Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

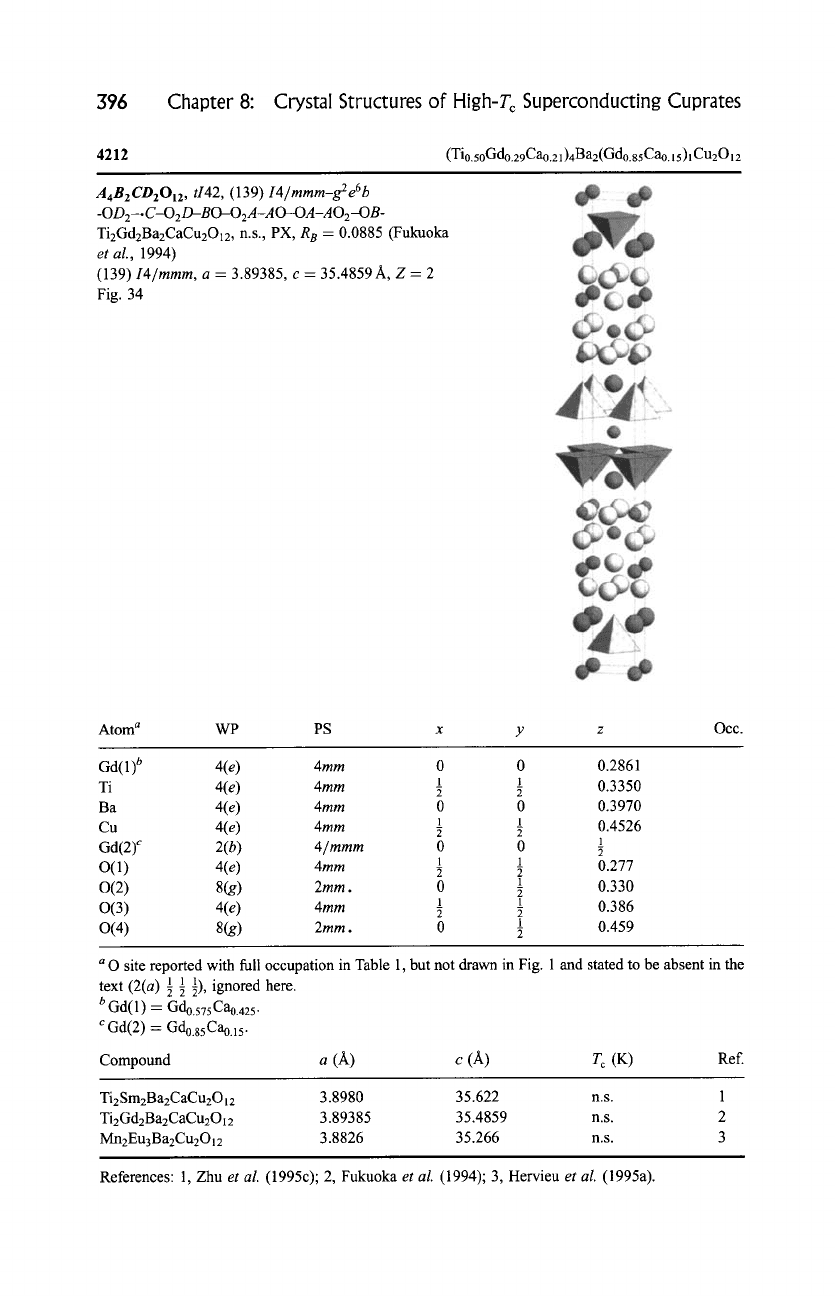
396
4212
Chapter
8:
Crystal Structures of High-Tc Superconducting Cuprates
(Tio.50Gdo.29Cao.21 )4Ba2(
Gdo.85Cao.
15)1Cu2 O12
A4B2CD2012 , ti42,
(139)
I4/mmm-gZe6b
-OD2-. C-O2 D-BO-O2A-A O-OA-A O2-OB -
TizGdzBazCaCu2012, n.s., PX, R B = 0.0885 (Fukuoka
et al.,
1994)
(139)
I4/mmm,
a = 3.89385, c = 35.4859 A, Z = 2
Fig. 34
Atom a WP PS x y z
Occ.
Gd(1)b 4(e)
4mm
0 0 0.2861
1 1 0.3350
Ti 4(e)
4mm ~
Ba 4(e)
4mm
0 0 0.3970
1 1 0.4526
Cu 4(e)
4mm ~
1
Gd(2) c 2(b)
4/mmm 0 0
1 1 0.277
O(1) 4(e)
4mm ~
1 0.330
0(2) 8(g)
2mm . 0
1 1
0.386
0(3) 4(e)
4mm ~
1 0.459
0(4) 8(g)
2mm . 0
a
O site reported with full occupation in Table 1, but not drawn in Fig. 1 and stated to be absent in the
text (2(a) 89 89 1), ignored here.
b Gd(1) = Gd0.575 Ca0.425 .
c Gd(2) = Gd0.85Ca0.15.
Compound a (A) c (A) T c (K) Ref.
Ti2Sm2Ba2CaCu2012 3.8980 35.622 n.s. 1
Ti2Gd2Ba2CaCu2012 3.89385 35.4859 n.s. 2
Mn2Eu3Ba2Cu2012 3.8826 35.266 n.s. 3
References: 1, Zhu
et al.
(1995c); 2, Fukuoka
et al.
(1994); 3, Hervieu
et al.
(1995a).
