Schweitzer P.A. Fundamentals of corrosion. Mechanisms, causes, and preventative methods
Подождите немного. Документ загружается.

Corrosion Mechanisms 9
2.2.1 Dissimilar Electrode Cells
Dissimilar electrode cells may be formed when two dissimilar metals
are in contact or due to the heterogeneity of the same metal surface. The
Daniel cell is an example of the former. In practice, a copper pipe con-
nected to a steel pipe or a bronze propeller in contact with the steel hull
of a ship provides an example of this type of corrosion cell. This is often
referred to as galvanic coupling, in which the less noble metal becomes
the anode. Galvanic corrosion is discussed further in Chapter 3. A cold
worked metal in contact with the same metal annealed leads to a similar
situation (i.e., the cold worked metal remaining anodic). On the same metal
surface, such type of cell formation may result from dissimilar phases and
impurities, grain boundaries, differentially strained areas, and scratches
or abrasions. In a single crystal, the different crystal faces differ in their
electrochemical characteristics because of the difference in their atomic
orientation and, as a result, one crystal face tends to become anodic com-
pared to the others.
2.2.2 Concentration Cells
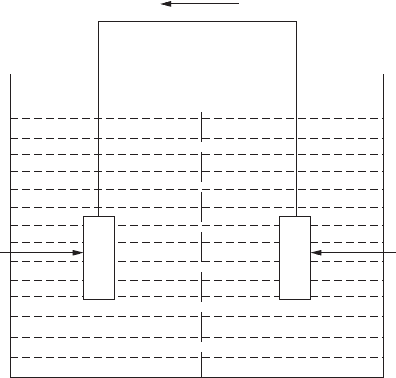
Concentration cells are formed when the electrodes are identical but are in
contact with solutions of differing composition. A salt concentration cell forms
when one electrode is in contact with a concentrated solution and the other
electrode with a dilute solution. On short-circuiting, the electrode in contact
with the dilute solution will be anodic (refer to Figure 2.1). The local variation
Cathode
CuCu
Anode
–+
Dilute CuSO
4
Conc. CuSO
4
FigurE 2.1
Salt concentration cell.
10 Fundamentals of Corrosion
of composition of the process stream inside the pipeline in a chemical plant
may lead to such a situation in practice.
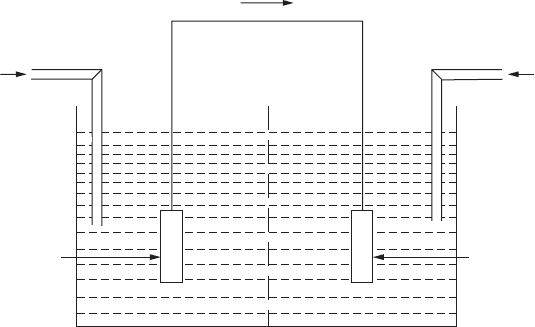
A differential aeration cell forms when the identical electrodes are exposed
to solutions of identical chemical composition that differ in oxygen content,
which is illustrated in Figure 2.2. The electrode in contact with the less aer-
ated or oxygenated solution will act as the anode.
Differential aeration cell formation is quite common in operation and is very
important from the viewpoint of practical corrosion damage. A metallic bucket
half lled with water tends to corrode just below the water line because of the
lower oxygen concentration compared to the area just above it near the water
line. Corrosion damage invariably becomes pronounced underneath a corrosion
product or at crevices where oxygen availability is low. Formation of concentra-
tion cells of both kinds accounts for the initiation of pits (discussed in Chapter 3)
in stainless steels and in some other metals and alloys exposed to seawater.
2.2.3 Differential Temperature Cells
Differential temperature cells are formed when electrodes of the same metal,
each of which is at a different temperature, are immersed in an electrolyte
of the same initial composition. Such a situation may arise in practice in
components of heat exchangers, boilers, and similar heat transfer equip-
ment. Polarity developed in an electrode varies from system to system. For
a copper electrode in a copper sulfate solution, the electrode at the higher
temperature is the cathode; but for lead, the situation is just the reverse. For
iron immersed in dilute aerated sodium chloride solutions, the hot electrode
is initially anodic to the colder metal, but the polarity may reverse with the
progress of corrosion.
Anode
N
2
Cathode
Air
or O
2
Dilute NaClDilute NaCl
Fe
+–
Fe
FigurE 2.2
Differential aeration cell.

