Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Surface gas nitriding: mechanical properties, morphology 483
17.3.3 Microhardness profiles
The microhardness is very high near the surface and falls gradually with the
distance from the surface. There is no significant difference in the microhardness
values for different initial roughness.
(g) Z scale = 2 µm,
R
a
= 0.036 µm
0 20.0 40.0 60.0
µm
60.0
40.0
20.0
0
(h) Z scale = 2 µm,
R
a
= 0.265 µm
0 20.0 40.0 60.0
µm
60.0
40.0
20.0
0
17.21
Continued
Titanium alloys: modelling of microstructure484
20
40
60
µm
(a)
20
40
60
µm
(b)
20
40
60
µm
(c)
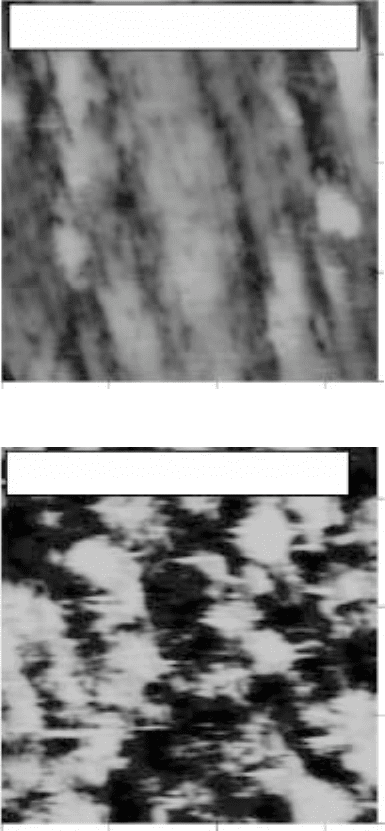
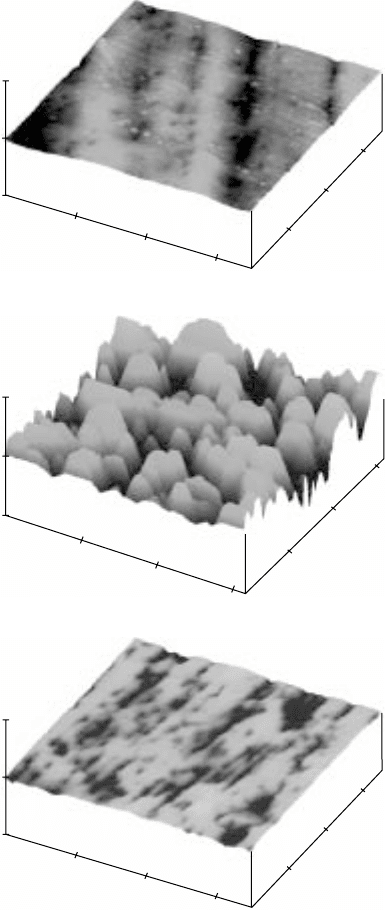
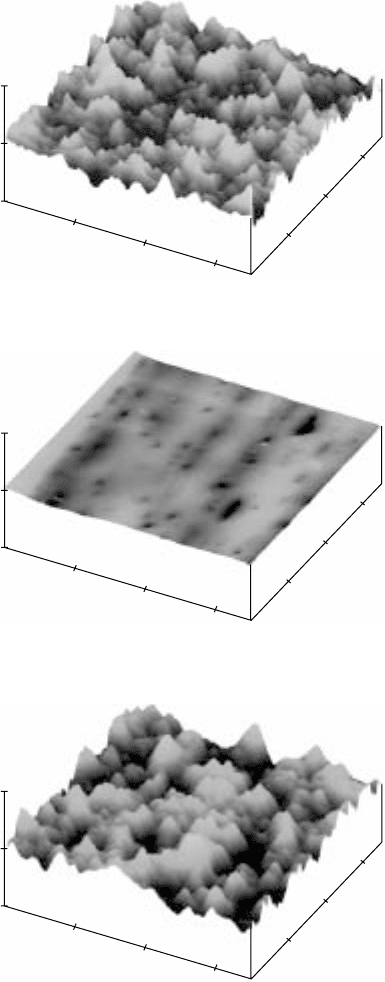
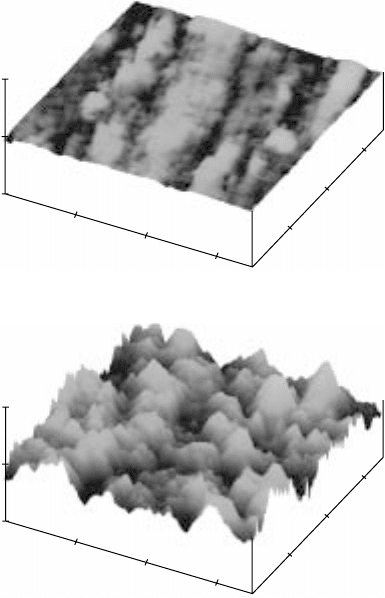
17.22
3D images of the surface morphology of polished Ti-6Al-2Sn-
4Zr-2Mo (a, c, e and g) before and (b, d, f and h) after gas nitriding at
different temperatures and for different periods of time. (a) and (b)
950 °C, 1 hour; (c) and (d) 950 °C, 3 hours; (e) and (f) 950 °C, 5 hours;
(g) and (h) 1050 °C, 5 hours. The
Z
scale for (b) is 4 µm/division and
for all the rest is 2 µm/division.