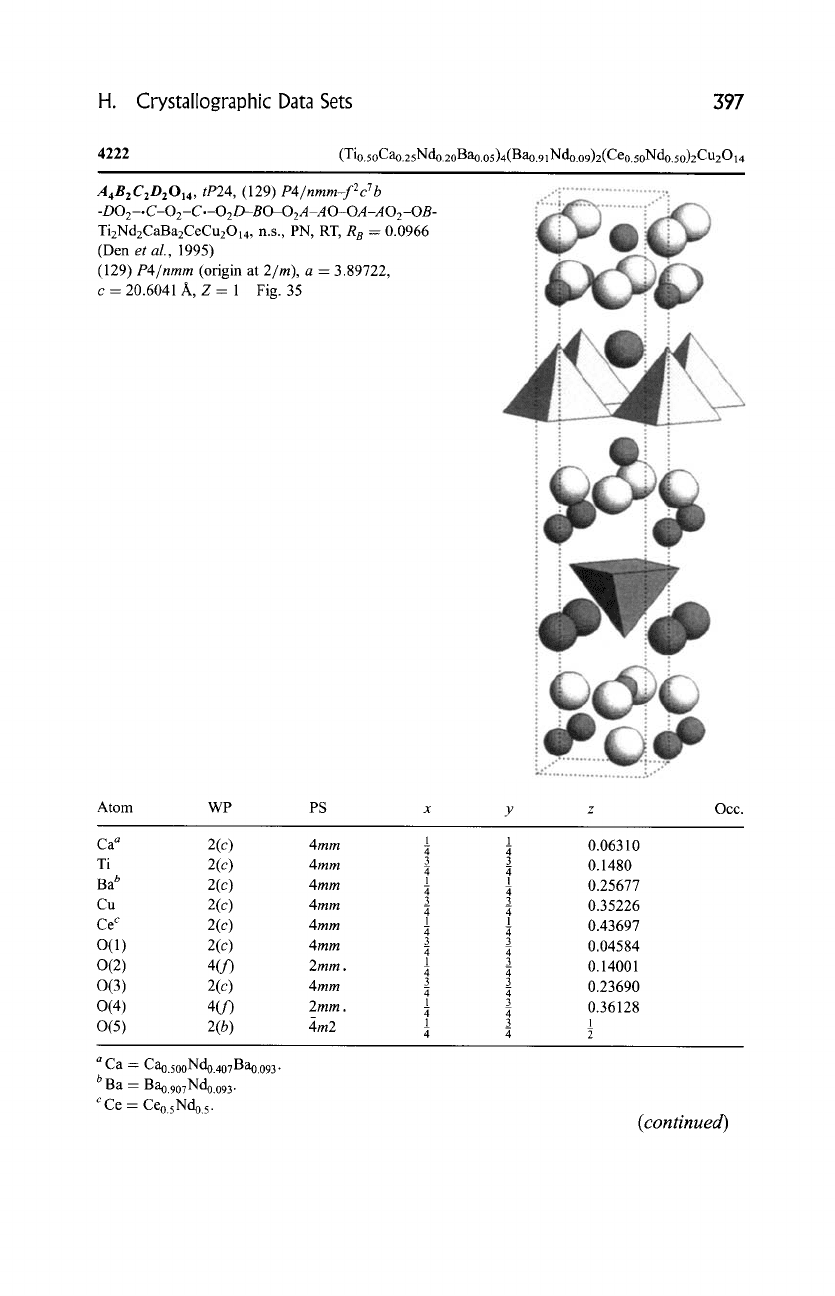
H. Crystallographic Data Sets 397
4222
(Tio.50Cao.25Ndo.20Bao.05)4(Bao.9 ]Ndo.09)2(Ceo.50Ndo.50)2Cu2014
A4B2C2D2014,
tP24, (129)
P4/nmm-f2c7b
-DO 2-. C-02--C.-O 2D-BO-O2A-AO-OA-AO2-OB-
TizNdzCaBazCeCu2014, n.s., PN, RT, R B = 0.0966
(Den
et al.,
1995)
(129)
P4/nmm
(origin at
2/m),
a = 3.89722,
c-20.6041A, Z=l Fig. 35
Atom WP PS x
Occ.
1 1
Ca a 2(c)
4mm -~ -4
Ti
3 3
~.~c : 4mm -4 -4
1 1
Bab 2(c)
4mm -4 -4
Cu
ot''~
3 3
:.a,c :
4 m m -4 -4
1 1
Ce c 2(c)
4mm -4 -4
3 3
O(1) 2(c)
4mm -4 -4
1 3
0(2) 4(f)
2mm . -4 -4
3 3
0(3) 2(c)
4mm -~ -4
1 3
0(4) 4(f)
2mm . -4 -4
1 3
0(5) 2(b) 4m2 -4
0.06310
0.1480
0.25677
0.35226
0.43697
0.04584
0.14001
0.23690
0.36128
1
2
a Ca - Cao.5ooNdo.4o7Bao.o93.
b Ba -- Bao.9o7Ndo.o93.
c Ce = Ceo.sNdo. 5.
(continued)
398 Chapter
8:
Compound
Crystal Structures of High-T~ Superconducting Cuprates
a (A) c (A) T~ (K) Ref.
Ti2NdzCaBazCeCu2014
Ti2 Sm2Ca 1.1BazCeo.9Cu2013.90
TizGdz.25CaBazCeo.vsCu2014
3.89722 20.6041 n.s. 1
3.8884 20.488 n.s. 2
3.8839 20.463 n.s. 3
References: 1, Den
et aL (1995);
2, Zhu
et al. (1995c);
3, Li
et al.
(1995a).