Corrosion Mechanisms 11
2.2.4 Oxygen Concentration Cells
The oxygen-reduction reaction that occurs in neutral or basic solutions, O
2
+
2Η
2
O + 4e
−
→ 40H
−
, plays a signicant role in many corrosion processes. It not
only contributes to sustaining a cathodic reaction, but also can induce one.
This occurs when substantial differences in dissolved oxygen content exist
at one area on the metal surface relative to another. The natural tendency
is to equal concentrations, and the means of achieving this by corrosion is
to lower the oxygen concentration at the region where it is the highest. The
oxygen-reduction reaction accomplishes this but the area where this occurs
becomes cathodic to the lower oxygen concentration region. Because of the
current ow created by this action, corrosion will occur at the anodic or low
oxygen concentration site.
2.2.5 Metal ion Concentration Cells
Metal ion concentration cells can also develop and fuel the corrosion process.
This situation arises when a signicant difference in metal ion concentration
exists over a metal surface. The tendency is to reach equilibrium ion con-
centration, and in a corrosive environment this is managed by putting more
metal ions into solution at the low-concentration area. This area becomes the
anode, and the current ow generated by this process can result in plating
out metal ions at the cathodic or high metal ion concentration region.
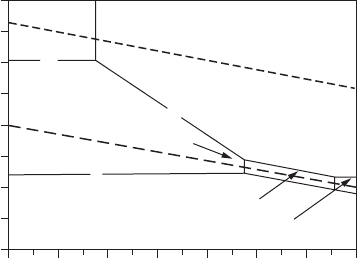
2.3 Potential–pH Diagrams
Potential–pH diagrams, also known as Pourbaix diagrams, are graphical rep-
resentations of the stability of a metal and its corrosion products as a func-
tion of the potential and pH (acidity or alkalinity) of the aqueous solution.
The potential is shown on the vertical axis and the pH on the horizontal axis.
Such diagrams are constructed from calculations based on the Nernst equa-
tion and the solubility data for various metal compounds. The potential–pH
diagram for an Fe–Η
2
Ο system is shown in Figure 2.3. In the diagram, the
horizontal lines represent pure electron transfer reactions dependent solely
on potential, but independent of pH:
1.
Fe Fe e=+
+−2
2
(2.3)
2.
Fe Fe e
2+
=+
+−3
(2.4)
12 Fundamentals of Corrosion
These lines extend across the diagram until the pH is sufciently high
to facilitate the formation of hydroxides, represented by vertical lines,
thereby reducing the concentration Fe
2+
and Fe
3+
ions. The boundary is
often set arbitrarily at the concentration of these ions at 10
−6
g-ions/liter,
which is indicative of a negligible dissolution or corrosion of the metal in
the medium.
The vertical lines in Figure 2.3 correspond to the reactions:
3.
Fe 2H OFeOHH
2+
2
+=
()
+
+
2
2
(2.5)
4.
Fe 3H OFeOHH
3+
2
+=
()
+
+
3
3
(2.6)
There is no electron transfer involved and the reactions are solely dependent
on pH.
The sloping lines in Figure 2.3 represent equilibria involving both electron
transfer and pH; for example:
5.
Fe 3H OFeOHHe
2+
2
+=
()
++
+−
3
3
(2.7)
6.
Fe 2H OHFeOHe
22
+= ++
−+−
32
(2.8)
1412108642
Fe
Potential E
H
(V)
0
–1.2
–0.8
–0.4
0
0.4
Hydrogen line
Oxygen line
0.8
1.2
Fe
+3
Fe(OH)
3
Fe(OH)
2
5
3
HFeO
2
–
Fe
+2
1
pH
2
FigurE 2.3
Potential–pH (Pourbaix) diagram for Fe–H
2
O system.
Corrosion Mechanisms 13
The hydrogen and oxygen are also shown in the diagram by the dotted lines.
The hydrogen line represents the equilibria:
2H +2e=Hinacidsolutions
+–
2
(2.9)
or
22 2
2
HO eHOH in neutraloralkalinesolu
2
+=
−
+
−
ttions
(2.10)
These two reactions are equivalent and their pH dependence of single elec-
trode potential is represented by:
EE pH
H/H
O
H/H
+
2
+
2
=−0059.
(2.11)
at pH = 0; that is, for [H
+
] = 1,
E
O
H+/H
2
= 0
and the slope is −0.059V. Similarly,
for oxygen equilibrium with water the corresponding reactions at lower and
higher pH are:
OHeHO
+
22
442++=
−
(2.12)
and
OHOe OH
22
244++=
−−
(2.13)
The pH dependence of single electrode potential is represented by:
EE pH
2
222
/H O
O
O/HO
O
=−0059.