Surface gas nitriding: mechanical properties, morphology 485
20
40
60
µm
(d)
20
40
60
µm
(e)
20
40
60
µm
(f)
17.22
Continued
Titanium alloys: modelling of microstructure486
The thickness of the nitrided layer can be estimated from the microhardness
profiles (see Section 17.1). As was discussed in that section, it can be supposed
that the nitrided layer ends where the microhardness value approaches the
core microhardness. For Ti-6Al-2Sn-4Zr-2Mo, gas nitrided at 950 °C for
5 hours, nitrided layers of about 200 µm have been obtained.
17.3.4 Summary
Gas nitriding significantly increases the surface roughness of Ti-6Al-2Sn-
4Zr-2Mo titanium alloy, up to the order of 25 times, except for the substrate
with high initial roughness already. The surface roughness after nitriding
depends on the initial surface roughness of the material. It increases with
increase of the initial roughness. The surface roughness increases with increase
of the nitriding time, but there is no significant change of the surface roughness
with increase of the temperature from 950 to 1050 °C. The increase of the
20
40
60
µm
(h)
17.22
Continued
20
40
60
µm
(g)
Surface gas nitriding: mechanical properties, morphology 487
surface roughness after gas nitriding is caused by the formation of titanium
nitride and titanium oxide on the surface of the materials. There is no significant
influence of the different initial surface roughness on the microhardness and
the thickness of the nitrided layer after nitriding.
17.4 Corrosion behaviour
One of the biggest advantages of titanium alloys is their good corrosion
resistance. Much research and testing have been carried out to assess the
corrosive behaviour of titanium alloys in different environments before and
after different types of thermo-chemical surface treatments, which can either
improve or worsen their corrosion resistance properties. The data in the
literature is quite diverse and inconsistent in this area. This section will
quantify the effect of the corrosive media and test temperature on the corrosion
behaviour of Ti-6Al-2Sn-4Zr-2Mo and Ti-8Al-1Mo-1V before and after the
surface thermo-chemical treatment of gas nitriding.
⇐
•
{100}
←
{101}
•
/←
• α-Ti(N) ⇓ TiN ↓ TiO
2
1050/5
950/5
950/3
950/1
•
/←
←
←
•
←
⇐
←
•
←
•
⇐
←
•
⇐
•
/←
•
/←
←
←
•
←
⇐
←
•
←
⇐
←
•
•
/←
←
←
←
•
•
•
←
⇐
←
←
←
•
⇐
{111}
•
{002}
←
{200}
•
{101}
←
{111}
⇐
{200}
←
{210}
←
{211}
•
{102}
←
{220}
⇐
{220}
•
{110}/
←
{002}
←
{310}
←
{301}
•
{103}/
←
{112}
⇐
{311}
40 50 60 70
2θ (°)
Intensity (c.p.s.)