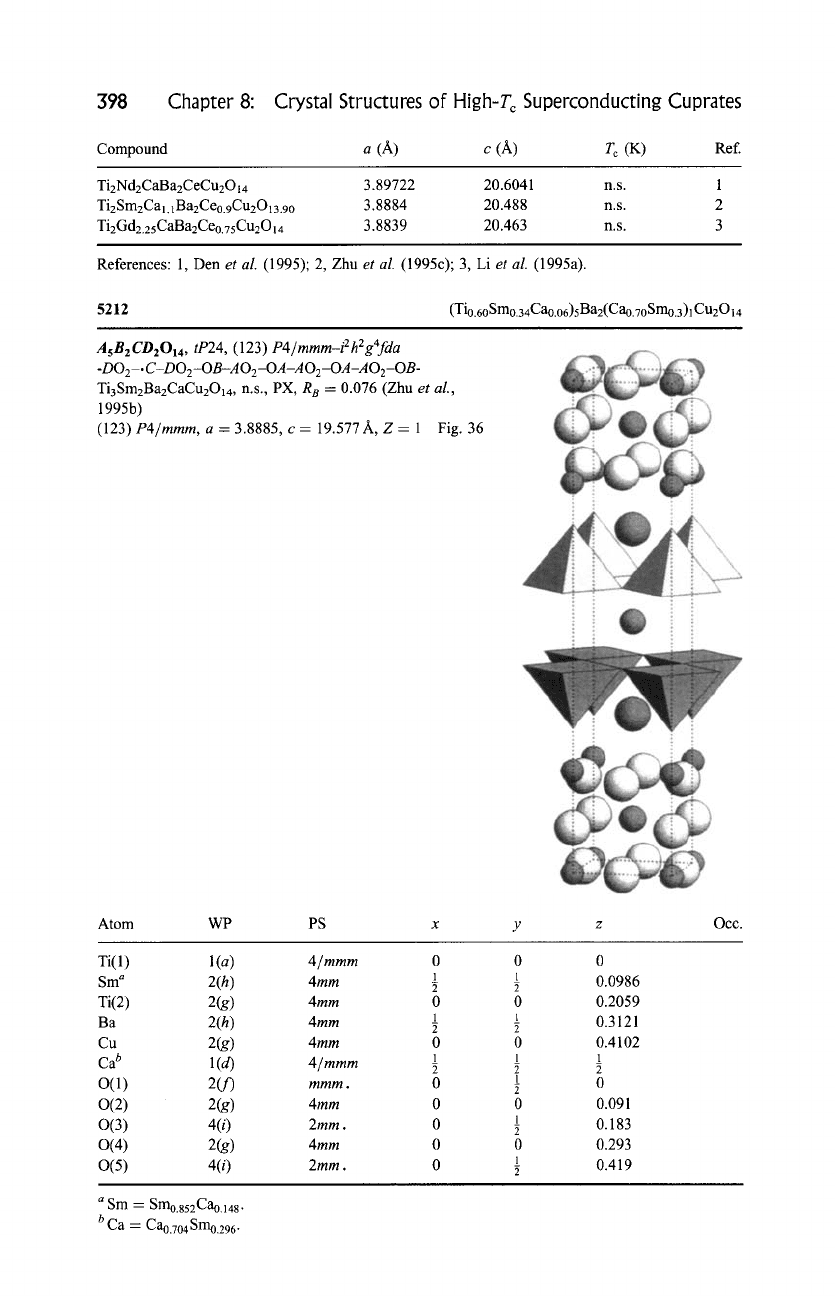
5212
(Tio.60 Smo.34Cao.06)5B a2(Cao.70 Smo.3)l Cu2014
A5B2CD2014 ,
tP24, (123)
P4/mmm-iZh2g4fda
-DO2-.
C-DO2-OB-A
O2-OA -A O2-OA-A 02-0B-
Ti3SmzBazCaCu2014, n.s., PX, R e -- 0.076 (Zhu
et aL,
1995b)
(123)
P4/mmm,
a = 3.8885, c = 19.577 A, Z = 1 Fig. 36
Atom WP PS x
Occ.
Ti(1) l(a)
4/mmm 0
1
Sm a 2(h)
4mm
Ti(2) 2(g)
4mm 0
1
Ba 2(h)
4mm
Cu 2(g)
4mm 0
1
Ca b l(d)
4/mmm
O(1) 2(f)
mmm. 0
0(2) 2(g)
4mm 0
0(3) 4(i)
2mm . 0
0(4) 2(g)
4mm 0
0(5) 4(i)
2mm . 0
0
0.0986
0.2059
0.3121
0.4102
1
2
0
0.091
0.183
0.293
0.419
a
Sm = Smo.852Cao.148.
b Ca = Cao.704
Smo.296.
