Seetharaman S. Fundamentals of metallurgy
Подождите немного. Документ загружается.

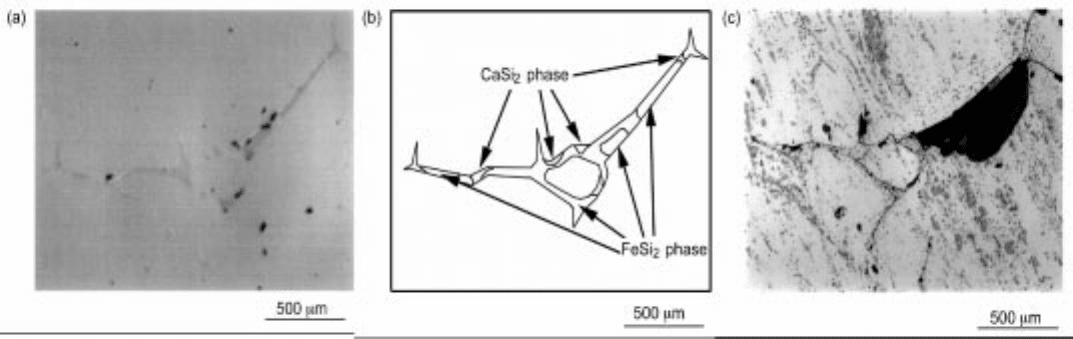
3.29 (a) Optical image of Si-Ca-Fe alloy (Si-0.929%Ca-1
.21%Fe, before acid leaching). (b) Microstructure of Si-Ca-Fe all
oy. (c) Optical
image of Si-Ca-Fe alloy (Si-0.929%Ca-1.21%Fe, 5 min. in aqu
a regia).
of the ternary eutectic of Si-CaSi
2
-FeSi
2
which is easily soluble in aqua regia.
On the other hand, when the ratio of calcium to iron is lower, FeSi
2
precipitates
as a secondary phase and most of the FeSi
2
precipitates remained after acid
leaching procedure. Figure 3.29(c) shows the optical image after acid leaching,
where a large piece of undissolved FeSi
2
was observed on the grain boundary.
These phenomena can be confirmed by following the solidification behaviour
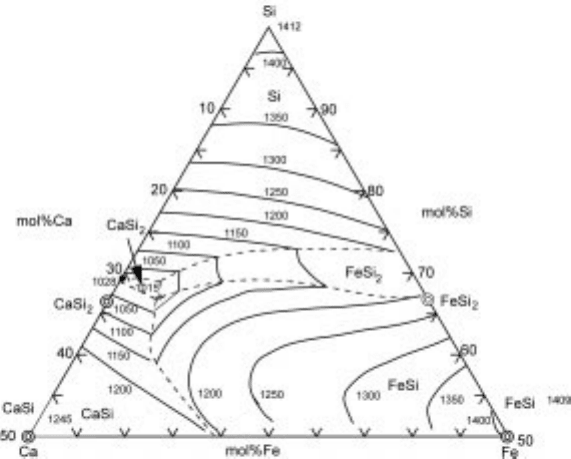
from phase diagram viewpoint. Since reliable data for the Si-Ca-Fe system were
not available, the ternary phase diagram was drawn as shown in Fig. 3.30 using
`Thermo-Calc', a thermodynamic database and software. From the diagram, the
secondary phase should be CaSi
2
when X
Ca
/X
Fe
> 6.5 and FeSi
2
when X
Ca
/X
Fe
<
6.5. This coincides with the experimental results that the optimum composition
for iron removal is X
Ca
/X
Fe
6±9 as shown in Fig. 3.27. Accordingly,
controlling the alloy composition to ensure the precipitation of CaSi
2
secondary
phase during cooling becomes the key for the removal of iron from silicon by
acid leaching procedure. As can be seen in the present example, comprehension
of phase diagram in view of solidification behaviour is helpful in developing a
new technology in materials science.
3.6 Conclusions
In this chapter, fundamentals of chemical potential diagrams and phase diagrams
were briefly reviewed and solidificat ion behaviour was discussed through phase
3.30 Phase diagram for the Ca-Si-Fe system.
Phase diagrams and phase transformations 107
diagram perspective by showing simplified examples together with recent
experimental work. Both diagrams are helpful in predicting a final product and
in verifying the processing conditions.
