Schweitzer P.A. Fundamentals of corrosion. Mechanisms, causes, and preventative methods
Подождите немного. Документ загружается.

Corrosion Mechanisms 19
2.5 Measuring Polarization
While polarization always leads to lower rates of corrosion, identifying the
effects of the environment on polarization of the corrosion circuit is use-
ful in predicting corrosion behavior. Measurement of the corrosion circuit
while the corrosion potential is varied is possible with the apparatus shown
in Figure 2.9.
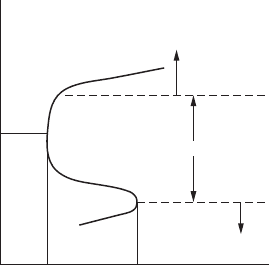
Turning to the example of iron corroding in hydrochloric acid solution,
if the iron sample is maintained at the natural corrosion potential of −0.2V,
no current will ow through the auxiliary electrode. The plot of this data
point in the study would equate to that of A or C in Figure 2.10. As the
potential is raised, anodically polarized, the current ow will increase and
curve AB will approximate the behavior of the true anodic polarization
curve. Alternatively, if the potential were lowered below −0.2V, the current
measurements would result in the curve CD and approximate the nature
of the cathodic polarization curve. By using the straight-line portion of the
Tafel regions of these curves, an approximation of the corrosion current
can be made.
Most often it is the anodic polarization behavior that is useful in under-
standing alloy systems in various environments. Anodic polarization tests
can be conducted with relatively simple equipment and the scans themselves
can be done in a short time. They are extremely useful in studying the active-
passive behavior that many materials exhibit. As the name suggests, these
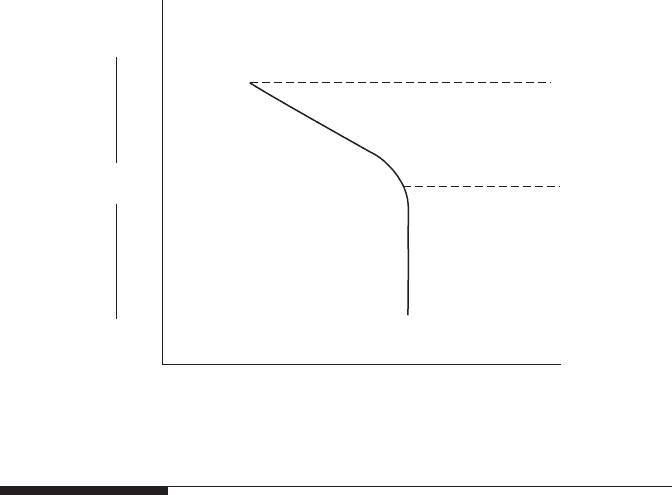
Concentration
polarization
Activation polarization
β
i
L
Log i
η
o
+
–
FigurE 2.8
Combined curve of activation and concentration polarizations for reduction process.
20 Fundamentals of Corrosion
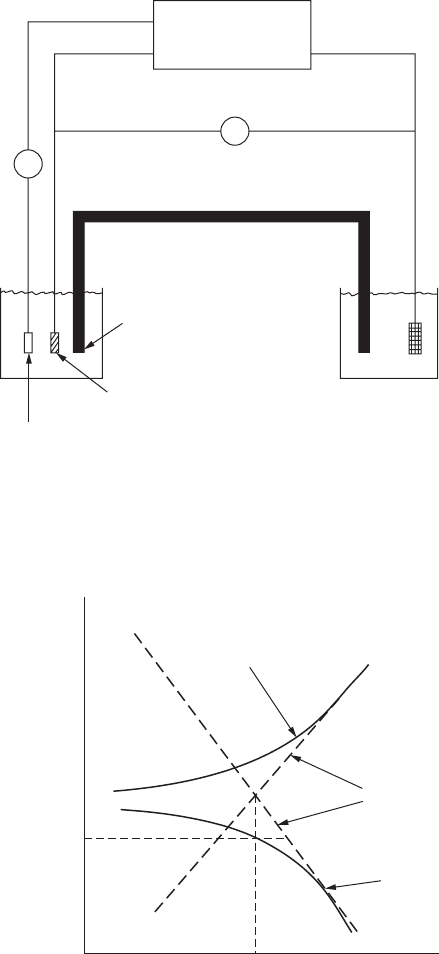
Reference
electrode
(Calomel)
Corrodent
Salt bridge
A
Potentiostat
Te st specimen
Auxiliary
electrode (Pt)
V
FigurE 2.9
Anode polarization measurement apparatus.