(2.14)
at pH = 0,
EV
O
O/HO
22
=1 226.
and at pH = 1 (i.e., for [OH
−
] = 1),
EV
O
O/HO
22
= 0401.
.
Here again, the slope of the line is −0.059V. Water is stable in the area designated
by these two lines. Below the hydrogen line it is reduced to hydrogen gas, and
above the oxygen line it is oxidized to oxygen.
The potential–pH diagram shows three clear-cut zones:
1. Immunity zone. Under these conditions of potential and pH, iron
remains in metallic form.
2. Corrosion zone. Under these conditions of potential and pH, iron cor-
rodes, forming Fe
2+
or Fe
3+
or HFeO
2
−
.

14 Fundamentals of Corrosion
3. Passive zone. Under these conditions of potential and pH, protective
layers of Fe(OH)
3
form on iron and further corrosion of iron does not
take place.
Such diagrams can be used for:
1. Predicting the spontaneous direction of reactions
2. Estimating the stability and composition of corrosion products
3. Predicting environmental changes that will prevent or reduce
corrosion
With reference to Figure 2.3, corrosion prevention can be achieved by lower-
ing the electrode potential down to the zone of immunity, raising the elec-
trode potential up to the region of passivity, or raising the pH or alkalinity of
the solution so that a passive lm is formed.
There are, however, limitations in using such diagrams. The most impor-
tant of these is that they represent equilibrium conditions and hence cannot
be used for predicting the rate of a reaction. The tacit assumption that cor-
rosion products (oxides, hydroxides, etc.) lead to passivity may not always
be true because they may not always precipitate on the metal surface. The
possibility of precipitation of other ions such as chlorides, sulfates, and phos-
phates has been ignored. Finally, the pH at the metal surface may vary dras-
tically because of side reactions, and a prediction of corrosion based on the
bulk pH of the solution may be misleading.
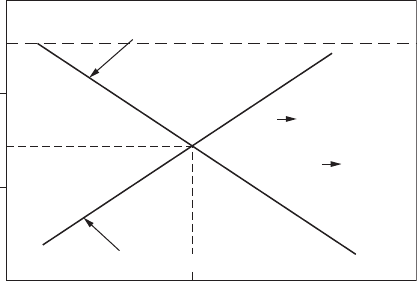
2.4 Polarization
At an intermediate resistance in the circuit, some current begins to ow and
the potentials of both half-cell reactions move slightly toward each other.
This change in potential is called polarization. The resistance in the circuit
depends on a number of factors, including the resistivity of the media,
surface lms, and the metal itself. The relationships between polarization
reactions at each half-cell are represented in Figure 2.4. The intersection of
the two polarization lines (curves) closely approximates the corrosion cur-
rent and the combined cell potentials for the freely corroding situation.
Once the corrosion current is determined, the corrosion density can be
calculated by determining the surface area. Using Faraday’s laws, a corro-
sion rate in terms of metal loss per unit time can be determined. However,
polarization data can be more useful than just estimating corrosion rates.
The extent of polarization can help predict the type and severity of cor-
rosion. As polarization increases, corrosion decreases. Polarization may
Corrosion Mechanisms 15
be preferable to either cathodic or anodic reactions. Understanding the
inuence of environmental changes on polarization can offer insight into
controlling corrosion. For example, in the iron–hydrochloric acid exam-
ple, hydrogen gas formation at the cathode can actually slow the reaction
(increased current resistance) by blocking access of hydrogen ions to the
cathode site. This results in cathodic polarization and lowers the current
ow and corrosion rate. If oxygen is bubbled through the solution, the
hydrogen is removed more rapidly by combining to form water and the
corrosion rate increases signicantly. Although this is an oversimplied
view of the effects of oxygen, it does indicate that the degree of polar-
ization can be affected by changes in the environment, either natural or
induced.
There are three basic causes of polarization, termed activation, concentra-
tion, and potential drop. Potential drop is the change in voltage associated
with effects of the environment and the circuit between the anode and cath-
ode sites. It includes the effects of the resistivity of the media, surface lms,
corrosion products, etc.
2.4.1 activation Polarization
Activation polarization arises out of a slow step in the electrode reaction
for which an activation energy in the form of an increment in potential is
required for the reaction to proceed. This will best be illustrated by the
hydrogen evolution reaction.
