Seetharaman S. Fundamentals of metallurgy
Подождите немного. Документ загружается.

and lines are reflected to the bottom of the prism to show the behaviour more
simply. Here, the solubility of each component in a solid phase is assumed to be
negligible for simplicity. When the liquid of a certain composition, X, is cooled
along path Xx, it reaches the liquidus surface at x, where the solid of
composition B starts to precipitate. This precipitated phase is called a primary
crystal and the boundaries which separate primary crystal regions are called
boundary lines, such as E
1
±E
0
, E
2
±E
0
and E
3
±E
0
in Fig. 3.18. They are
obviously the intersections of liquidus surfaces and correspond to the `creeks' in
the map described in the Section 3.3 .3. The reflected triangle in the figure can be
divide into three regions of AE
1
E
0
E
2
, BE
3
E
0
E
1
and CE
2
E
0
E
3
, which are named
the primary phase fields of A, B and C, respectively.
Once the primary crystal starts to precipitate, the liquid composition
changes to one with a lowering A concentration at a constant ratio of B and C
along path x±y until it reaches another liquidus surface, namely the boundary
line E
1
±E
0
. While the liquid composition is cooled along path x±y, it is (singly)
saturated with B and the composition x should be positioned on the tie-line A±
y in the reflected plane of the figure. After the liquid composition reached
another liquidus surface, it varies along the boundary line E
1
±E
0
, increasing C
concentration and coprecipitating the solid phases A and B. The secondary
precipitate B can be quoted as a secondary crystal, but may precipitate as a
two-phase secondary microconstituent composed of A and B. Thus the liquid
composition during solidification along E
1
±E
0
is doubly saturated with A and
B. Also, the composition X must be kept inside the triangle ABy surrounded by
three tie-lines. This is called a three-phase triangle indicating that three phases
coexist throughout the triangle keeping every chemical potential constant.
