameters for the diffusion coefficient for nitrogen
in α-Ti in the temperature range 900–1400 °C as follows: A = 1.2 × 10
–6
m
2
/sec and Q = 189.45 kJ/mole. Similar values were given by Fromm and
Gebhardt (1980) (A = 1.2 × 10
–6
m
2
/sec and Q = 178 kJ/mole) for the same
temperature interval and by Smithells et al. (1992) (A = 1.2 × 10
–6
m
2
/sec
Titanium alloys: modelling of microstructure516
and Q = 176.9 kJ/mole) for the temperature range 900–1570 °C. Different
data for the diffusion parameters were suggested by Metin and Inal (1989),
A = 9.6 × 10
–5
m
2
/sec and Q = 214.7 kJ/mole. The diffusivity of nitrogen in
α-Ti is calculated using Eq. [16.1] and the parameters reported in various
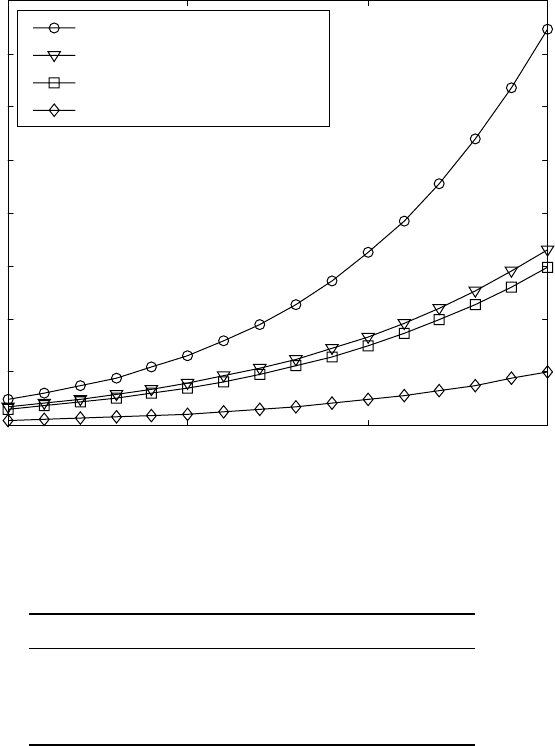
sources (Fig. 18.12).
The calculations are carried out for a temperature range of 850–1000 °C,
the usual temperature interval for nitriding of titanium alloys. Some of the
results are in agreement, but there are also significant differences between
the diffusion coefficients suggested by the various authors. For further
comparison, the diffusivity of nitrogen in α-Ti is calculated for a temperature
of 950 °C using the data from various authors and the results are compared
Metin and Inal (1989)
Smithells
et al
. (1992)
Fromm and Gebhardt (1980)
Samsonova (1976)
× 10
–13
1.6
1.4
1.2
1
0.8
0.6
0.4
0.2
0
Diffusion coefficiet (m
2
/sec)
850 900 950 1000
T
(°C)
18.12
Diffusion coefficients of nitrogen in α-Ti according to various
authors.
Table 18.1
Diffusion coefficients of nitrogen in α-Ti at
950 °C according to different authors
Source
D
, m
2
/s (×10
–14
)
Samsonova (1976) 0.973
Fromm and Gebhardt (1980) 2.999
Smithells
et al
. (1992) 3.341
Metin and Inal (1989) 6.496

Nitriding: modelling of hardness profiles and the kinetics 517
in Table 18.1.
There is up to six times difference between calculated diffusion coefficients
based on different sources. The modelling results will be different depending
on which one of the diffusion coefficients is used in the calculations. In the
models here, experimental data are compared with modelling results using
different diffusion coefficients.
18.2.4 Analytical solutions
Mathematical modelling of the diffusion processes in materials is carried out
by solving the fundamental differential equation of diffusion for the appropriate
initial and boundary conditions. If the diffusion is one-dimensional, i.e. there
is a gradient of concentration only along one axis (the case in nitriding of
titanium) and if the diffusion coefficient is constant, the general diffusion
equation can be simplified to:
∂
∂
∂
∂
C
t
D
C
x
=
2
2
[18.1]
where C is the concentration of the diffusing element (nitrogen), t is time and
x is the space coordinate.
Both analytical and numerical approaches are used, depending on the
nature of the problem. Analytical solutions are simpler but are applicable
only for ideal initial and boundary conditions. In spite of this, they can be
applied to model different phenomena in materials science. One significant
disadvantage is that analytical solutions are difficult to find for moving
boundary problems, and where there is a point of interruption. Here, analytical
solutions are used for the first and approximate calculation of the nitrogen
gradient in the diffusion zone and the layer thickness under different
thermodynamic conditions. In order to apply a simple analytical solution, it
is assumed that there is no compound layer formed. This assumption is quite
reasonable for approximate calculations, and has experimental justification.
