Povh B., Rith K., Scholz Ch., Zetsche F. Particles and Nuclei: An Introduction to the Physical Concepts
Подождите немного. Документ загружается.

252 17 The Structure of Nuclei
300
200
100
0
+20 0 –20
B
/
[
MeV
]
1.
2.
n
/
1.
2.
12
/
C
Counts
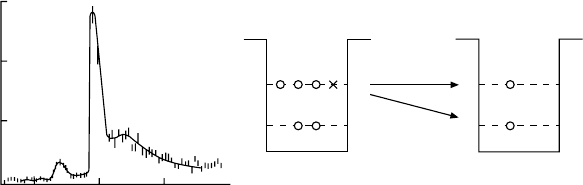
Fig. 17.3. The pion spectrum from the reaction K
−
+
12
C → π
−
+
12
Λ
Cfora
kaon momentum of 720 MeV/c [Po81]. The pion counting rate at 0
◦
is plotted as
a function of the transferred energy B
Λ
, which may be interpreted as the binding
energy of the Λ in the nucleus. Peak no. 1 corresponds to binding energy B
Λ
=0
and peak no. 2, which is the
12
Λ
C ground state, has a binding energy of 11 MeV.
We have the following explanation for this. The Pauli principle prevents a
proton or a neutron in the nucleus from occupying a lower energy level that is
already “taken” – the states in the nucleus get filled “from the bottom up”. If
we, however, change a neutron into a Λ particle, then this can occupy any of
the states in the nucleus. The Λ does not experience the individual presence
of the nucleons, but rather just the potential that they create. This potential
is, it should be noted, shallower than that which the nucleons experience.
This is because the Λ-nucleon interaction is weaker than that between the
nucleons themselves. That this is the case may also be seen from the lack of
any bound state formed from a Λ and a single nucleon.
The spectrum of Fig. 17.3 now makes sense: the protons and neutrons in
the
12
C nucleus occupy 1s and 1p energy levels and should one of the neutrons
in a 1p state be transformed into a Λ, then this can also take up a 1p state.
In this case the binding energy of the Λ is close to zero. Alternatively it can
land in a 1s state and it then has a binding energy of about B
Λ
≈ 11 MeV.
The smeared out peak with B
Λ
< 0 can be interpreted as arising from
the transformation not of weakly bound neutrons near the Fermi level, but
rather of deeper lying neutrons.
The Λ one-particle states may be seen even more clearly in heavier nuclei.
Systematic investigations, based upon the reaction
π
+
+A→
Λ
A+K
+
, (17.21)
have yielded the binding energies of the 1s states and, furthermore, those of
the excited p, d and f states for various nuclei as shown in Fig. 17.4. This
shows the dependence of these binding energies upon the mass number A in
the nuclei concerned.
17.3 The Shell Model 253
0 0.05 0.10 0.15 0.20
30
25
20
15
10
5
A
-2/3
89
Y
/
51
V
/
40
Ca
/
28
Si
/
16
O
/
13
C
/
12
C
/
s
/
p
/
d
/
MeV
[
]
B
/
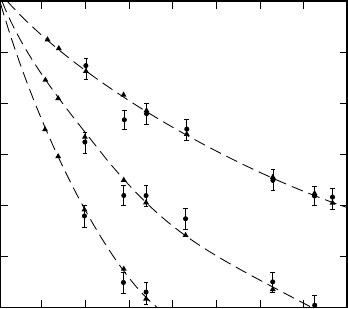
Fig. 17.4. The binding energy of Λ particles in hypernuclei as a function of the
mass number A [Ch89]. The symbols s
Λ
,p
Λ
and d
Λ
refer to the state of the Λ in
the nucleus. The triangles which are connected by the dashed lines are theoretical
predictions.
In this way it is seen that the Λ hyperons occupy discrete energy levels,
whose binding energies increase with the mass number. The curves shown are
the results of calculations assuming both a potential with uniform depth V
0
≈
30 MeV and that the nuclear radius increases as R = R
0
A
1/3
[Po81, Ch89].
The scale A
−2/3
corresponds then to R
−2
and was chosen because B
Λ
R
2
is
almost constant for states with the same quantum numbers, cf. (16.13).