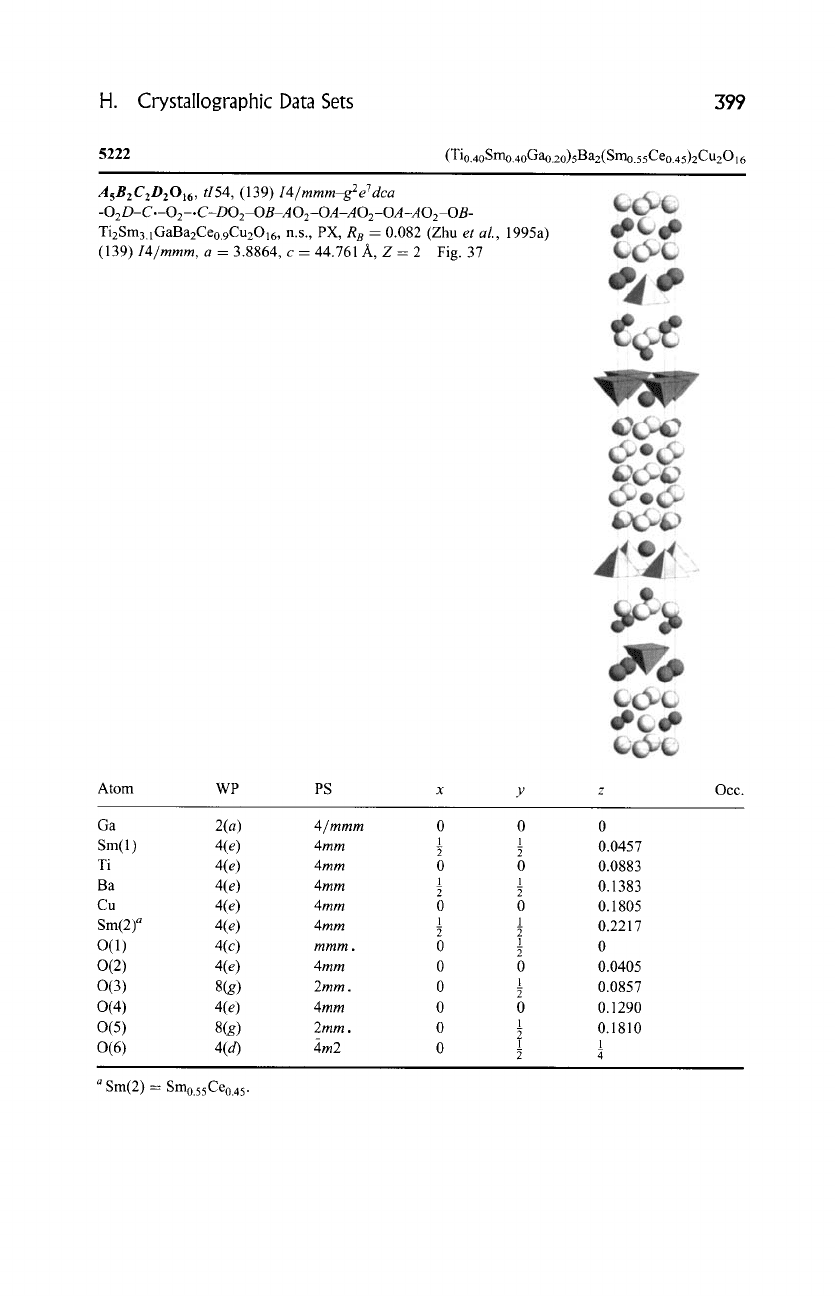
H. Crystallographic Data Sets
399
5222
(Tio.40 Smo.40Gao.20)5 B a2 (Smo.55Ceo.45)2Cu2016
AsBzCzDzO16, ti54, (139) I4/mmm-g2e7dca
-0 2D-C.-O
2-.C-DO 2-OB-AO
2-OA-AO 2-OA-AO 2-OB-
TizSm3.1GaBazCeo.9Cu2016, n.s., PX, R B = 0.082 (Zhu et al., 1995a)
(139)
I4/mmm, a = 3.8864, c = 44.761 A, Z = 2 Fig. 37
Atom WP PS x y
Occ.
Ga 2(a)
4/mmm 0 0
1 1
Sm(1) 4(e) 4mm ~
Ti 4(e) 4ram 0 0
Ba
A [ "~
1 1
-,~ e ~ 4mm ~
Cu 4(e) 4mm 0 0
1 1
Sm(2) a 4(e) 4mm ~
1
O(1) 4(c) mmm . 0
0(2) 4(e) 4mm 0 0
1
0(3) 8(g) 2mm . 0
0(4) 4(e) 4mm 0 0
1
0(5) 8(g) 2mm . 0
1
0(6) 4(d) ,~m2 0
0
0.0457
0.0883
0.1383
0.1805
0.2217
0
0.0405
0.0857
0.1290
0.1810
1
_
4
a Sm(2) - Smo.55Ceo.45.
400
Chapter 8: Crystal Structures of High-Tr Superconducting Cuprates
1. Other Basic Structures
3223, 3234, 3245: Superconducting oxycarbonates with two (C,Cu)O
layers were reported by Kawashima
et al.
(1994b,c). In the proposed structural
models, the two (C,Cu)O layers are separated by a BaO layer that in the
present classification is also considered as an
additional
layer. Superconducting
transition temperatures of 91, 113, and 110 K were measured for the nominal
compositions CBa2.sCa2.sCu4Oll.6 (3223), Co.6Ba2Ca4Cus.5013.2 (3234), and
Co.sBa2Ca4Cus.5013 (3245), respectively. An iodine-intercalated (Bi,I)-3223
compound, (Bi2I)Sr2Ca2Cu3Oy (a-5.40, c-22.01A, T c - 100K) was
reported by Xiang
et al.
(1991).
3232, 3242, 3252: A series of nonsuperconducting cuprates with the
general formula
(Pb2Cu)Sra(YI_xCex)mCu206+2m+6,
where m = 3 (3232), 4
(3242) and 5 (3252), was described by Tokiwa
et al.
(1991).
0011 (Sro.9Lao.1)lCUl02
CDO2,
tP4, (123) P4/mmm-fda
-DO2-.C-
Sro.9Lao.lCuO2, a T c -- 42K, PN, RT,
Rwp
= 0.160
(Jorgensen
et al.,
1993)
(123)
P4/mmm,
a = 3.95068, c = 3.40902A, Z = 1
Fig. 38
Atom WP PS
Occ.
Cu l(a)
4/mmm
Sr b l(d)
4/mmm
0 2(f) mmm.
a
Prepared at 5 GPa.
b Sr = Sr0.9Lao.1; full occupation confirmed.
(continued)
H. Crystallographic Data Sets
401
Compound a (A) c (A) T c (K) Ref.
Sro.8Bao.zCuO2 a . ..... 90 1
Sro.63Cao.27CuO2 a
3.902 3.350 110 2
Sro. 14Cao.86CuO 2
3.8611 3.1995 n.s. 3
Sro.6375Cao. 2125Ndo. 15CUO2
b 3.9196 3.3505 34 4
Sro.9Lao.lCuO2 c 3.947 d 3.412 d 43 5
Sro.85Pro.