Finally, it reaches the eutectic composition at a certain temperature, namely
eutectic point, E
0
. Here, the liquid is saturated with three solid phases, and the
degree of freedom becomes zero. This situation is the so-called four-phase
equilibria, and the three-phase tertiary microconstituent composed of A, B and
t
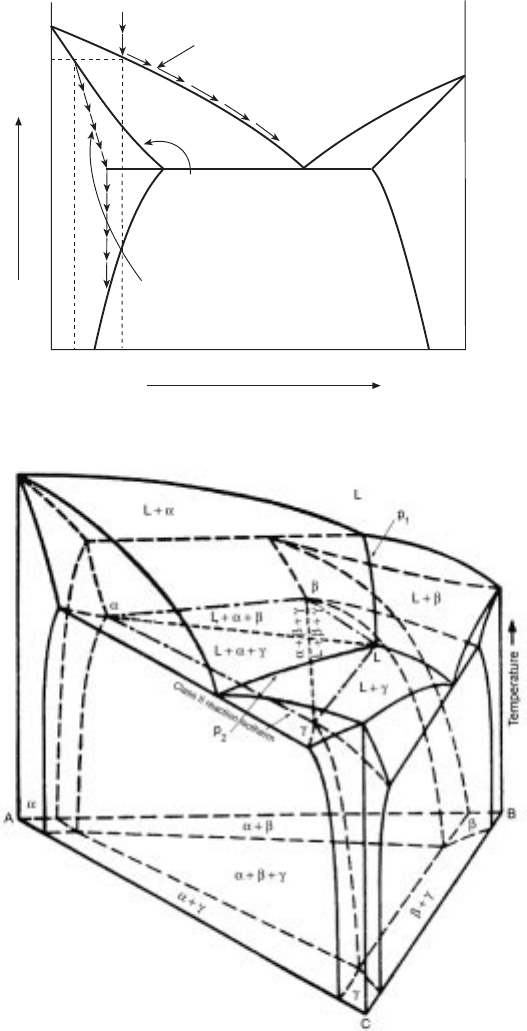
T
Cooling of liquid
Evolution of heat by a solidification
Eutectic isotherm
Cooling of solid
L L + a L + a + b a + b
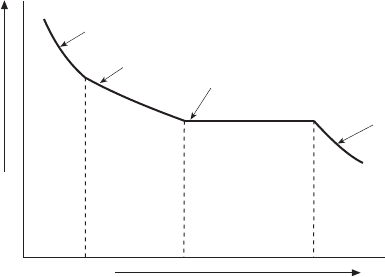
3.17 Cooling curve for the alloy shown in Fig. 3.16.
96 Fundamentals of metallurgy
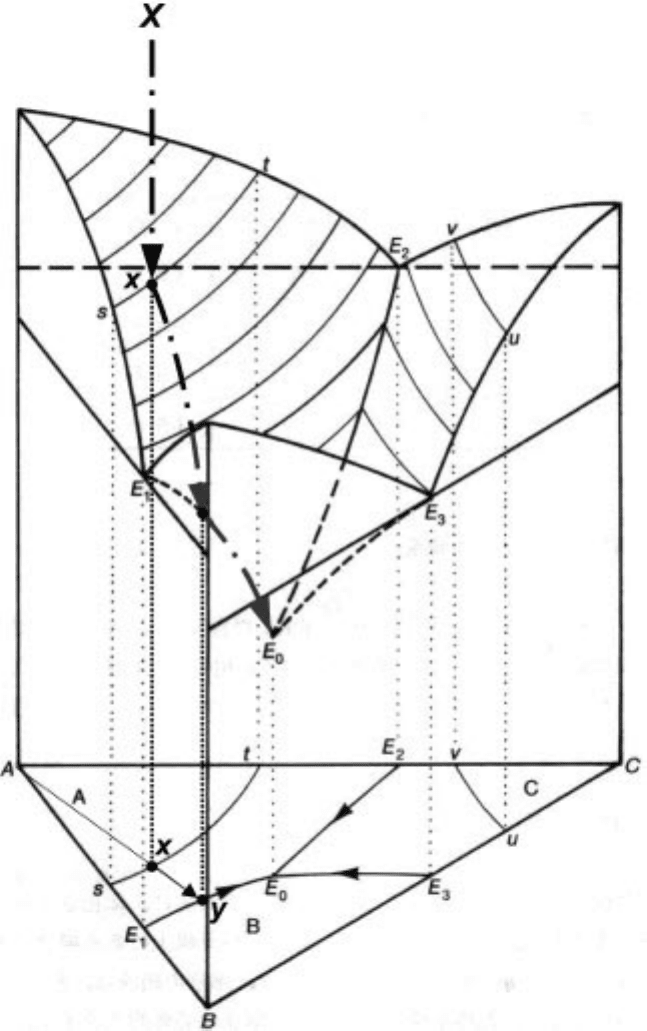
3.18 Phase diagram for the ternary eutectic system A-B-C.
Phase diagrams and phase transformations 97
C is precipitated until the liquid phase diminishes. The cooling curve is shown
in Fig. 3.19.
For most of the alloys, solid phases have some solubility of the other
components. During the precipitation of primary crystals, their composition in
equilibrium with the liquid can be given by the tie-line at any temperature. This
is demonstrated in Fig. 3.20. After the liquid becomes saturated with primary
and secondary crystals, their compositions are determined by the extremities of
the three-phase region as shown in Fig. 3.21. The fraction of their amounts can
be given by applying the lever rule to the three-phase triangle.