The agreement between the calculated binding energies of the Λ particles
and the experimental results is amazing, especially if one considers how simple
the potential well is. The Λ moves as a free particle in the well although the
nucleus is composed of densely packed matter.
17.3 The Shell Model
The consequences that we have drawn from the spectroscopy of the hyper-
nuclei can be directly applied to the nucleons and we may assume that each
nucleon occupies a well-defined energy level.
The existence of these discrete energy levels for the nucleons in the nucleus
is reminiscent of the atomic electron cloud. The electrons move in the atom
in a central Coulombic potential emanating from the atomic nucleus. In the
nucleon, on the other hand, the nucleons move inside a (mean field) potential
produced by the other nucleons. In both cases discrete energy levels arise
which are filled up according to the dictates of the Pauli principle.
254 17 The Structure of Nuclei
100
80
60
40
20
0
Z
0 20 40 60 80 100 120 140 160
N
E
1
[MeV]
> 5.0
2.5...5.0
1.0...2.5
0.5...1.0
< 0.5
126
82
82
50
28
20
8
2
28
20
8
2
50
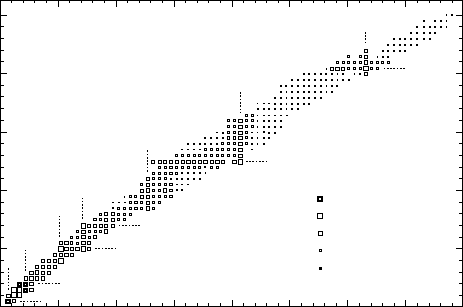
Fig. 17.5. The energy E
1
of the first excited state of even-even nuclei. Note that it
is particularly big for nuclei with “magic” proton or neutron number. The excited
states generally have the quantum numbers J
P
=2
+
. The following nuclei are
exceptions to this rule:
4
2
He
2
,
16
8
O
8
,
40
20
Ca
20
,
72
32
Ge
40
,
90
40
Zr
50
(0
+
),
132
50
Sn
82
,
208
82
Pb
126
(3
−
)and
14
6
C
8
,
14
8
O
6
(1
−
). E
1
is small further away from the “magic” numbers –
and is generally smaller for heavier nuclei (data from [Le78]).
Magic numbers. In the atomic case we can order the electrons in “shells”.
By a shell we mean that several energy levels lie close together clearly sepa-
rated from the other states. Matters seem to be similar in nuclei.
It is an observed fact that nuclides with certain proton and/or neutron
numbers are exceptionally stable (cf. Fig. 2.4) [Ha48]. These numbers (2, 8,
20, 28, 50, 82, 126) are known as magic numbers. Nuclei with a magic proton
or neutron number possess an unusually large number of stable or very long
lived nuclides (cf. Fig. 2.2). If a nucleus has a magic neutron number, then
a lot of energy is needed to extract a neutron from it; while if we increase
the neutron number by one then the separation energy is much smaller. The
same is true of protons. It is also found that a lot of energy is needed to
excite such nuclei (Fig. 17.5).
These jumps in the excitation and separation energies for individual nu-
cleons are reminiscent of chemistry: the noble gases, i.e., those with full shells,
are particularly attached to their electrons, while the alkali metals, i.e., atoms
with just one electron in their outermost shell, have very small separation
(ionisation) energies.
The doubly magic nuclei, those with both magic proton and magic neutron
numbers, are exceptionally stable. These are the following nuclides:
4
2
He
2
,
16
8
O
8
,
40
20
Ca
20
,
48
20
Ca
28
,
208
82
Pb
126
.

17.3 The Shell Model 255
The existence of these magic numbers can be explained in terms of the so-
called shell model. For this we need first to introduce a suitable global nuclear
potential.
Eigenstates of the nuclear potential. The wave function of the particles
in the nuclear potential can divided into two parts: a radial one R
n
(r),
which only depends upon the radius, and a part Y
m
(θ, ϕ) which only depends
upon the orientation (this division is possible for all spherically symmetric
potentials; e.g., atoms or quarkonium). The spectroscopic nomenclature for
quarkonium is also employed for the quantum numbers here (see p. 174):
n with
n =1, 2, 3, 4, ··· number of nodes + 1
=s, p, d, f, g, h, ··· orbital angular momentum .