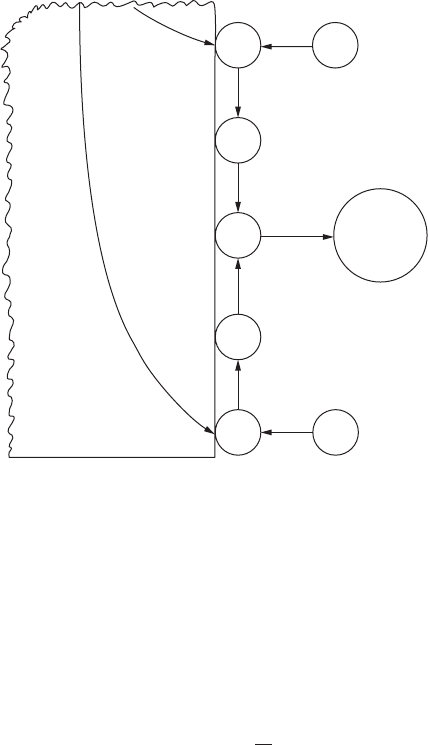
The hydrogen evolution reaction consists of several steps as shown
in Figure 2.5. Either the electron transfer step (step 2) or the formation of
Current, i
i
corr
2H
+
+ 2e
H
2
Fe Fe
2+
+ 2e
–0.5
–0.3
–0.1
Potential, E
E (H
2
/H
+
) Cathodic polarization curve
0.1
E
corr
E (Fe/Fe
2+
)
Anodic polarization curve
FigurE 2.4
Polarization of iron in acid.
16 Fundamentals of Corrosion
hydrogen molecules (step 3) is deemed the slowest step in the reaction
sequence, and the rate of overall reaction will depend on how fast or slow it
proceeds. Therefore, to have a higher rate of reaction, expressed in terms of
increased current density, an increase in potential should be effected. The
relationship between reaction rate and change in potential (overvoltage) is
expressed by the Tafel equation:
ηβ
a
o
log
i
i
=±
(2.15)
where η
a
is overvoltage polarization (in volts), and β is a constant, called the
Tafel constant (also expressed in volts), and is usually on the order of 0.1V.
A graphical representation of Equation 2.15, as applied to the hydrogen
evolution reaction, with a β slope of 0.1V is shown in Figure 2.6. It can be
noted from the graph that 0.1V change in overvoltage can effect a tenfold
increase or decrease in the reaction rate.
Dissolution reactions (anodic) in corrosion are usually controlled by
activation polarization where the solution of ions is the probable rate-con-
trolling step. Hydrogen evolution reactions (cathodic reactions) are con-
trolled by activation polarization when the concentration of hydrogen ions
is high.
H
+
–
H
+
H
–2
H
H
+
H
+
–H
2
H
2
H
2
H
2
H
2
1
3
3
2–
Zinc
4
1
FigurE 2.5
Steps involved in hydrogen reduction reaction.
Corrosion Mechanisms 17
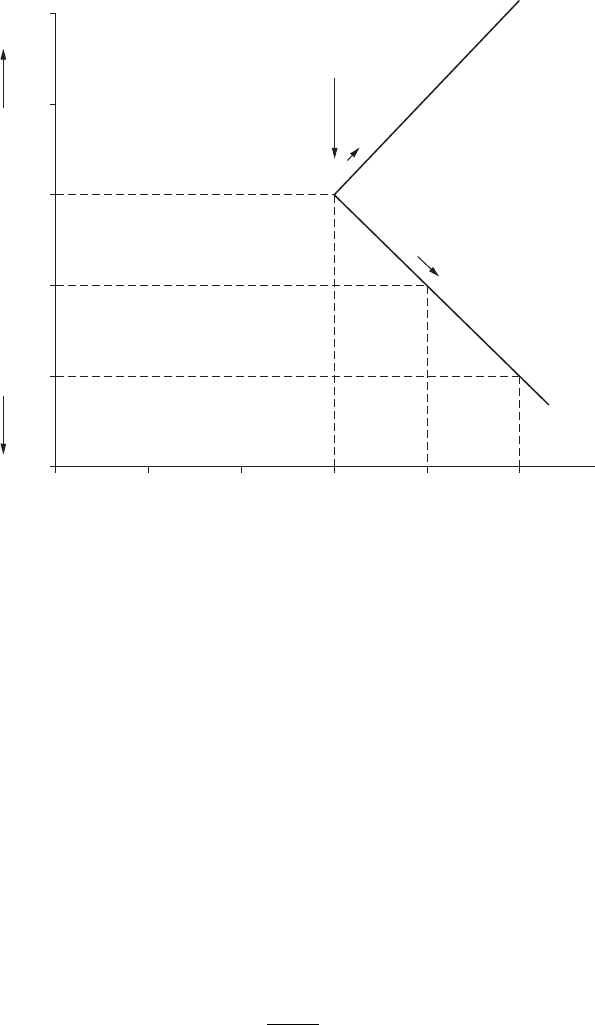
2.4.2 Concentration Polarization
A buildup or depletion of ions at the electrode surface as a result of reac-
tion will change the value of the electrode potential according to the Nernst
equation. For example, for a corroding zinc electrode, the concentration of
zinc will increase with dissolution in the vicinity of the electrode. The value
of a
oxid
in the equation increases, causing the electrode potential to shift in a
positive direction.