We often treat the systems in non-equilibrium states, such as supercooling,
appearance of metastable phases, non-uniformity due to slow diffusion or slow
reaction processes, etc. Such conditions are preferable or artificially created in
producing various new materials, such as low T
C
superconductors, semi-
conductor compounds, new glasses and ceramics, etc. They are developed with
profound consideration on relations among the phases whether they are stable or
metastable. Henceforth, fundamental studies on phase diagrams should be
continued for the future development of materials science and engi neering.
3.7 References
1. Phase Diagram of Binary Iron Alloys, H. Okamoto (ed.) (1993), ASM International,
Materials Park, OH.
2. Physical Chemistry of High Temperature Technology, E.T. Turkdogan (1980),
Academic Press, New York, NY.
3. Phase Diagram for Ceramists, vol. I, E.M. Levin, C.R. Robbins and H.F. McMurdie
(eds) (1964), American Ceramic Society, Westerville, OH.
4. Phase Diagrams in Metallurgy , F.N. Rhines (1956), McGraw-Hill Book Co.,
Columbus, OH.
5. Binary Alloy Phase Diagrams, T.B. Massalski and H. Okamoto (eds) (1990), ASM
International, Materials Park, OH.
6. T. Yoshikawa and K. Morita (2003), J. Electrochem. Soc., 150, G465.
7. R.C. Miller and A. Savage (1956), J. Appl. Phys., 27, 1430.
8. D. Navon and V. Chernyshov (1957), J. Appl. Phys., 28, 823.
9. V.N. Lozvskii and A.I. Udyanskaya (1968), Izv. Akad. Nauk SSSR, Neorg. Mater.,
4, 1174.
10. T.L. Chu and S.S. Chu (1983), J. Electrochem. Soc., 130, 455.
11. R.H. Hopkins and J. Rothatgi (1986), J. Cryst. Growth, 73, 67.
12. T. Sakata, T. Miki and K. Morita (2002), J. Japan Inst. Metals, 66, 459.
108 Fundamentals of metallurgy

4.1 Introduction
4.1.1 The need for thermo-physical property data
Surveys of the requirements of industry show that there is an urgent need for
reliable data for the thermo-physical properties of the materials involved in
high-temperature processes (metals, slags and refractories). This need arises
from the fact that thermo-physical property data have proved extremely useful in
improving both process control and product quality. Physical property data are
beneficial in two ways:
1. In the direct solution of industrial problems.
2. As input data in the mathematical modelling of processes.
One example of the direct use of physical property data is in the case of
`Variable weld penetration' in gas tungsten arc (GTA sometimes known as TIG)
welding of steels. Some applications require a large number of welds (e.g. heat
exchangers). In these cases the welding conditions providing good weld
penetration are established in preliminary trials. However, sometimes another
batch of the materials (fully matching the materials specification) had to be used
and the resulting welds were very shallow and consequently, w eak.
Compositional differences between the two batches were very small. Subsequent
work
1±3
showed that:
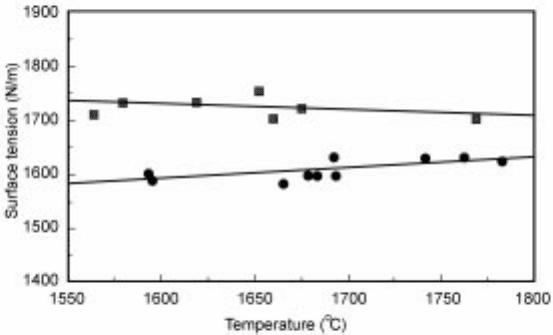
(i) good weld penetration was associated with lower surface tensions () and a
positive temperature depend ence (d/dT) and a sulphur content of >50 ppm
of the steel; and
(ii) shallow weld penetration was associated with a high surface tension, a
negative (d/dT) and a sulphur content of <30 ppm (Fig. 4.1).
The effect of sulphur on the surface tension () and its temperature dependence
(d/dT) of steel is shown in Fig. 4.2.
4
The heat transfer in the weld pool is
determined by the fluid flow and there are several forces affecting the fluid flow,
4
Measurement and estimation of physical
properties of metals at high temperatures
K C M I L L S , Imperial College, London, UK
namely, buoyancy, Lorenz, aerodynamic drag and thermo-capillary (Marangoni)
forces.