The energy is independent of the m quantum number, which can assume
any integer value between ±. Since nucleons also have two possible spin
directions, this means that the n levels are in fact 2·(2+1) times degenerate.
The parity of the wave function is fixed by the spherical wave function Y
m
and is just (−1)
.
Since the strong force is so short-ranged, the form of the potential ought
to follow the density distribution of the nucleons in the nucleus. For very light
nuclei (A
<
∼
7) this would mean a Gaussian distribution. The potential can
then be approximated by that of a three dimensional harmonic oscillator. The
Schr¨odinger equation can be solved analytically in this particularly simple
case [Sc95]. The energy depends upon the sum N of the oscillating quanta in
all three directions as follows
E
harm. osc.
=(N +3/2) · ω =(N
x
+ N
y
+ N
z
+3/2) · ω, (17.22)
where N is related to n and by
N =2(n − 1) + . (17.23)
Hence states with even N have positive parity and those with odd N negative
parity.
Woods-Saxon potential. The density distribution in heavy nuclei can be
described by a Fermi distribution, cf. (5.52). The Woods-Saxon potential is
fitted to this density distribution:
V
centre
(r)=
−V
0
1+e
(r−R)/a
. (17.24)
States with the same N but different n values are no longer degenerate
in this potential. Those states with smaller n and larger are somewhat
lower. The first three magic numbers (2, 8 and 20) can then be understood
as nucleon numbers for full shells:
256 17 The Structure of Nuclei
N 012233444···
n 1s 1p 1d 2s 1f 2p 1g 2d 3s ···
Degeneracy 2 610214618102···
States with E ≤ E
n
2 8 18 20 34 40 58 68 70 ···
This simple model does not work for the higher magic numbers. For them
it is necessary to include spin-orbit coupling effects which further split the
n shells.
Spin-orbit coupling. We may formally introduce the coupling of the spin
and the orbital angular momentum (16.8) in the same manner as for the
(atomic) electromagnetic interaction. We therefore describe it by an addi-
tional s term in the potential:
V (r)=V
centr
(r)+V
s
(r)
s
2
. (17.25)
The combination of the orbital angular momentum and the nucleon spin s
leads to a total angular momenta j = ±/2 and hence to the expectation
values
s
2
=
j(j +1)− ( +1)− s(s +1)
2
=
(
/2forj = +1/2
−( +1)/2forj = − 1/2 .
(17.26)
This leads to an energy splitting ∆E
s
which linearly increases with the
angular momentum as
∆E
s
=
2 +1
2
·V
s
(r) . (17.27)
It is found experimentally that V
s
is negative, which means that the j =
+1/2 is always below the j = − 1/2 level, in contrast to the atomic case,
where the opposite occurs.
Usually the total angular momentum quantum number j = ±1/2ofthe
nucleon is denoted by an extra index. So, for example, the 1f state is split
into a 1f
7/2
and a 1f
5/2
state. The n
j
level is (2j + 1) times degenerate.
Figure 17.6 shows the states obtained from the potential (17.25). The
spin-orbit splitting is separately fitted to the data for each n shell. The
lowest shells, i.e., N =0,N =1andN = 2, make up the lowest levels and
are well separated from each other. This, as we would expect, corresponds
to the magic numbers 2, 8 and 20. For the 1f shell, however, the spin-orbit
splitting is already so large that a good sized gap appears above 1f
7/2
.This
in turn is responsible for the magic number 28. The other magic numbers can
be understood in a similar fashion.