Tafel
region
True
Y
D
Current, Log i
X
C
A
Measured
B
Potential
FigurE 2.10
Anodic and cathodic polarization curve.
Corrosion Mechanisms 21
materials can exhibit both a highly corrosion-resistant behavior and that of
a material that corrodes actively, while in the same corrodent. Metals that
commonly exhibit this type of behavior include iron, titanium, aluminum,
chromium, and nickel. Alloys of these materials are also subject to this type
of behavior.
Active-passive behavior depends on the material–corrodent combination
and is a function of the anodic or cathodic polarization effects that occur in
that specic combination. In most situations where active-passive behavior
occurs, there is a thin layer at the metal surface that is more resistant to the
media than the underlying metal. In stainless steels, this layer is composed
of various chromium and/or nickel oxides that exhibit substantially differ-
ent electrochemical characteristics than the underlying alloy. If this resistant,
or passive layer is damaged while in aggressive media, active corrosion of
the freshly exposed surface will occur. The damage to this layer can be either
mechanical or electrochemical in nature.
The behavior of iron in nitric acid underscores the importance of recog-
nizing the nature of passivity. Iron is resistant to corrosion in nitric acid at
concentrations around 70%. Once passivated under these conditions, it can
also exhibit low rates of corrosion as the nitric acid is diluted. However, if this
passive lm is disturbed, rapid corrosion will begin and repassivation will not
be possible until the nitric acid concentration is raised to a sufcient level.
2.5.1 anodic Polarization
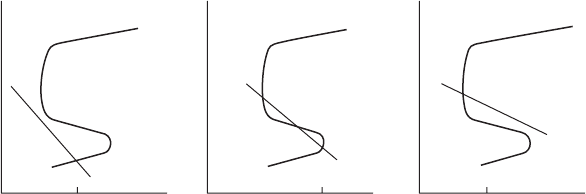
Active-passive behavior is schematically represented by the anodic polar-
ization curve as shown in Figure 2.11. Starting at the base of the plot, the
curve starts out with a gradually increasing current as expected. However,
Transpassive
Passive
Active
i
crit
Log i
i
pass
E
pp
E
p
B
Volts
A
FigurE 2.11
Anodic polarization curve for material exhibiting active-passive behavior.
22 Fundamentals of Corrosion
at point A there is a dramatic polarizing effect that drops the current to a
point where corrosion is essentially halted. As this potential is increased
further, there is little change in current ow until the next critical stage B,
where a breakdown of the passive lm occurs and the current again begins
to rise.
Even with an established anodic polarization behavior, the performance of
a material can vary greatly with relatively minor changes in the corrodent.
This is also illustrated in Figure 2.12. Frame 1 illustrates the case where the
anodic and cathodic polarization curves intersect much the same as in mate-
rials with no active-passive behavior. The anode is actively corroding at a
high but predetermined rate.
Frame 2 represents the condition often found perplexing when using
materials that exhibit active-passive behavior. With relatively minor
changes within the system, the corrosion current could be very low when
the material is in the passive state or very high when active corrosion
begins.
Frame 3 in Figure 2.12 typies the condition sought after when using mate-
rials in the passive state. In this example, the cathodic polarization curve
intersects only in the passive region, resulting in a stable and low corrosion
current. This type of system can tolerate moderate upset conditions without
the onset of accelerated corrosion.
The anodic polarization technique is also useful in studying the effects of
variations in the environment and the benets of alloy additions. As illus-
trated in Figure 2.13, temperature increases cause a shift of the curve to
higher currents. Increasing chromium content in steel expands the passive
region signicantly, and adding molybdenum raises the potential required
for the initiation of pitting-type attack. The presence of chloride or other
strong oxidizing ions will shrink the passive region.
Frame 3Frame 2
i
c
i
c
i
c
Frame 1
FigurE 2.12
Schematic representation of a material with active-passive behavior in different corrosive
environments.
Corrosion Mechanisms 23
2.6 Other Factors Affecting Corrosion
Temperature can have a signicant inuence on the corrosion process. This
is not unexpected because it is an electrochemical reaction and reaction rates
do increase with increasing temperature. There are additional inuences on
corrosion other than the corrodent itself.