15CUO2 e
3.942 3.393 39 6
Sro.84Ndo. 16CUO2 e 3.944d 3.383 d 40 6
Sro.92Smo.o8CuO2 r 3.942 d 3.399 d 44 7
Sro.92Gdo.osCuO2 c 3.941 d 3.393 d 44 7
CaCuO2 f 3.8556 3.1805 n.s. g 8, 9
a
Prepared at 6 GPa; nominal composition.
b Prepared at 2 GPa.
c Prepared at 3 GPa; nominal composition.
d Value taken from figure.
e
Prepared at 2.5 GPa; nominal composition.
fPrepared at 1 GPa; cation content
Cao.98_o.99CuO 2
from microprobe analysis.
g Onset of diamagnetic signal (79 K) not confirmed by resistivity measurements.
References: 1, Takano
et al.
(1991); 2, Azuma
et al.
(1992); 3, Siegrist
et al.
(1988b); 4, Alonso and
Lapertot (1995); 5, Er
et al.
(1991); 6, Smith
et al.
(1991); 7, Ikeda
et al.
(1993); 8, Karpinski
et al.
(1994); 9, Karpinski (1997).
0011 compounds are usually referred to as infinite-layer compounds. The
first crystal structure was reported for the composition
Cao.86Sro.14CuO2,
the
refinement being carried out in space group
P4/mmm
(Siegrist
et al.,
1988b).
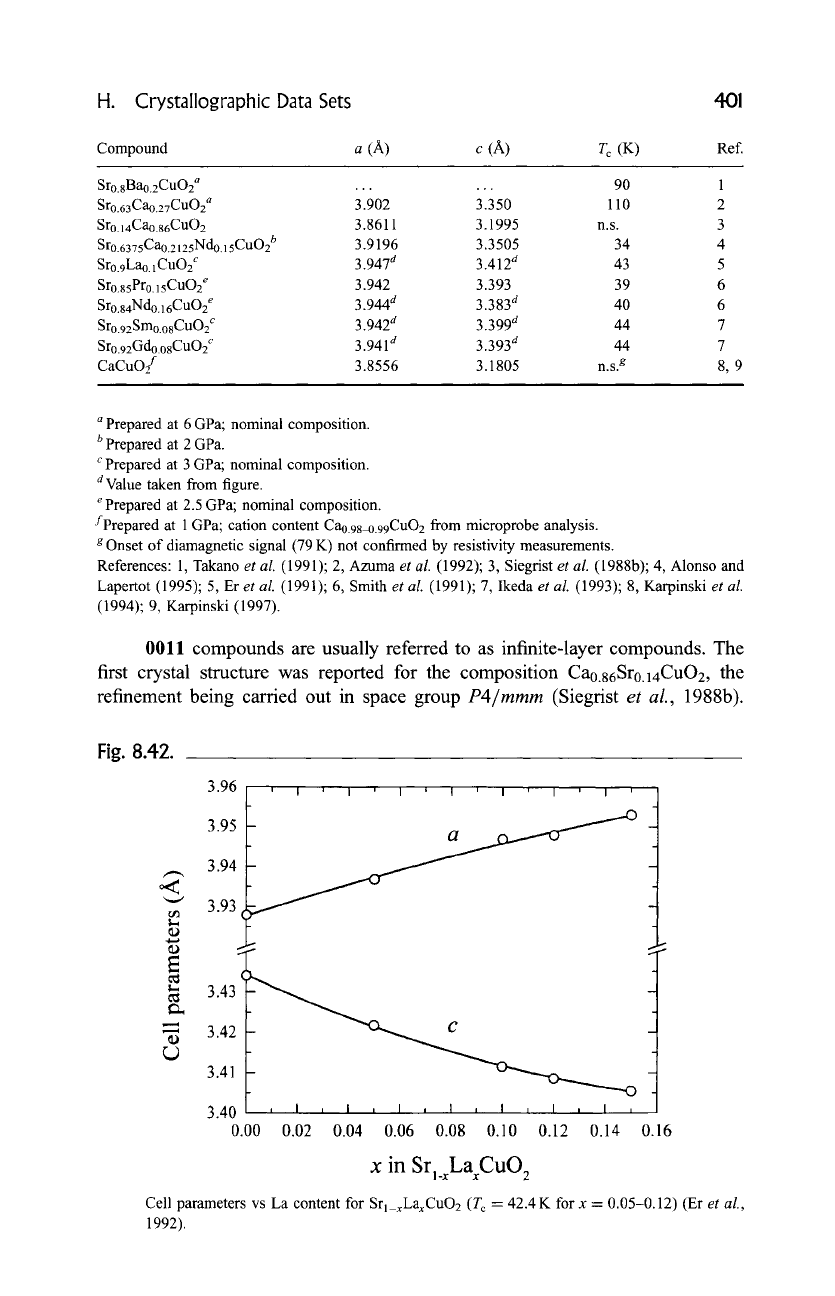
Fig. 8.42.
3.96 , , , i , i , i , ~ , ~ , i
3.95
3.94
'~" 3.93
~ 3.43
"- 3.42
3.41
c
"'---O
, ..I i I , I , I , I i. I , [ ,
0.02 0.04 0.06 0.08 0.10 0.12 0.14
3.40
0.00 0.16
x
in Srl_xLaCuO 2
Cell parameters vs La content for Srl_xLaxCuO2 (T c = 42.4 K for x - 0.05-0.12) (Er
et al.,
1992).
402
Chapter 8: Crystal Structures of High-T~ Superconducting Cuprates
Superconductivity was observed for the electron-doped compounds
Sro.84Ndo.16CuO2 and Sro.8sPro.15CuO2 (Smith
et al.,
1991). For the structural
refinement on Sro.9Lao.lCuO2 (compound first prepared by Er
et al.
(1991), a
model with oxygen atoms in the
separating
layer (l(b)0 0 89 was tested and
rejected (Jorgensen
et al.,
1993). The cell parameters of Srl_xLaxCuO2 vs the La
content are presented in Fig. 8.42. Srl_xCaxCuO2 is not superconducting;
however, superconductivity was claimed for hole-doped (Sro.7Cao.3)l_xCuO2
(Azuma
et al.,
1992; Hiroi
et al.,
1993a), as well as for its structural analogue
in the Sr-Ba-Cu-O system (Takano
et al.,
1991). Superconductivity observed for
samples of nominal composition (Sro.7Cao.3)l.lCuO2 (prepared at 5.7 GPa) was
stated to be due to the presence of
Sr3Cu205+ 6
(0212) and
Sr4Cu307+ 6
(0223)
impurities (Shaked
et al.,
1995).