1±3
However, the Marangoni forces are dominan t becau se of the huge
temperature gradients across the surface of the weld pool. In steels with
S < 30 ppm (d/dT) is negative (Fig. 4.2) and thermo-capillary flow (high to
low ) is radially-outward taking hot liquid to the periphery of the weld where
melt-back produces a shallow weld (Fig. 4.3). For steels containing more than
50 ppm S the thermo-capillary forces are inward and the hot liquid is forced
down the weld and melt-back occurs in the bottom of the pool giving a deep
weld. Surface tension measurements played an important part in solving this
problem.
Mathematical modelling has proved a valuable tool in improving process
control and product quality. There are several types of mathematical models,
e.g. those based on thermodynam ics, kinetics and heat and fluid flow of the
process. In this review we are mainly concerned in the modelling of the heat and
fluid flow in the process.
Defects in a casting can result in the scrapping of a casting. The cost of
scrapping has been estimated to be greater than 2 billion US$ per annum.
Mathematical models of the heat and fluid flow have been developed to predict
the locations of defects. It has been shown that the accurate prediction of defects
requires accurate thermo-physical data for the alloy being cast.
5
Similar models
have been developed for the prediction of micro-structure (e.g. dendrite arm
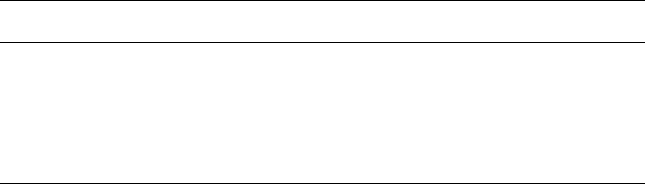
spacing) and Fig. 4.4 shows the sensitivity of local solidification time to changes
in the various properties
6
of aluminium alloys. It can be seen that it is
particularly sensitive to the thermal conductivity value used.
The properties needed for modelling fluid flow and heat and mass transfer are
as follows.
4.1 Surface tension±temperature relations for two stainless steels exhibiting
good penetration (lower curve, S content > 50 ppm) and poor penetration
(upper curve, S content < 30 ppm).
110 Fundamentals of metallurgy
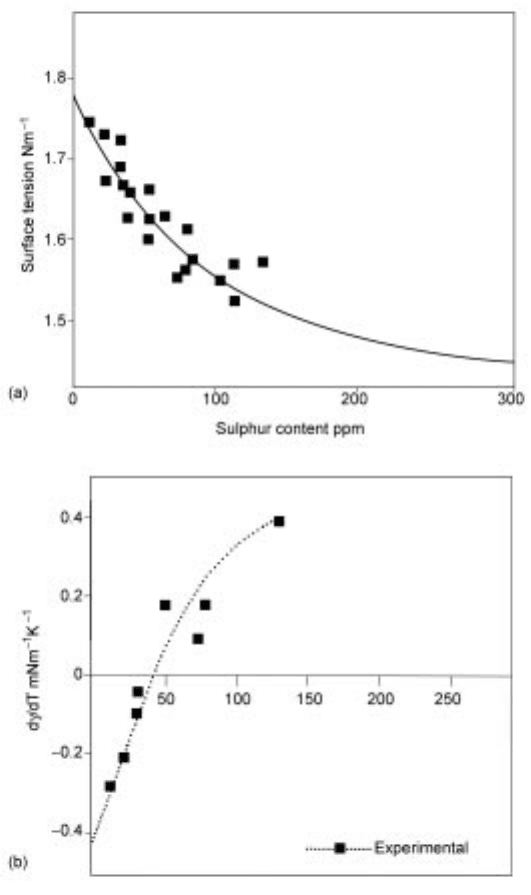
4.2 The effect of sulphur content on (a) surface tension () at 1923K and (b)
its temperature dependence (d/dT).