Experimental results with nitrided layers in titanium show that the thickness
of the compound layer is within a range of 1–20 µm, while the diffusion
zone is in the range of hundreds of micrometers, depending on the temperature
and time of nitriding. Thus, the diffusion zone contributes to more than 95%
of the entire layer. Hence, if the concentration profile and the thickness of
the diffusion zone are modelled, the results can be used for estimation of the
layer thickness. The first simple solution can be obtained assuming simple
initial and boundary conditions from first order:
C(x, 0) = C
0
[18.2a]
C(0, t) = C
s
[18.2b]

Titanium alloys: modelling of microstructure518
The first condition, Eq. [18.2a], means that the initial concentration of nitrogen
in the alloy is C
0
. This value can be obtained from the alloy bulk composition
analysis. The second condition, Eq. [18.2b], implies that the concentration of
nitrogen at the surface accepts value C
s
from the very beginning of the
nitriding and is maintained at this constant value throughout the process. The
immediate jump of the concentration of nitrogen is not justified because, in
reality, the concentration of nitrogen on the surface gradually increases from
C
0
to C
s
during the early period of the process. However, the condition of
maintaining the concentration at the surface at constant value C
s
(after it has
been reached) is correct. It should be reiterated here that the above analytical
solution concerns only the diffusion zone. The concentration of nitrogen at
the top of the diffusion zone (at the interface between the compound layer
and the diffusion zone) is kept constant and is equal to the maximum solubility
of nitrogen in α-Ti. This value can be taken from the Ti–N phase diagram.
Solving Eq. [18.1] in initial and boundary conditions Eq. [18.2], one can
obtain:
C(x, t) = C
0
+ (C
s
– C
0
)(1 – erf(η)) [18.3a]
where
η =
2
x
Dt
[18.3b]
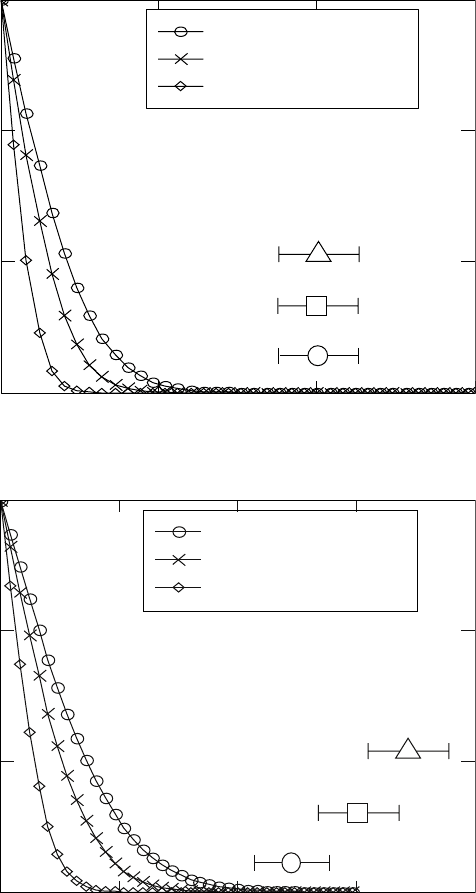
Using these equations, the nitrogen profile in the diffusion zone is calculated
after nitriding at 950 °C for 1, 3 and 5 hours. For C
s
, a value of 6 wt.% is
used, which is taken from the Ti–N phase diagram. Nitrogen concentration
profiles are calculated for diffusion coefficients suggested by different authors
(see Section 18.2.3) and the results are compared with experimental results
for the thickness of the nitrided layer after nitriding at the same temperature
and time (Fig. 18.13). The experimental values are obtained from microhardness
profiles.
The best correspondence between experimental and calculated values for
the layer thicknesses is after using the diffusion coefficient given by Metin
and Inal (1989). This coefficient is therefore used in the further simulations.
A more realistic analytical solution can be derived by applying boundary
conditions from third order. If the flux of nitrogen atoms across the boundary
interface (J
N
) is proportional to the difference between the equilibrium (C
s
)
and the current (C(0, t)) concentration of nitrogen at the boundary, the boundary
condition can be written as:
JD
Ct
x
CCt
Ns
= –
(0, )
= ( – (0, ))
∂
∂
α
[18.4]
where α is a coefficient in m/s.
This condition traces the realistic evolution of the nitrogen profile in the
Nitriding: modelling of hardness profiles and the kinetics 519
Nitrogen concentration (wt.%)
6
4
2
0
Metin and Inal (1989)
Smithells
et al
. (1992)
Samsonova (1976)
Experimental
data ranges
Ti-8-1-1
Ti-6-2-4-2
Ti-6-4
0 50 100 150
Depth (µm)
(a)
Nitrogen concentration (wt.%)
6
4
2
0
Metin and Inal (1989)
Smithells
et al
. (1992)
Samsonova (1976)
Experimental
data ranges
Ti-8-1-1
0 50 100 150 200
Depth (µm)
(b)
Ti-6-2-4-2
Ti-6-4
18.13
Calculated profiles for nitrogen concentration in the diffusion
layer using different diffusion coefficients compared with
experimental data, for layer thickness. Nitriding temperature 950 °C.
Nitriding time: (a) 1 h; (b) 3 h; (c) 5 h.