This then is the decisive difference between the nucleus and its atomic
cloud: the s coupling in the atom generates the fine structure, small correc-
tions of the order of α
2
, but the spin-orbit term in the nuclear potential leads
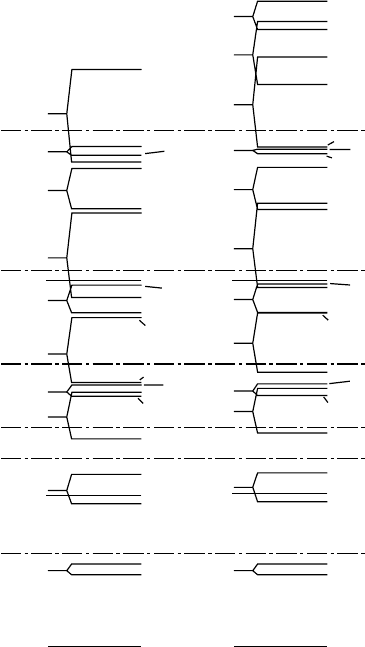
17.3 The Shell Model 257
126
82
50
28
20
8
1p
2s
1d
1f
2p
1g
2d
3s
1h
2f
3p
1i
3d
2g
1i
3p
2f
1h
3s
2d
1g
2p
1f
1d
2s
1p
3/2
1/2
5/2
1/2
3/2
7/2
3/2
5/2
9/2
1/2
5/2
7/2
11/2
3/2
1/2
7/2
9/2
5/2
3/2
1/2
13/2
9/2
11/2
5/2
7/2
3/2
11/2
1/2
13/2
5/2
3/2
7/2
9/2
1/2
3/2
11/2
5/2
7/2
9/2
5/2
3/2
1/2
7/2
3/2
1/2
5/2
1/2
3/2
1/2
1s
Protons
1/2
1s
Neutrons
Fig. 17.6. Single par-
ticle energy levels calcu-
lated using (17.25) (from
[Kl52]). Magic numbers ap-
pear when the gaps between
successive energy shells are
particularly large. This dia-
gram refers to the nucleons
in the outermost shells.
to sizeable splittings of the energy states which are indeed comparable with
the gaps between the n shells themselves. Historically speaking, it was a
great surprise that the the nuclear spin-orbit interaction had such important
consequences [Ha49, G¨o55].
One particle and one hole states. The shell model is very successful
when it comes to explaining the magic numbers and the properties of those
nuclei with “one nucleon too many” (or too few).
Those nuclei with mass number between 15 and 17 form a particularly
attractive example of this. Their excited states are shown in Fig. 17.7. The
15
Nand
15
O nuclei are so-called mirror nuclei, i.e., the neutron number of the
one is equal to the proton number of the other and vice versa. Their spectra
are exceedingly similar, both in terms of where the levels are and also in terms
of their spin and parity quantum numbers. This is a consequence of the isospin
independence of the nuclear force: if we swap protons and neutrons the strong
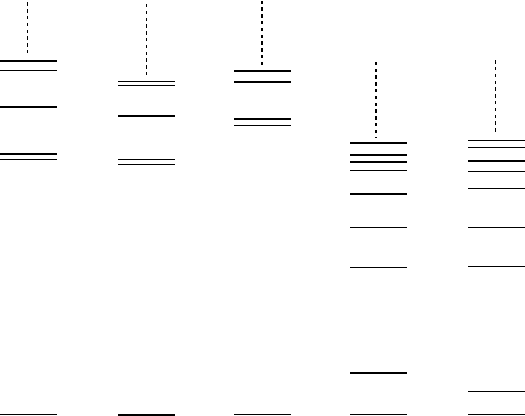
258 17 The Structure of Nuclei
force essentially does not notice it. The small differences in the spectra can
be understood as electromagnetic effects. While the energy levels of
16
Odo
not resemble those of its neighbours, the
17
Oand
17
F nuclei are, once again,
mirror nuclei and have very similar excitation spectra. It is striking that the
nuclei with mass numbers 15 and 16 require much more energy to reach their
first excited states than do those with mass number 17.
These spectra can be understood inside the shell model. The
16
O nucleus
possesses 8 protons and 8 neutrons. In the ground state the 1s
1/2
,1p
3/2
and 1p
1/2
proton and neutron shells are fully occupied and the next highest
shells, 1d
5/2
, are empty. Just as in atomic physics the angular momenta of
the particles in a full shell add up to zero and the overall parity is positive.
The ground state of
16
O has then the quantum numbers J
P
=0
+
. Since the
gap between the 1p
1/2
and 1d
5/2
energy shells is quite large (about 10 MeV)
there are no easily reachable excitation levels.