0021
(Ndo.92Ceo.08)2CUl O3.92
C2/)O4, tI14, (139)
I4/mmm-edca
-OO2-.C-O2-C.-
Ndl.845Ceo.155CuO3.922, T c ----
20 K, PN, RT, R 8 = 0.0247
(Izumi
et aL,
1989b)
(139)
I4/mmm,
a = 3.9469, c = 12.0776 A, a Z = 2 Fig. 39
Atom WP PS x y z
Occ.
Cu 2(a)
4/mmm 0 0 0
1 1 0.1475
Nd b 4(e)
4mm ~
1 0
O(1) 4(c)
mmm. 0
1 1
0(2) 4(d) 4m2 0 ~
0.992
0.969
a
Additional reflections indicate a small fraction of superstructure (2~/2a, 2~/2a, c).
bNd = Ndo.9225Ce0.0775.
(continued)
H. Crystallographic Data Sets 403
Compound a (A) c (A) T c (K) Ref.
Pr1.85Ceo.
15CUO4-6
3.9620 12.151 22 a 1, 2
Prl. 85Tho. 15CUO4- 6 b ...... 23 3
Ndl.85Ceo. 15CUO4_ 6
3.9450 12.078 24 1, 2
Ndl.85Tho.15CuO4_6 b ...... 20 4
Nd2CuO3.6Fo.4 c 3.961 12.13 21 d 5
Sml.85Ceo.15CuO4_6 3.9191 11.904
18 a
1, 2
Eul.85Ceo.15CuO4_ 6 3.9077 11.8409 13 a 1, 2
Gdl.ssCeo.15CuO4_ 6 3.9010 11.832 n.s. 1, 2
Tml.s3Cao.17CuO3.99 e 3.831 11.642 30 6
a
Value taken from figure.
b Nominal composition.
C Nominal composition, oxygen and fluorine content Nd2CuO3.74Fo.28 from chemical analysis.
d From resistivity measurements (onset), T c = 27 K for Nd2CuO3.7Fo.3 (nominal composition).
e Prepared at 6 GPa.
References: 1, Uzumaki
et al. (1991);
2, Xue
et al. (1990);
3, Markert
et al.
(1989); 4, Markert and
Maple (1989); 5, James
et al.
(1989); 6, Zhu
et al.
(1994).
0021 compounds are usually referred to as T' phases. R2CuO 4 compounds
with R = Pr, Nd, Sm, and Eu crystallize with a Nd2CuO4-type structure
(I4/mmm)
(Mfiller-Buschbaum, 1975). The structure of
Gd2CuO4
shows orthor-
hombic distortions
(Acam,
a = b = 5.500, c = 11.871 A), caused by the rotation
of the CuO4 squares (Braden
et al.,
1994; Galez and Collin, 1990). Different
superstructures (~/2a, ~/2a, r 2~/2a, ~/2a, c, and 2~/2a, 2~/2a, 2r referring to
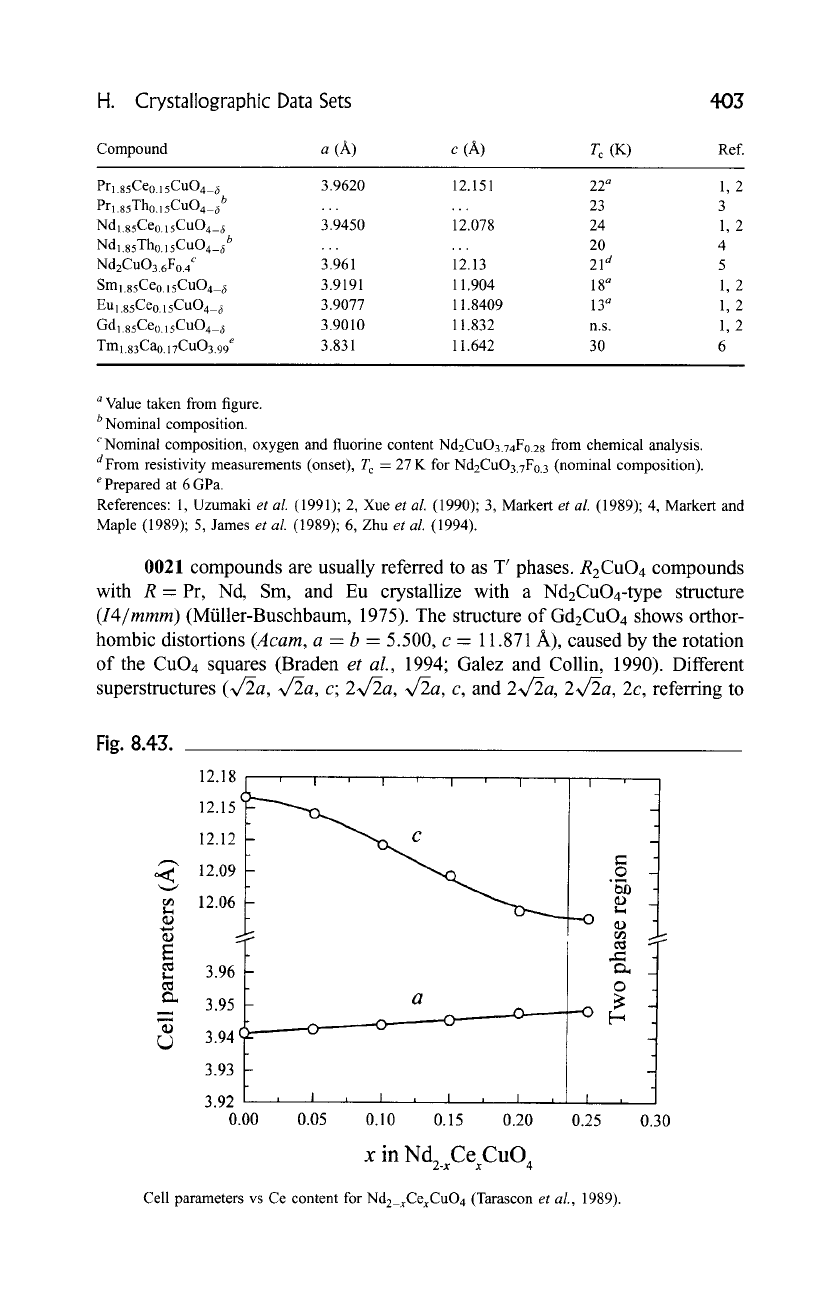
Fig. 8.43.