Titanium alloys: modelling of microstructure520
diffusion zone. The nitrogen concentration at the boundary increases gradually
from zero (or any other value) to the equilibrium concentration (C
s
), and is
kept at this value afterwards. These are the real conditions for the evolution
of the diffusion zone during nitriding of titanium alloys. One disadvantage of
this boundary condition is that the coefficient α is unknown. It depends on
many factors and varies significantly for different conditions. To obtain the
α coefficient, experimental data are necessary.
The analytical solution of Eq. [18.1] at boundary condition Eq. [18.4] is:
Cxt C
t
D
t
D
t
D
( , ) = erfc( ) – exp +
2
erfc +
s
2
η
α
αη
η
α
[18.5]
This solution is used to model the evolution of nitrogen in the diffusion zone
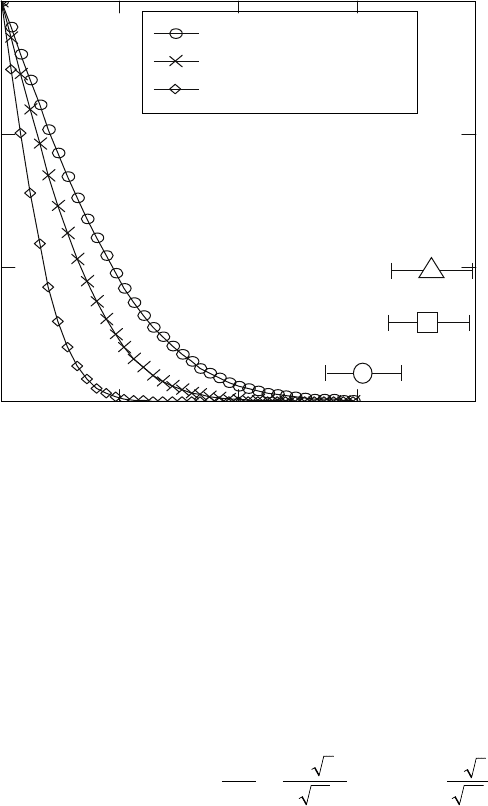
during nitriding of Ti-6Al-4V, Ti-6Al-2Sn-4Zr-2Mo and Ti-8Al-1Mo-1V alloys.
The value of α is obtained by fitting the modelling results to experimentally
observed nitrided layers. The best correspondence between modelling and
experimental results is obtained for α = 2.55 × 10
–8
m/sec. The simulation
results after nitriding at 900 and 950 °C for different periods of time are
shown in Figs. 18.14 and 18.15, respectively. The concentration of the nitrogen
at the surface gradually increases as the concentration gradient develops.
Nitrogen concentration (wt.%)
6
4
2
0
Metin and Inal (1989)
Smithells
et al
. (1992)
Samsonova (1976)
Experimental
data ranges
Ti-8-1-1
0 50 100 150 200
Depth (µm)
(c)
Ti-6-2-4-2
Ti-6-4
18.13
Continued
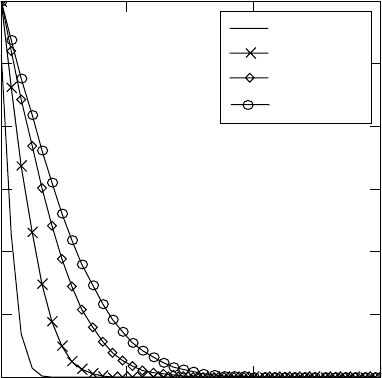
Nitriding: modelling of hardness profiles and the kinetics 521
The concentration of nitrogen at the surface reaches a value of 4.5 wt.%
after nitriding for 10 min at both temperatures, but is still not in equilibrium.
After nitriding for one hour, again at both temperatures, the nitrogen
concentration in the α phase at the surface already reaches the equilibrium
value of 6 wt.%. This means that titanium nitride should be formed already.
These simulation results are in agreement with the experimental observations
from the X-ray analysis. The diffraction patterns show the presence of titanium
nitride after nitriding at 950 °C for one hour. Good correspondence between
modelling and experimental results regarding the layer thickness evolution is
observed.
Finally, it should be stated once again that the analytical solutions described
above can be used to describe the evolution of the diffusion zone only. It is
possible to apply some analytical solutions to model the reaction diffusion
and formation of the entire (including compounds) layers in certain conditions.
However, it is preferable to use numerical simulation for modelling the
entire physical model.
18.2.5 Numerical simulations
In addition to the analytical solutions, a mathematical model and program
package for numerical simulation of the nitriding process in titanium alloys
have been developed. The mathematical model, based on the physical model
Nitrogen concentration (wt.%)
6
5
4
3
2
1
0
0 50 100 150
Depth (µm)
10 min
60 min
180 min
300 min
18.14
Calculated profiles for nitrogen concentration in the diffusion
layer for different times of nitriding at 900 °C.

Titanium alloys: modelling of microstructure522
Nitrogen concentration (wt.%)
6
5
4
3
2
1
0
0 50 100 150 200
Depth (µm)
10 min
60 min
180 min
300 min
18.15
Profiles for nitrogen concentration in the diffusion layer for
different times of nitriding at 950 °C, compared with experimental
micrographs, of Ti-6Al-2Sn-4Zr-2Mo nitrided at the same conditions.
180 min
60 min
300 min