400
100
0
900
400
100
0
900
400
100
0
1600
400
0
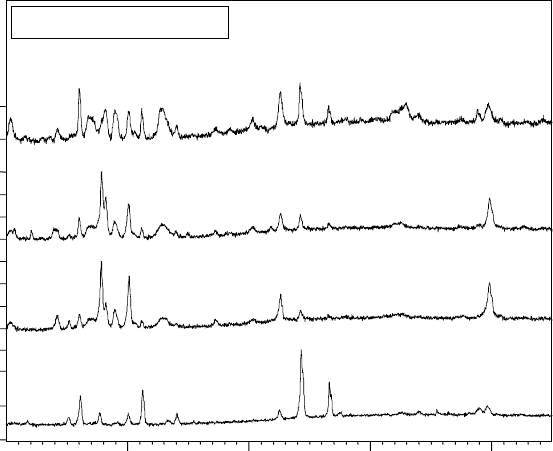
17.23
X-ray diffraction patterns of Ti-6Al-2Sn-4Zr-2Mo after gas
nitriding at 950 °C for 1, 3 and 5 hours and at 1050 °C for 5 hours.
Titanium alloys: modelling of microstructure488
17.4.1 Basic principles of titanium alloys corrosion
General corrosion of titanium alloys
Titanium, like any other metal, is subject to corrosion in certain environments.
The types of corrosion that have been observed on titanium may be classified
under the following general headings: general corrosion, crevice corrosion,
stress corrosion cracking, anodic breakdown pitting, and galvanic corrosion.
This section (Section 17.4) is aimed specifically at the general corrosion
resistance of titanium alloys before and after surface gas nitriding. General
corrosion is characterised by a uniform attack over the entire exposed surface
of the metal. The severity of this type of attack can be expressed by the
corrosion rate. General corrosion in aqueous media may take the form of
mottled or severely roughened metal surfaces. When titanium is fully passive,
corrosion rates are typically around 0.04 mm/yr, much lower than 0.13 mm/yr
maximum rate accepted by engineers. This very low corrosion rate is attributed
to a titanium oxide film on the surface. The exact process will be explained
next in this section. As a result, titanium is often designed with a zero
corrosion allowance in passive environments. However, in some environments,
(c)
100 µm
(d)
500 µm
(b)
100 µm
(a)
100 µm
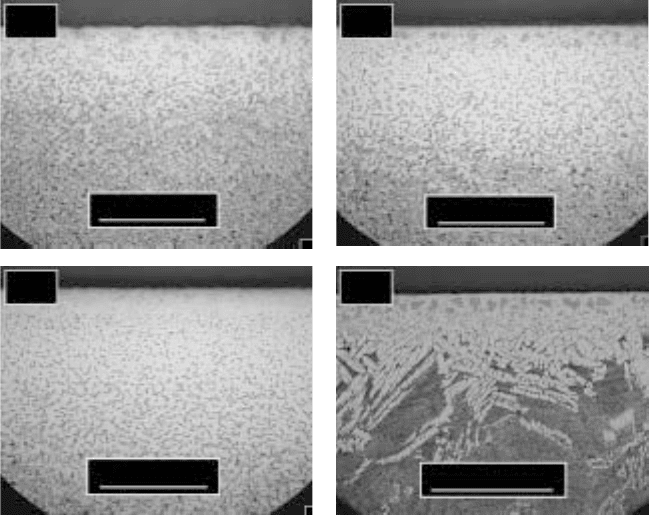
17.24
Microstructure of Ti-6Al-2Sn-4Zr-2Mo. (a) 950 °C, 1 hour;
(b) 950 °C, 3 hours; (c) 950 °C, 5 hours; (d) 1050 °C, 5 hours.
Surface gas nitriding: mechanical properties, morphology 489
titanium may experience an oxide growth, characterised by a coloured surface
and slight weight gain.
General corrosion really becomes a concern in reducing acid environments,
especially if the acidity and temperatures begin to rise. In strong or hot
reducing acids, the oxide film on the surface will deteriorate and break
down, so leaving the metal surface susceptible to corrosion.
Mechanism of corrosion resistance
The excellent corrosion resistance of titanium alloys is due to the formation
of a very stable, continuous, highly adherent, and protective oxide film on
the metal surfaces. Because titanium metal itself is highly reactive and has
an extremely high affinity for oxygen, these beneficial surface oxide films
form spontaneously and instantly, when fresh metal surfaces are exposed to
air, or moisture. In fact, a damaged oxide film can generally heal itself
instantaneously if at least traces (that is, parts per million) of oxygen or
water (moisture) are present in the environment.