,,,,a
E
12.18 , i ,
12.15 (-~,~,0~
- ~
12.12 -
12.09 -
12.06 -
2
3.96
3.95
3.94
3.93
3.92 , I i
0.00 0.05
III
"--v-..._
a
I
O
~
ea)
---o
ra~
O
I , , I , I , I ,
0.10 0.15 0.20 0.25
x in Nd2_ CexCuO4
Cell parameters vs Ce content for Nd2_xCexCuO 4 (Tarascon
et al.,
1989).
0.30
404
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
the tetragonal cell) were proposed by Bordet et al. (1992) for compounds with
R = Y, Tb, Dy, Ho, Er, and Tm, synthesized at high pressure, first reported by
Okada et aL (1990). Superconductivity could be induced by partly substituting Pr,
Nd, Sm, or Eu by Ce or Th and annealing under reducing conditions (Tokura et
aL, 1989b; Markert et aL, 1989). An O site with occupancy 0.06 was refined in
the separating layers of oxidized Nd2_xCexCuO 4 (Schultz et al., 1996). The cell
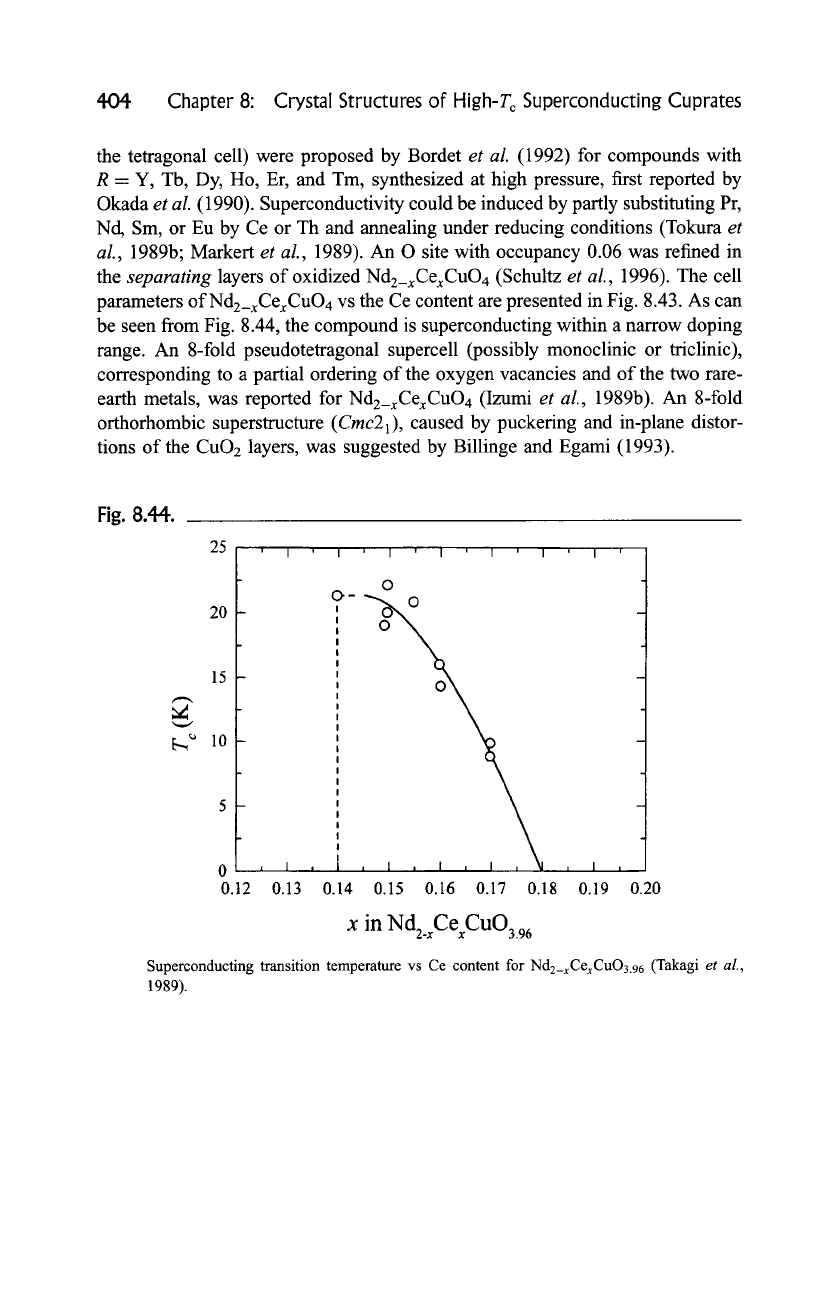
parameters ofNd2_xCexCuO4 vs the Ce content are presented in Fig. 8.43. As can
be seen from Fig. 8.44, the compound is superconducting within a narrow doping
range. An 8-fold pseudotetragonal supercell (possibly monoclinic or triclinic),
corresponding to a partial ordering of the oxygen vacancies and of the two rare-
earth metals, was reported for Nd2_xCexCuO 4 (Izumi et aL, 1989b). An 8-fold
orthorhombic superstructure (Cmc21), caused by puckering and in-plane distor-
tions of the CuO2 layers, was suggested by Billinge and Egami (1993).