The relative velocities between the component and the media can have a
direct effect on the corrosion rate. In some instances, increasing the veloc-
ity of the corrodent over the sufface of the metal will increase the corrosion
rate. When concentration polarization occurs, the increased velocity of the
media will disperse the concentrating species. However, with passive mate-
rials, increasing the velocity can actually result in lower corrosion rates. This
occurs because the increased velocity shifts the cathodic polarization curve
such that it no longer intersects the anodic polarization curve in the active
corrosion region, as shown in Figure 2.14.
Increasing
temperature
Increasing chloride
Increasing
chromium
Increasing
molybdenum
Log iLog i
V
V
Log iLog i
VV
FigurE 2.13
Effects of environment and alloy content on anodic polarization behavior.
24 Fundamentals of Corrosion
The surface nish of the component also has an impact on the mode and
severity of the corrosion that can occur. Rough surfaces or tight crevices can
facilitate the formation of concentration cells. Surface cleanliness can also be
an issue, with deposits or lms acting as initiation sites. Biological growths
can behave as deposits or change the underlying surface chemistry to pro-
mote corrosion.
Other variations within the metal surface on a microscopic level inu-
ence the corrosion process. Microstructural differences such as secondary
phases or grain orientation will affect the way corrosion manifests itself.
For corrosive environments where grain boundaries are attacked, the grain
size of the material plays a signicant role in how rapidly the material’s
properties deteriorate. Chemistry variations in the matrix of weld deposits
are also a factor.
Radiation can have an effect on a material’s properties. The effect on metal-
lic materials is very gradual and not very pronounced. Stresses, either resid-
ual or applied, impact the mode of corrosion and lower the energy needed
for corrosion to begin. Stress is a requirement for stress corrosion cracking
(which will be discussed later) or corrosion fatigue, but can also inuence the
rate of general corrosion.
Finally, time is a factor in determining the severity of corrosion. Corrosion
rates are expressed using a time dimension. Some corrosion processes are
violent and rapid while most are so slow as to be imperceptable on a day-to-
day basis. Equipment is planned to provide a useful service life. A chief goal
in understanding corrosion is the proper selection of materials, equipment,
processes, or controls to optimize our natural and nancial resources.
Decreasing
concentration
polarization
Log i
V
FigurE 2.14
Increased corrodent velocity can shift the cathodic polarization curve such that passive behav-
ior can be induced.

Corrosion Mechanisms 25
2.7 Corrosion Rate Measurement
Measurement of corrosion is essential for the purpose of material selec-
tion. The compatibility of a metal with its environment is a prime require-
ment for its reliable performance. Corrosion rate measurement may
become necessary for the evaluation and selection of materials for a spe-
cic environment, a given denite application, or for the evaluation of
a new or old metal or alloys to determine the environments in which
they are suitable. Often, the corrosive environment is treated to make it
less aggressive, and corrosion rate measurements of a specic material
in untreated environments will reect the efcacy of the treatment. In
addition, corrosion rate measurement is also essential in the study of the
mechanisms of corrosion.
2.7.1 Corrosion rate Expressions
Corrosion involves dissolution of metal, as a result of which the metal-
lic part loses its mass (or weight) and becomes thinner. Corrosion rate
expressions are therefore based on either weight loss or penetration into
the metal.
The most widely used weight expression, based on weight loss, is mg/
dm
2
/day (mdd) and the rate expression on penetration is inch penetration/
year (ipy) and mils penetration/year (mpy). One mil is one thousandth of
an inch. The last expression is very convenient because it does not involve a
decimal point or zeroes. Thus, 0.002 ipy is simply expressed as 2 mpy. The
expression is readily calculated from the weight loss of the metal specimen
during the corrosion test by the empirical formula:
mpy
534W
DAt
=
(2.18)
where W is weight loss (mg), D is density of metal (g/cm
3
), A is area of speci-
men (in.
2
), t is exposure time (h), and mdd and mpy are convertible through
the following multiplying factors:
mpy
1.44 mdd
density
=
(2.19)
mdd=mpy(0.696) (density)
(2.20)
Conversion factors for the above equations are given in Table 2.2.