The two nuclei with A= 17 both have a single extra nucleon in the 1d
5/2
shell. The spin and parity of the nucleus are completely fixed by this one
nucleon. The 2s
1/2
shell happens to be just a little above the 1d
5/2
shell
and as small an energy as 0.5 MeV suffices to excite this single nucleon to
7.30
7.16
6.32
5.30
5.27
6.86
6.79
6.18
5.24
5.18
7.12
6.92
6.13
6.05
1-
2+
3-
0+
3
/
2+
5
/
2+
3
/
2-
1
/
2+
5
/
2+
5
/
2+
3
/
2+
3
/
2-
5
/
2+
1
/
2+
5.70
5.38
5.08
4.55
3.85
3.06
7
/
2-
3
/
2-
3
/
2+
1
/
2-
5
/
2-
3
/
2-
1
/
2+
5
/
2+
0.87
1
/
2-
1
/
2-
0+
0.50
5
/
2+
1
/
2+
5.67
5.49
5.00
4.64
3.86
3.10
7
/
2-
3
/
2-
3
/
2+
1
/
2-
5
/
2-
3
/
2-
15
N
8
7
15
O
7
8
16
O
8
8
17
O
9
8
17
F
8
9
Fig. 17.7. Energy levels of the
15
N,
15
O,
16
O,
17
Oand
17
F nuclei. The vertical axis
corresponds to the excitation energy of the states with the various ground states
all being set equal, i.e., the differences between the binding energies of these nuclei
are not shown.

17.3 The Shell Model 259
the next shell. The nuclear quantum numbers change from 5/2
+
to 1/2
+
in
this transition. The excited nucleon later decays, through photon emission,
into the lowest possible state. Just as we talk of valence electrons in atomic
physics, so these nucleons that jump between shells are known as valence
nucleons. The 1d
3/2
shell is about 5 MeV above the 1d
5/2
one and this an
amount of energy is required to reach this state.
The A = 15 ground states lack one nucleon in the 1p
1/2
shell. One speaks
of a hole and uses the notation 1p
−1
1/2
. The quantum numbers of the hole
are those of the nucleus. Thus the ground states of these nuclei have the
quantum numbers J
P
=1/2
−
. If a nucleon from the 1p
3/2
shell is excited
into the vacant state in the 1p
1/2
, and in some sense fills the hole, a hole is
then created in the 1p
3/2
shell. The new nuclear state then has the quantum
numbers J
P
=3/2
−
.
Magnetic moments from the shell model. If in the shell model we
associate spin and orbital angular momentum to each individual nucleon, then
we can understand the magnetic moment of the nucleus from the sum over
the nucleonic magnetic moments based upon their spin and orbital angular
momenta:
µ
nucleus
= µ
N
·
1
A
i=1
{
i
g
+ s
i
g
s
} . (17.28)
Note that
g
=
1 for protons
0 for neutrons
(17.29)
and (from 6.7 etc.):
g
s
=
+5.58 for protons
−3.83 for neutrons.
(17.30)
Recall our five nuclei with mass numbers from 15 to 17. The magnetic
moment of
16
O is zero, which makes perfect sense since in a full shell the
spins and angular momenta add up to zero and so the magnetic moment
must vanish.
We are in a position to make quantitative predictions for one particle
and one hole states. We first assume that the nuclear magnetic moment is
determined by that of the single nucleon or hole
µ
nucleus
=
1
ψ
nucleus
|g
+ g
s
s|ψ
nucleus
·µ
N
. (17.31)
The Wigner-Eckart theorem tells us that the expectation value of every vector
quantity is equal to its projection onto the total angular momentum, which
here means the nuclear spin J:
µ
nucleus
= g
nucleus
· µ
N
·
J
(17.32)
260 17 The Structure of Nuclei
where
g
nucleus
=
JM
J
|g
J + g
s
sJ|JM
J
JM
J
|J
2
|JM
J
. (17.33)
Since the nuclear spin J in our model is nothing but the total angular mo-
mentum of our single nucleon j and we have
2j = j
2
+
2
− s
2
2sj = j
2
+ s
2
−
2
(17.34)
we see that
g
nucleus
=
g
{j(j +1)+(+1) − s(s+1)} + g
s
{j(j +1)+s(s+1) − (+1)}
2j(j +1)
.