In the practical solidification processes of a system with more than two
components, however, equilibrium solidification is exceptional and solidus
curves or surfaces will be depressed due to the non-uniformity of the solid
t
T
Cooling of liquid
Evolution of heat by B solidification
Eutectic isotherm
Cooling of solid
Evolution of heat by A + B solidification
L L + B L + A + B L + A + B + C A + B + C
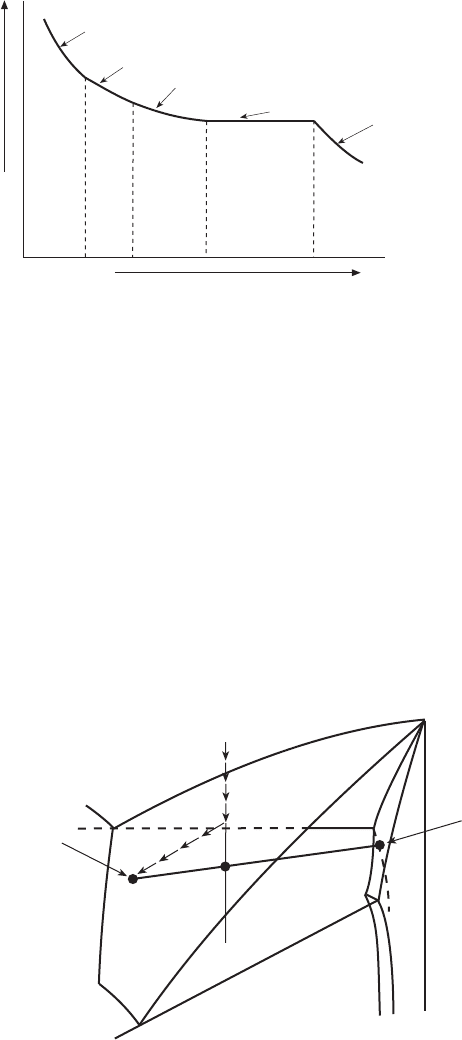
3.19 Cooling curve for the alloy shown in Fig. 3.18.
Equilibrium
solid
composition
Equilibrium
liquid
composition
Average alloy
composition
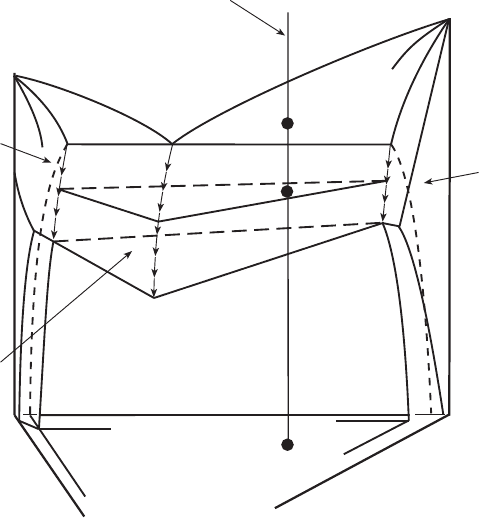
3.20 Solid composition during primary solidification.
98 Fundamentals of metallurgy
phase. Accordingly, the average composition of the solid inside will lie below
the equilibrium curves or surfaces, although the equilibrium solidus lines or
curves still demonstrate the composition in equilibrium with the liquid phase
(Fig. 3.22). Thus, when considering the average composition of solid phase s in
ternary systems, apparent solidus surfaces will be depressed and the three-phase
regions will also be enlarged. The eutect ic temperature will not be affected, but
the apparent tie-line will be lengthened showing the average compositions of
primary and secondary microconstituents in a no n-equ ilibrium eutectic
microstructure.
Although phase relations in practical systems may not follow equilibria as
mentioned, such alloys can be treated as in the equilibrium state, and a number
of phase transformations can be described in ternary systems. Four-phase
equilibrium is one of the distinctive cases. The first representative case is a
decomposition of a single phase upon cool ing to form three new phases, which
was already pointed out in Fig. 3.18, such eutectic and eutectoid reactions.
L $
$
a
b
Three-phase
triangle
(a + b + L)
Average alloy
composition
3.21 Solid composition during secondary solidification.
Phase diagrams and phase transformations 99
A
T
l
b a
B
X
B
T
L
a
b
Average solid
composition
Equilibrium
solid
composition
Liquid composition
3.22 Non-equilibrium solidification for the binary eutectic system A-B.
3.23 Phase diagram for the ternary system A-B-C based on two eutectic and
one peritectic binaries.