For the hydrogen evolution reaction, the higher rate of discharge of hydro-
gen ions at the electrode surface brings down the value of a
oxid
and the
electrode potential, according to the Nernst equation, shifts in a negative
direction. However, the rate of discharge of hydrogen ions at the electrode
surface depends on the diffusion of hydrogen ions from the bulk of the solu-
tion to the surface:
i=
DnFC
x
l
(2.16)
1001010.10.01
–0.3
–0.2
–0.1
0
0.1
2H
+
H
2
H
2
2H
+
+ 2e
–
2e
–
+
Noble
Active
Overvoltage η
a
Volts
0.2
Current Density
i
o
H
2
/H
+
FigurE 2.6
Activation polarization curve of a hydrogen electrode.
18 Fundamentals of Corrosion
where i
l
is called the limiting diffusion current density (amp/cm
2
), D is the
diffusion coefcient for Η
+
ion, n is the number of electrons transferred, F is
the Faraday number, C is the bulk concentration of Η
+
ions in the solution,
and x is the thickness of the diffusion layer adjacent to the electrode surface
through which the concentration of the reacting species (H
+
ions) changes
from C in the bulk to zero at the electrode surface.
A mathematical expression for concentration polarization involves i
l
and
is given by:
η
c
l
RT
F
i
i
=−
23
1
.
log
(2.17)
where η
c
is overvoltage due to concentration polarization (in volts). A graphi-
cal representation of the equation is shown in Figure 2.7.
It can be seen from the graph in Figure 2.7 as well as from Equation
2.17 that as i approaches i
l
, η
c
tends to innity. As evident from Equation
2.16, factors such as increasing velocity (smaller x), increasing temperature
(higher D), and increasing concentrations will increase the value of i
L
, that
is, a shift in the vertical position of the curve in Figure 2.7 more toward
the right.
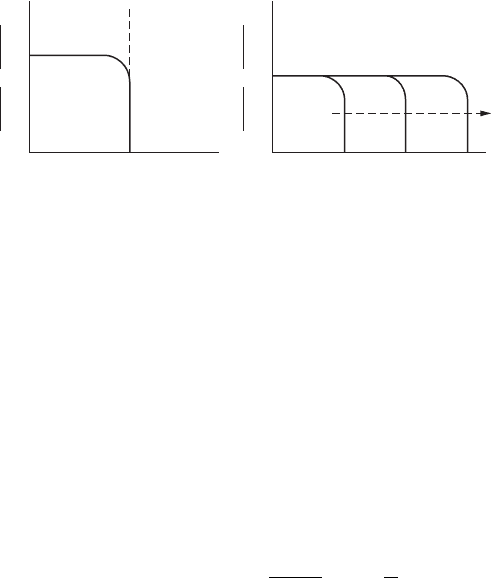
There is no question of concentration polarization when the supply of
reacting species is abundant. Hence, in metal dissolution reactions, its effect
is negligible as the supply of metal atoms for dissolution is unlimited. On
the other hand, for a hydrogen evolution reaction, concentration polarization
becomes signicant in the solutions of low Η
+
concentration. More often, the
reduction process is controlled by a combined polarization — that is, activa-
tion polarization at lower reaction rates and concentration polarization at
higher reaction rates — as i approaches i
l
. A graphical representation of such
combined polarization is shown in Figure 2.8.
Increasing velocity
Increasing temperature
Increasing concentration
Log iLog i
(b)(a)
i
L
t
i
L
c
i
i
L
L
o
o
η
c
+
–
v
η
c
+
–
FigurE 2.7
(a) Concentration polarization curve for reduction process, and (b) effect of environmental
variations on concentration polarization curve.