(17.35)
The magnetic moment of the nucleus is defined as the value measured
when the nuclear spin is maximally aligned, i.e., |M
J
| = J. The expectation
value of J is then J and one finds
|µ
nucleus
|
µ
N
= g
nucleus
· J =
g
±
g
s
− g
2 +1
· J for J = j = ±
1
2
.
(17.36)
There are many different ways to measure nuclear magnetic moments,
e.g., in nuclear magnetic spin resonance or from optical hyperfine structure
investigations [Ko56]. The experimental values [Le78] of the magnetic mo-
ments can be compared with the predictions of (17.36).
µ/µ
N
Nucleus State J
P
Model Expt.
15
Np-1p
−1
1/2
1/2
−
−0.264 −0.283
15
On-1p
−1
1/2
1/2
−
+0.638 +0.719
17
On-1d
5/2
5/2
+
−1.913 −1.894
17
Fp-1d
5/2
5/2
+
+4.722 +4.793
The magnetic moments of the A =15 and A =17nucleican,wesee,
be understood in a single particle picture. We should now perhaps admit to
having chosen the example with the best agreement between the model and
experiment: firstly these nuclei are, up to one single nucleon or hole, doubly
magic and secondly they have a relatively small nucleon number which means
that effects such as polarisation of the remainder by the valence nucleon are
relatively tiny.
We assume for nuclei with odd mass number whose incomplete shells con-
tain more than one nucleon or hole that the total nucleon magnetic moment
is due to the one unpaired nucleon [Sc37]. The model then roughly repro-
duces the experimental trends, but disagreements as big as ±1µ
N
and larger
appear for many nuclei. The magnetic moment is, generally speaking, smaller
than expected. The polarisation of the rest of the nucleus from the unpaired
nucleon tends to explain this [Ar54].
17.4 Deformed Nuclei 261
17.4 Deformed Nuclei
The shell model approximation which assumes that nuclei are spherically
symmetric objects – plus, of course, the additional spin-orbit interaction – is
only good for those nuclei which are close to having doubly magic full shells.
For nuclei with half full shells this is not the case. In such circumstances the
nuclei are deformed and the potential is no longer spherically symmetric.
It was already realised in the 1930’s, from atomic spectroscopy, that nuclei
are not necessarily always spherical [Ca35, Sc35]. Deviations in the fine struc-
ture of the spectra hinted at a non-vanishing electrical quadrupole moment,
i.e. that the charge distribution of the nuclei was not spherically symmetric.
Quadrupole moments. The charge distribution in the nucleus is described
in terms of electric multipole moments. Since the odd moments (e.g., the
dipole and octupole) have to vanish because of parity conservation, the elec-
tric quadrupole moment is the primary measure of in how far the charge
distribution, and hence the nucleus, deviate from being spherical.
The classical definition of a quadrupole moment is
Q =
3z
2
− x
2
(x)d
3
x. (17.37)
An ellipsoid of diameter 2a in the z direction and diameter 2b in the other
two directions (Fig. 3.9), with constant charge density (x) has the following
quadrupole moment:
Q =
2
5
Ze
a
2
− b
2
. (17.38)
For small deviations from spherical symmetry, it is usual to introduce a
measure for the deformation. If the average radius is R =(ab
2
)
1/3
and the
difference is ∆R = a −b then the quadrupole moment is proportional to the
deformation parameter
1
δ =
∆R
R
(17.39)
and we find
Q =
4
5
ZeR
2
δ. (17.40)
Since the absolute value of a quadrupole moment depends upon the charge
and size of the nucleus concerned, we now introduce the concept of the reduced
quadrupole moment to facilitate the comparison of the deformations of nuclei
with different mass numbers. This is a dimensionless quantity and is defined
as the quadrupole moment divided by the charge Ze and the square of the
average radius R :
1
We skip over the exact definition of the deformation parameter here; (17.38) and
(17.39) are approximations for small deformations.