4
100 Fundamentals of metallurgy
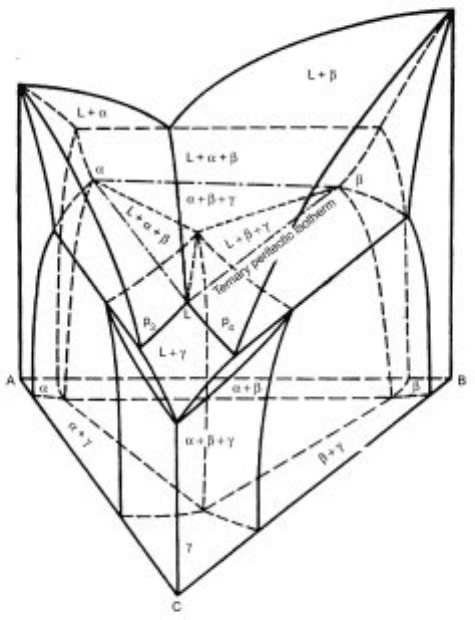
Another type of four-phase equilibrium is a decomposition of two phases on
cooling to form two new phases, which can be explained by a typical phase
diagram shown in Fig. 3.23.
4
The system is composed of one peritectic and
two eutectic binaries. Two boundary lines (alterative and crystallization
curves of L ! and L ! ), p
1
and p
2
, descend from each binary
with increasing amount of the third component and meet somewhere in the
middle, where the reaction, L ! , occurs at a constant temperature
until the phase diminishes. During this stage, four-phase equilibria are
attained. Thereafter, three-phase equilibria among L, and will be kept on
cooling.
One more case is shown in Fig. 3.24.
4
When two alterative curves forming
phase, p
3
and p
4
L ! and L ! , ascend from binaries with
increasing amounts of the third component and meet as shown in the figure,
where the reaction, L ! , occurs at a constant temper ature.
3.24 Phase diagram for the ideal ternary peritectic system A-B-C.
4
Phase diagrams and phase transformations 101
3.5 Examples of solidification behaviour from a
phase diagram perspective
Various metals are often refined by oxidation of impurities followed by their
removal into slag phases. In steel refining treatments, several impurities, such
as phosphorus, silicon, carbon, etc., are removed during oxygen blowing
processes. As can be seen in the Ellingham diagram, oxidation refining of
silicon is hopeless because it will be preferentially oxidized to most of the
impurity elements, such as iron, titanium, etc. On the other hand, the
solubilities of most impurity elements in solid silicon are extremely low.
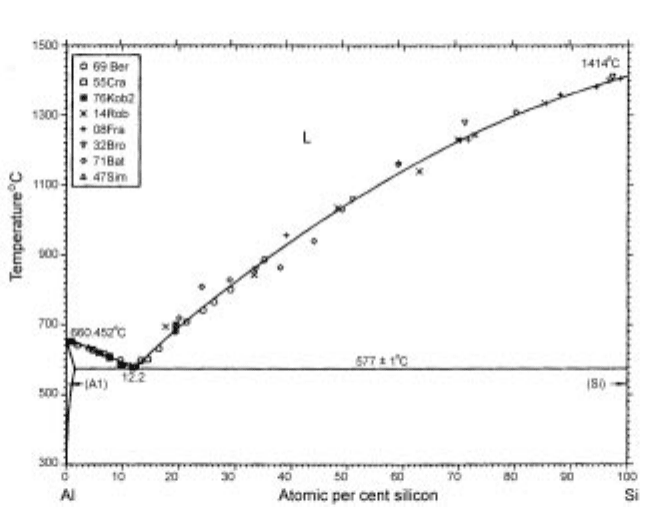
Figure 3.25 shows the Al-Si binary phase diagram
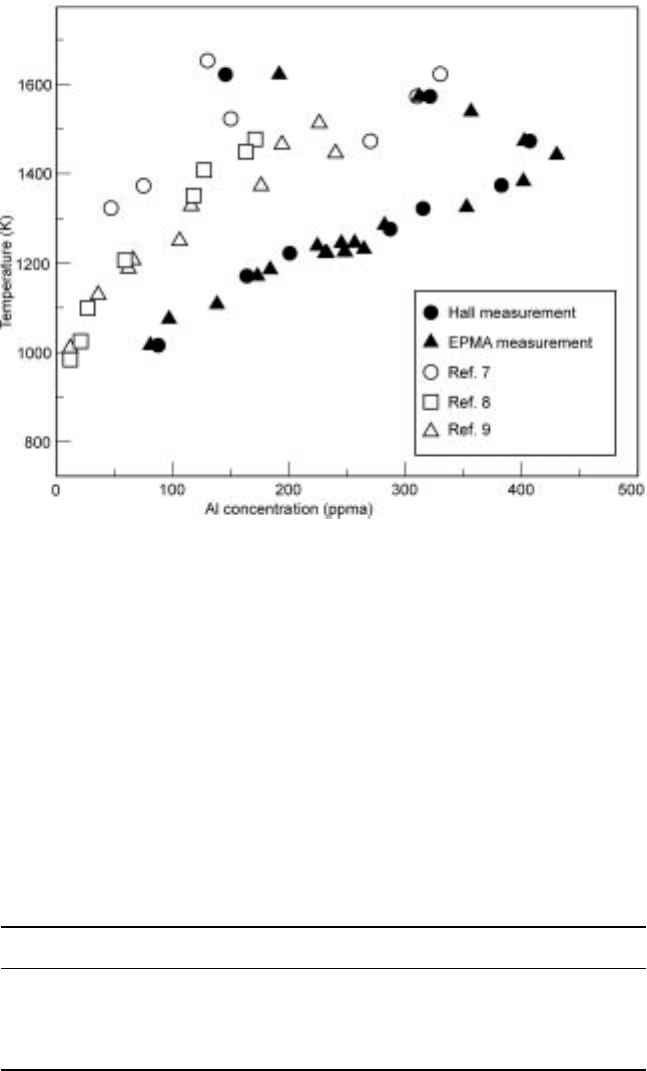
5
and the solubility curve of
aluminum in solid silicon is reported as shown in Fig. 3.26.
6
Although there are
discrepancies among the reported data, the solubility of aluminium in solid
silicon can be found to be lower than 100 ppmw at 1000K. For example, when