The composition of this film varies from TiO
2
at the surface to Ti
2
O
3
and
TiO at the metal interface. Oxidising conditions promote the formation of
TiO
2
, so that in such environments the film is primarily TiO
2
. This film is
transparent in its normal thin configuration, and not detectable by visual
means. A study of the corrosion resistance of titanium is basically a study of
the properties of the oxide film, which is attacked only by certain substances,
under, for example, anhydrous conditions in the absence of a source of
oxygen.
Therefore, the successful non-corrosive properties of titanium and its
alloys can be expected in mildly reducing to highly oxidising environments,
in which protective oxide films spontaneously form and remain stable. Titanium
exhibits excellent resistance to atmospheric corrosion in both marine and
industrial environments.
The following is some background information on the effects of some
corrosive media.
Salt solutions
Titanium alloys are very resistant to almost all salt solutions over the pH
range of 3 to 11, and to temperatures that exceed their boiling point. Titanium
can withstand exposure to solutions of chlorides, bromides, iodides, sulfites,
sulfates, borates, phosphates, cyanides, carbonates, bicarbonates, and
ammonium compounds. The corrosion rate values for titanium alloys in this
variety of salt solutions are generally less than 0.03 mm/yr. Titanium alloys
are frequently used in many process streams, brines, and seawater, because
of their good resistance to the chlorides typically found in them.
Titanium alloys: modelling of microstructure490
Reducing acids
This category includes hydrochloric, sulfuric, hydrobromic, hydriodic,
hydrofluoric, phosphoric, sulfamic, oxalic, and trichloroacetic acids. The
corrosion resistance of titanium alloys in reducing acid solutions is very
sensitive to the acid concentration, solution temperature and purity of the
acid solution, as well as to the alloy composition. With increase of the
temperature, or the concentration of the reducing acid solution, the protective
oxide film on the surface of the material may break down, which would
result in severe general corrosion. Various oxidising species can effectively
inhibit the corrosion of titanium in reducing acid environments, when present
in small concentrations. These inhibiting species often occur as natural process
stream constituents or contaminants, and do not need to be intentionally
introduced to achieve passivation.
Enhancing the corrosion resistance of titanium alloys
Basically, the only methods of increasing the corrosion resistance of titanium
in reducing environments are:
(i) increasing the surface oxide film thickness by anodising or thermal
oxidation
(ii) anodically polarising the alloy with a more noble metal, in order to
maintain the surface oxide film
(iii) applying precious metal surface coatings
(iv) alloying titanium with certain elements
(v) adding oxidising species to the reducing environment to permit oxide
film stabilisation.
Of these five methods, the last two are the most practical, and are widely
used in service.
Thermo-chemical nitriding of titanium alloys may also improve their
corrosion resistance. The problem after these types of treatments is that if the
nitrided surface has become pitted, the corrosion is higher for the nitrided
material, because of the potential difference between core and case. Many
researchers have studied the corrosion behaviour of titanium alloys before
and after nitriding (Gokul Lakshmi et al., 2002; Meletis, 2002; Takahashi
and Kimura, 2001).
Considering phase transformations taking place during the thermo-chemical
treatment of gas nitriding, one can expect that these newly-formed surface
layers would affect the corrosion properties.
Surface gas nitriding: mechanical properties, morphology 491
17.4.2 Influence of the alloy composition and
surface gas nitriding
The two alloys discussed in this section are the near-α titanium alloys Ti-
8Al-1Mo-1V and Ti-6Al-2Sn-4Zr-2Mo. Normally, the additions of
molybdenum and vanadium improve the corrosion resistance of titanium
alloys, and aluminium content worsens it. These two alloys have different
alloy composition, and they would have different corrosion behaviour in
various media.
0.5
M NaCl
In 40 °C solution, Ti-8Al-1Mo-1V shows better corrosion resistance in
comparison with Ti-6Al-2Sn-4Zr-2Mo. At the higher medium temperature
of 80 °C, the relative corrosion resistance between the two alloys is the
opposite, and the weight loss increases under each condition except for Ti-
6Al-2Sn-4Zr-2Mo nitrided at 950 °C for 5 hours. Gas nitriding worsens the
corrosion resistance of Ti-6Al-2Sn-4Zr-2Mo at both medium temperatures,
but improves the corrosion resistance of Ti-8Al-1Mo-1V.