26 Fundamentals of Corrosion
TabLE 2.2
Conversion Factors from Inches per Year (ipy) to
Milligrams per Square Decimeter per Day (mdd)
Metal
Density
(g/cm
3
)
0.00144
Density
(× 10
-3
)
696 ×
Density
Aluminum 2.72 0.529 1890
Brass (red) 8.75 0.164 6100
Brass (yellow) 8.47 0.170 5880
Cadmium 8.65 0.167 6020
Columbium 8.4 0.171 5850
Copper 8.92 0.161 6210
Copper-nickel (70/30) 8.95 0.161 6210
Iron 7.87 0.183 5480
Duriron 7.0 0.205 4870
Lead (chemical) 11.35 0.127 7900
Magnesium 1.74 0.826 1210
Nickel 8.89 0.162 6180
Monel 8.84 0.163 6140
Silver 10.50 0.137 7300
Tantalum 16.6 0.0868 11550
Titanium 4.54 0.317 3160
Tin 7.29 0.198 5070
Zinc 7.14 0.202 4970
Zirconium 6.45 0.223 4490
Note: Multiply ipy by (696 × density) to obtain mdd. Multiply
mdd by (0.00144/density) to obtain ipy.

27
3
Forms of Metallic Corrosion
The mechanisms of metallic corrosion were discussed in Chapter 2. The
various mechanisms can result in different forms or types of corrosion, as
discussed in this chapter. The primary forms of corrosion are as follows:
General (uniform) corrosion•
Intergranular corrosion•
Galvanic corrosion•
Crevice corrosion•
Pitting corrosion•
Erosion corrosion•
Stress corrosion cracking•
Biological corrosion•
Selective leaching•
Hydrogen damage•
Liquid metal attack•
Exfoliation•
Corrosion fatigue•
Filiform corrosion•
Each form of corrosion and the conditions responsible for its initiation
are discussed.
3.1 General (Uniform) Corrosion
Although other forms of attack must be considered in special circumstances,
uniform attack is one form most commonly confronting the user of metals
and alloys. Uniform (or general) corrosion, which is the simplest form of cor-
rosion, is an even rate of metal loss over the exposed surface. It is generally
thought of as metal loss due to chemical attack or dissolution of the metallic
component into metallic ions. In high-temperature situations, uniform metal
loss is usually preceded by its combination with another element rather than
28 Fundamentals of Corrosion
its oxidation to a metallic ion. Combination with oxygen to form metallic
oxides, or scale, results in the loss of material in its useful engineering form;
scale ultimately akes off to return to nature.
A metal resists corrosion by forming a passive lm on the surface. This
lm is formed naturally when the metal is exposed to the air for a period
of time. It can also be formed more quickly by chemical treatment. For
example, nitric acid, if applied to austenitic stainless steel, will form this
protective lm. Such a lm is actually corrosion but once formed, it prevents
further degradation of the metal, provided that the lm remains intact. It
does not provide an overall resistance to corrosion because it may be sub-
ject to chemical attack. The immunity of the lm to attack is a function of
the lm composition, temperature, and the aggressiveness of the chemical.
Examples of such lms are the patina formed on copper, the rusting of iron,
the tarnishing of silver, the fogging of nickel, and the high-temperature oxi-
dation of metals.
There are two theories regarding the formation of this lm. The rst theory
states that the lm formed is a metal oxide or other reaction compound. This is
known as the oxide lm theory. The second theory states that oxygen is adsorbed
on the surface, forming a chemisorbed lm. However, all chemisorbed lms
react over a period of time with the underlying metal to form metal oxides.
Oxide lms are formed at room temperature. Metal oxides can be classied as
network formers, intermediates, or modiers. This division can be related to
thin oxide lms on metals. The metals that fall into network-forming or inter-
mediate classes tend to grow protective oxides that support anion or mixed
anion/cation movement. The network formers are noncrystalline, whereas the
intermediates tend to be macrocrystalline at low temperatures.
3.1.1 Passive Film on iron
Iron in iron oxides can assume a valence of two or three. The former acts as
a modier and the latter as a network former. The iron is protected from the
corrosion environment by a thin oxide lm 1 to 4 mm in thickness with a com-
position of
Fe O/Fe O
23 34
.
This is the same type of lm formed by the reac-
tion of clean iron with oxygen or dry air. The
Fe O
23
layer is responsible for
the passivity, while the Fe
3
O
4
provides the basis for the formation of a higher
oxidation state. Iron is more difcult to passivate than nickel because with iron
it is not possible to go directly to the passivation species
Fe O
23
. Instead, a
lower oxidation state of Fe
3
O
4
is required and the lm is highly susceptible to
chemical dissolution. The
Fe O
23
layer will not form until the Fe
3
O
4
phase
has existed on the surface for a reasonable period of time. During this time, the
Fe
3
O
4
layer continues to form.