74 mass% Al-Si molten alloy was cooled from above liquidus temperature,
solid silicon with only 100 ppmw Al starts to precipitate at 1000K, which
means that 99.995% of aluminium was excluded by solidification at the initial
stage. This principle of solidification refining makes the ultra high purification
of silicon for semiconductors easier. Although some elements, such as
phosphorus and boron, have high solubility in solid silicon, most impurities can
3.25 Phase diagram for the Al-Si binary system.
102 Fundamentals of metallurgy
be removed by the one-directional solidification refining due to their small
segregation coefficients, which are tabulated in Table 3.1.
Herewith, an example of solidification behaviour of a ternary silicon-based
alloy is considered. Since solubilities of most impurities in solid silicon are
negligibly small, they tend to concentrate at the grain boundaries of primary
crystals of silicon almost free from such impurities. By making use of this
tendency, a new refining process
10
has been proposed, combined with the acid
leaching procedure. Although efficiency of impurity removal strongly depends
on its segregation coefficient, pure silicon grains remain after condensed
impurities at the grain boundary are washed away by acid dissolution. In order to
promote the selective dissolution of the grain boundaries by leaching process, a
3.26 Solubility of aluminium in solid silicon reported by several researchers.
Table 3.1 Segregation coefficients of impurities in silicon
Impurity Segregation coefficient Impurity Segregation coefficient
B 8.00 10
ÿ1
Fe 6.40 10
ÿ6
P 3.50 10
ÿ1
Ti 2.00 10
ÿ6
C 5.00 10
ÿ2
Cu 8.00 10
ÿ4
Al 2.80 10
ÿ3
Phase diagrams and phase transformations 103
leachate must corrode the boundary phase, but its amount may not be enough in
the case of silicon. Hence, the addition of an acid soluble element which forms
intermetallic compounds or eutectic microstructure with silicon might be
effective. One of the promising elements to form such phases is calcium as
shown in the following paragraph.