Despite the above differences, the corrosion resistance of these alloys in
0.5
M NaCl at both solution temperatures may be concluded as excellent,
with or without the nitriding, considering that the average weight loss values
never reach 0.0005 g/cm
2
after 1500 hours of corrosion test. The level of
weight loss in this salt solution is insignificant in comparison to the weight
loss in acidic media. These results are partly in contradiction to the results
given by Fleszar et al. (2000), where it was reported that plasma nitriding
significantly increased the corrosion resistance of Ti-6Al-3Mo-2Cr.
4.9
M HCl
The weight loss increases for all conditions with the time prolongation and
the increase of the temperature, except that there is no significant influence
of the temperature on the corrosion behaviour of the untreated Ti-8Al-1Mo-
1V alloy (Fig. 17.25).
The nitrided Ti-8Al-1Mo-1V shows, in general, better performance than
the nitrided Ti-6Al-2Sn-4Zr-2Mo. The weight loss values of the untreated
condition are similar for the two alloys at 20 and 40 °C. At 80 °C, Ti-8Al-
1Mo-1V shows a better performance in 4.9
M HCl in comparison to Ti-6Al-
2Sn-4Zr-2Mo, probably due to the advantageous combination of the alloying
elements in the former. These data are in contradiction with the results
obtained for Ti-6Al-4V by Galvanetto et al. (2002).
Gas nitriding worsens the corrosion resistance of these alloys and especially
of Ti-6Al-2Sn-4Zr-2Mo. The reducing acid solution is very aggressive and
Titanium alloys: modelling of microstructure492
Weight loss (g/cm
2
)
0.012
0.01
0.008
0.006
0.004
0.002
0
0 1000 2000 3000 4000
Time (h)
(a)
Ti-6242, nitrided
Ti-6242, untreated
Ti-811, nitrided
Ti-811, untreated
Weight loss (g/cm
2
)
0.012
0.01
0.008
0.006
0.004
0.002
0
0 500 1000 1500 2000 2500 3000
Time (h)
(b)
Ti-6242, nitrided
Ti-6242, untreated
Ti-811, nitrided
Ti-811, untreated
Weight loss (g/cm
2
)
0.02
0.016
0.012
0.008
0.004
0
0 500 1000 1500
Time (h)
(c)
Ti-6242, nitrided
Ti-6242, untreated
Ti-811, nitrided
Ti-811, untreated
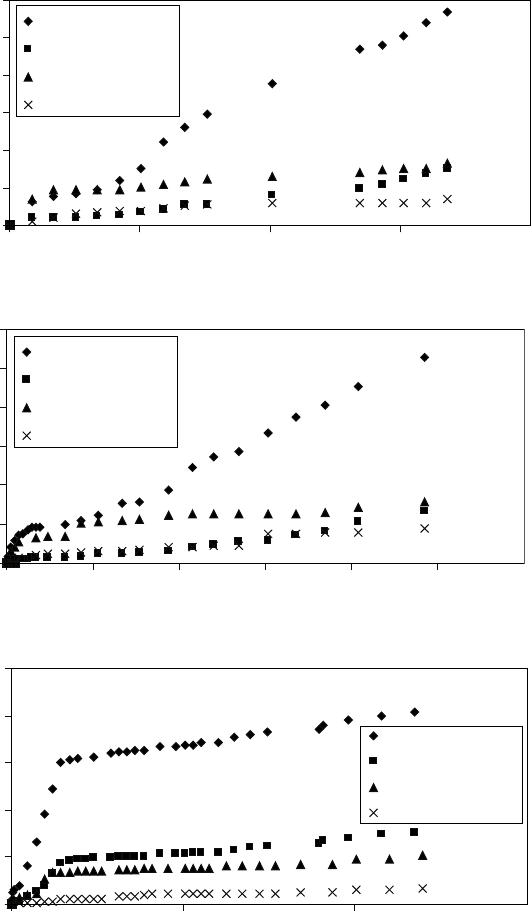
17.25
Weight loss versus time of Ti-6Al-2Sn-4Zr-2Mo and Ti-8Al-1Mo-
1V, untreated and nitrided (950 °C for 5 hours), after holding them in
4.9
M HCl at (a) room temperature, (b) 40 and (c) 80 °C.