4
Measurement and estimation of physical properties of metals 111
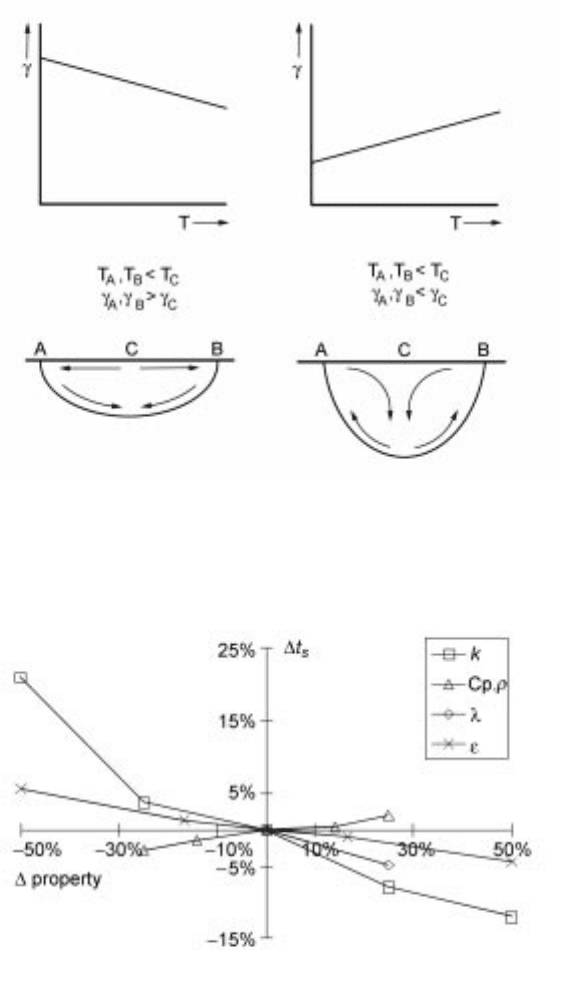
4.3 Schematic diagram illustrating the mechanism for variable weld
penetration showing fluid flow in the weld pool for a steel with S < 30 ppm on
the left and for a steel with S content >50 ppm on the right.
1,2,3
4.4 Sensitivity of loc al solidification time to thermal conductivity, (k) the
parameter (density heat capacity, (Cp. )) latent heat (denoted )
6
and
emissivity ().
112 Fundamentals of metallurgy
Heat flow Fluid flow Mass transfer
Heat capacity, enthalpy Density Diffusion coefficient
Thermal conductivity/diffusivity Viscosity
(Electrical conductivity) Surface/Interfacial
Emissivity tension
Optical properties (slags and glasses)
These data are needed for alloys, slags, glasses and fluxes. The measurements
are time-consuming and require considerable expertise. Consequently, it is an
enormous task to provide all the therm o-physical data for all materials involved
in the various industrial processes. Thus, considerable effort has been devoted to
the est imation of physical properties. Usually these estimations are based on the
chemical composition since this is usually available on a routine basis.
4.2 Factors affecting physical properties and their
measurement
4.2.1 Structure
Some physical properties are very dependent upon the structure of the sample.
The effect of structure is greatest in the case of viscosity and, in fact, visc osity
measurements have been used in conjunction wi th other data to determine the
structure of melts. The effect of structure on property values is in the following
hierarchy: viscosity > thermal conductivity > electrical conductivity > density
(small) > enthalpy (usually has little effect). Structural effects tend to be much
larger for slags and glasses than for metals.
Both X-ray and neutron diffraction have proved very useful in determining
the structure of solids. In crystalline solids the atoms have well-defined
positions. The array of atoms interferes with the passage of X-rays which are
scattered in all directions except those predicted from Braggs law:
2d sin (4.1)
where is the wavelength, d is the distance between two layers of crystals and
is the angle of diffraction. X-ray diffraction patterns for a gas show a constant
scattering intensity with no maxima due to the random distribution of atoms.
However, X-ray diffraction patterns for liquids exhibit a few max ima and minima
(Fig. 4.5) indicating that atoms are randomly distributed in an approximately
close-packed array (short range order) but have little long range order due to the
thermal excitation and motion.