Fig. 8.44.
25 ' ,
20
15
~,,~ 10
5
0
0.12
I ' I ' I "' .... ] ' l ' I ' I '
1 I ,
0.13 0.19 0.20
o
9-
I
i
I
i
I
I
I
I
I
I
I
I
I
I
I
,,
!
I
I
I
I
i,. .I , I , I , I , ~I . j
i
0.14 0.15 0.16 0.17 0118
x in
Nd2.xCexCuO3.96
Superconducting transition temperature vs Ce content for
Nd2_xCexCuO3.96
(Takagi
et al.,
1989).
H. Crystallographic Data Sets 40S
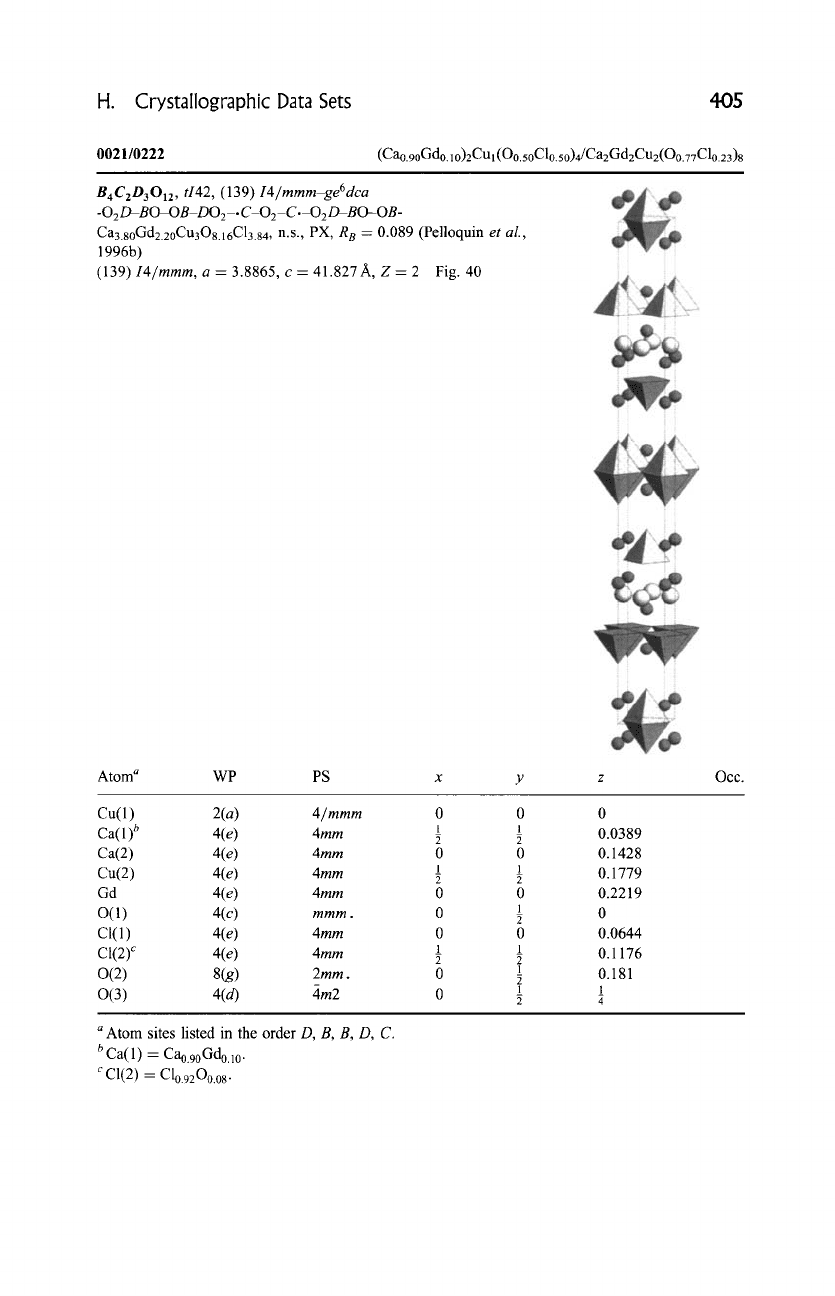
0021/0222
(Cao.90Gdo. 1 o)2CUl (O0.50Clo.50)4/Ca2Gd2Cu2(O0.77Clo.23)8
B4C2D3012, ti42, (139)
I4/mmm-ge6dca
-02D-BO-OB-DO 2-.
C-0 2-C.-O 2D-BO-OB-
Ca3.goGd2.2oCu308.16C13.84, n.s., PX, R 8 = 0.089 (Pelloquin
et al.,
1996b)
(139)
I4/mmm,
a = 3.8865, c = 41.827 A, Z = 2 Fig. 40
Atom a WP PS x y
Occ.
Cu(1) 2(a) 4/mmm
0 0
1 1
Ca(l) b 4(e)
4mm ~
Ca(2) 4(e)
4ram 0 0
1 1
Cu(2) 4(e)
4mm ~
Gd 4(e)
4mm 0 0
1
O(1) 4(c)
mmm. 0
CI(1) 4(e)
4mm 0 0
1 1
C1(2) c 4(e)
4mm ~
1
0(2) 8(g)
2mm . 0
1
0(3) 4(d) 4m2 0
0
0.0389
0.1428
0.1779
0.2219
0
0.0644
0.1176
0.181
1
_
4
a
Atom sites listed in the order D, B, B, D, C.
b Ca(l) -- Cao.9oGdo.lo.
c C1(2) = Clo.92Oo.o8.