Recently, a new mass produc tion process of solar grade silicon has been
developed in order to solve the rapidly increasing demand of solar cells.
Metallurgical grade silicon (>98%) is selected as a starting material and the final
purity of solar grade silicon should be higher th an 6N through some
metallurgical refining treatments. Among others, iron is one of the most
harmful elements for the solar grade silicon since it shortens the lifetime and
drastically lowers the efficiency of solar cells. As mentioned previously,
however, iron is less favourable to be oxidized than silicon, and oxidation
refining is not suitable as well as vacuum refining for high vapour pressure
species. Hence, the only effective way is solidification refining using a
segregation coefficient as low as 10
ÿ6
.
11
However, another pretreatment at the
stage of met allurgical grade silicon may be helpful for reducing the
solidification refining cost. Accordingly, iron removal from metallurgical grade
silicon by acid leaching was investigated.
12
Figure 3.27 shows the experimental
results in which Si-Ca-Fe alloys with various compositions were subjected to
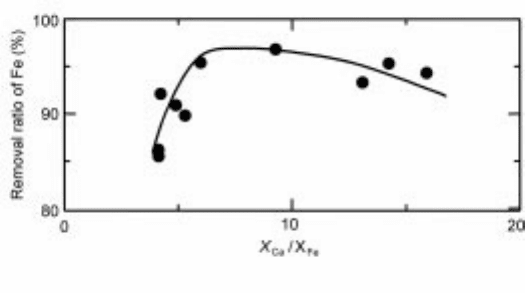
acid leaching procedure using aqua regia. Calcium was added to form an acid
soluble grain boundary. As seen in the figure, there seems to be an optimum
ratio of calcium to iron content. Optical images of Si-Ca-Fe alloys of two
different compositions before and after acid leaching are shown in Figs 3.28(a),
(c) and 3.29(a), (c). The sample shown in Figs 3.28(a), (b) and (c) has higher
calcium to iron ratio and calcium silicide, CaSi
2
, seems to have precipitated as a
secondary phase during cool ing, after silicon precipitated as a primary phase.
Iron silicide phase, FeSi
2
, was considered to exist as a part of the microstructure
3.27 The relationship between ratio of calcium to iron content and removal
ratio of iron.
104 Fundamentals of metallurgy
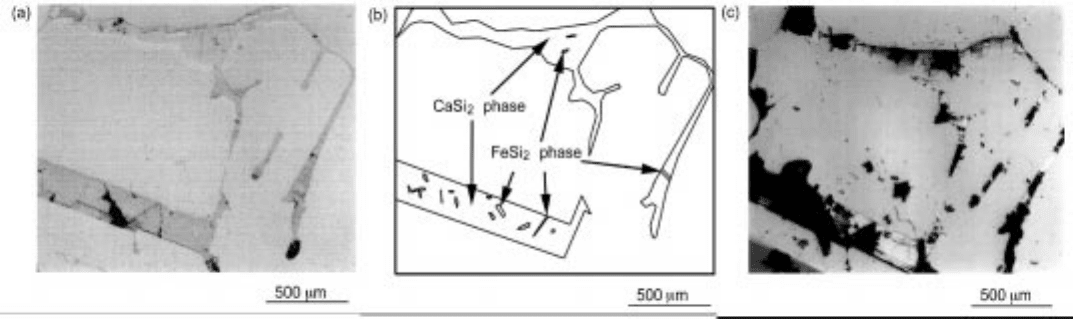
3.28 (a) Optical image of Si-Ca-Fe alloy (Si-8.64%Ca-0.7
56%Fe, before acid leaching). (b) Microstructure of Si-Ca-Fe all
oy. (c) Optical
image of Si-Ca-Fe alloy (Si-8.64%Ca-0.756%Fe, 5 m
in. in aqua regia).