7
The principal parameters used to describe
structure are derived from X-ray and neutron diffraction data, namely:
· Pair distribution factor (g(r)) where r is the radius and g(r) is the probability
of finding another atom at a certain position (Fig. 4.6a); in real liquids g(r) is
Measurement and estimation of physical properties of metals 113
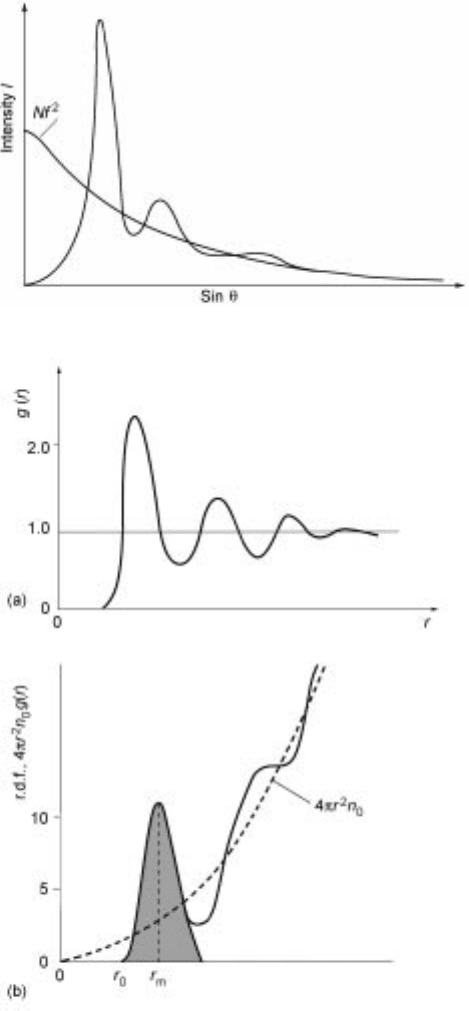
4.5 X-ray diffraction pattern for a liquid.
7
4.6 (a) Pair distribution function (g(r)) of a metal near its melting point and (b)
radial distribution function (rdf): the number of nearest neighbours can be
determined from the area of the hatched region.
114 Fundamentals of metallurgy
affected by attraction and repulsion forces (of other atoms).
·
Radial distribution factor (rdf) can be calculated from g(r); the hatched area
in Fig. 4.6b is a measure of the number of nearest neighbours (i.e.
coordination number).
Other parameters used to describe the str ucture are summarised by Iida and
Guthrie.
7
The situation is much mor e complicated in alloys since for a binary
alloy three distribution factors are needed, namely, (g(r))
11
, (g(r))
22
and (g(r))
12
.
Structure of slags and glasses
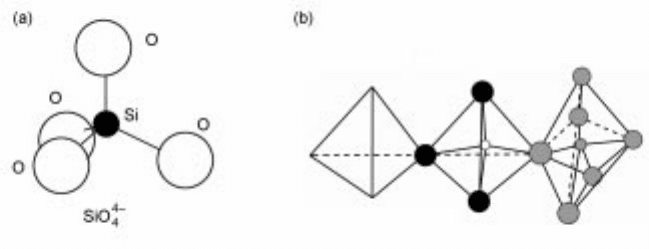
Slags and glasses are polymers in the form of chains, rings etc made up of
SiO
4
4ÿ
tetrahedral units. They have the following characteristics:
8±11
· Each Si (with a valence of 4) is surrounded (tetrahedrally) by 4 O ions (with a
valence of 2) each connecting to 2Si ions (Fig. 4.7).
· In SiO
2
these SiO
4
4ÿ
tetrahedra are connected in a three-dimensional
polymerised structure (Fig. 4.8) and the oxygens are predominantly bridging
oxygens (denoted O
o
).
· Cations such as Ca
2+
, Mg
2+
etc. tend to break up the Si-O bonds and de-
polymerise the melt by forming non-bridging oxygens (NBO denoted O
ÿ
)
and free oxygens (denoted O
2ÿ
), i.e. not bound to Si.
· Other cations such as Al
3+
, P
5+
, Ti
4+
can fit into the Si polymeric chain but
need to maintain char ge balance, e.g. if an Al
3+
is incorporated into a Si
4+
chain it must have a Na
+
(or half* of a Ca
2+
) sitting near the Al
3+
to maintain
local charge balance.
· Smaller cations such as Mg
2+
tend to give a wider distribution of chain
lengths than larger cations such as Ba
2+
.
4.7 Schematic drawings of (a) tetrahedral arrangement of Si-O bonds and (b)
silicate chain with bridging O (O
o
) shown in black, non-bridging O (O-) as
hatched and tetrahedrally-coordinated cations as open.
* The Ca
2+
would charge balance two neighbouring Al
3+
ions.
Measurement and estimation of physical properties of metals 115
