Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.


366
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
refinement of the average structure of
Co.32Ba2Ca3Cu4.68Oll.06, two
pairs of O
sites were considered in the
additional
and
bridging
layers, corresponding to an
occupation of the cation site in the
additional
layer by C and Cu, respectively
(Shimakawa
et al.,
1994a). A superconducting, carbon-free 1234 compound
(T c = 117.1 K) was reported for the nominal composition
Agl_xBa2Ca3Cu4+xOll_ • (Ihara
et al.,
1994a). The average structure was
described in space group
P4/mmm
(a= 3.8635, r 18.ILIA), but body-
centered tetragonal superstructures (2a, 2c) with ordered arrangements of
copper and silver atoms were suggested for x = 0.25 and x = 0.75. Wu
et al.
(1994b) and Matsuhata
et al.
(1994) stated that the amount of Ag in the
compound was negligible and explained the superstructure by the ordering of
vacancies on the Cu site in the
additional
layer. Cu-1234 compounds, with
structural features similar to those described earlier, were reported by Jin
et al.
(1994), Wu
et al.
(1994a), Ihara
et al.
(1994b), and Alario-Franco
et al.
(1994b).
In the last article, the superstructure was shown to be orthorhombic (2a, a, 2c),
but the same authors later detected small amounts of carbon in their samples
(Alafio-Franco
et al.,
1994a). In the refinement of the average structure of
Ba2Ca3Cu4.6Olo.8, not only the Cu site, but also the O sites in the
additional
and
bridging
layers were found to be partly vacant (Akimoto
et al.,
1995). The
orthorhombic space group
A2mm
(or monoclinic
Am
or
A2/m)
was proposed for
the description of a 4-fold superstructure by Matsuhata
et al.
(1995).
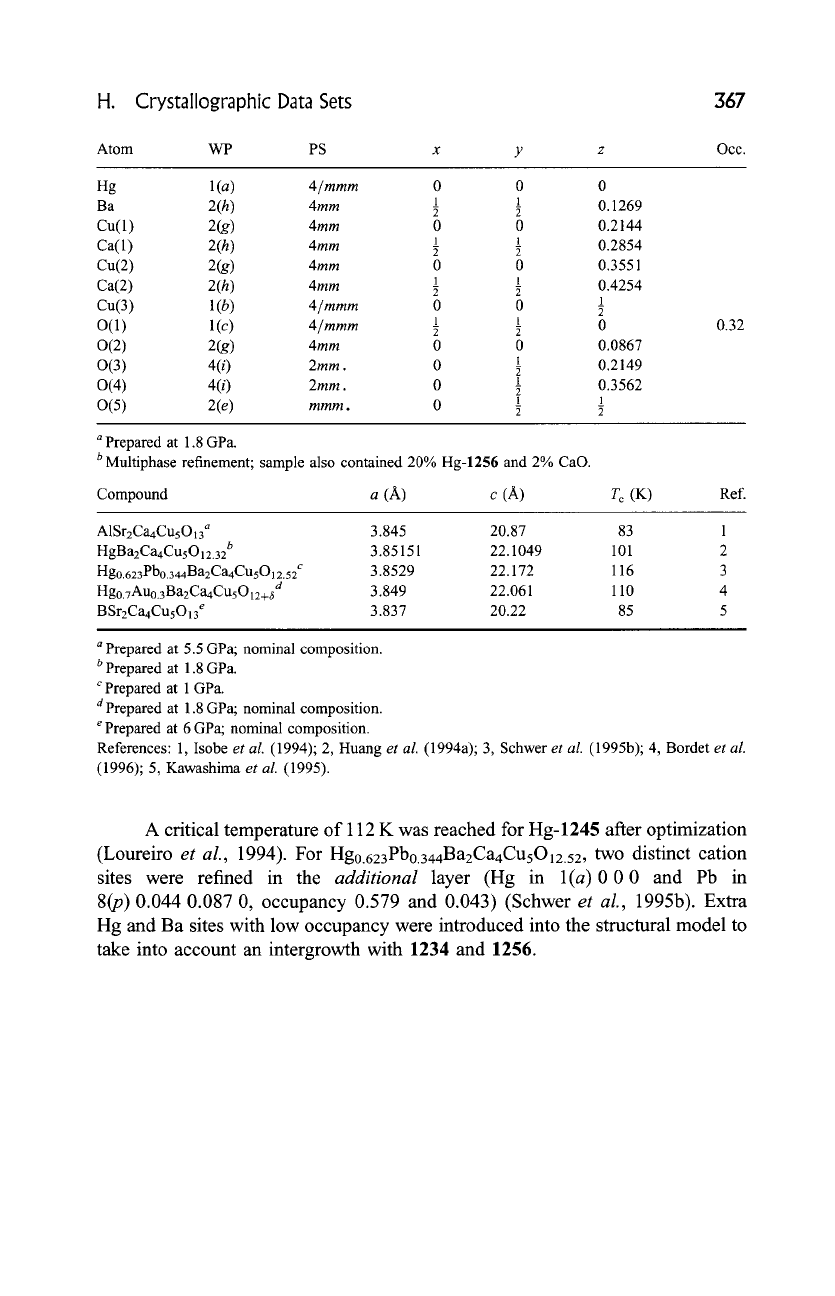
1245 HglBa2Ca4Cu5012.32
AB2C4D5012+8 ,
tP24, (123)
P4/mmm-ieh3 g 3 e(c)ba
-DO2-.
C-DO 2-" C-DO2-" C-DO2-. C-DO2-OB-A .-O B-
HgBa2Ca4CusO12.32, a T c = 101 K, PN, RT,
Rwp
= 0.0640 b
(Huang
et al.,
1994a)
(123)
P4/mmm,
a = 3.85151, c = 22.1049 A, Z = 1
Fig. 20
H. Crystallographic Data Sets
367
Atom WP PS x y z
Occ.
Hg l(a)
4/mmm 0 0 0
1 1
0.1269
Ba 2(h)
4mm ~ -~
Cu(1) 2(g)
4mm
0 0 0.2144
1 1
0.2854
Ca(l) 2(h)
4mm ~
Cu(2) 2(g)
4mm
0 0 0.3551
1 1
0.4254
Ca(2) 2(h)
4mm ~
1
Cu(3) l(b)
4/mmm 0 0
1 1 0
O(1) l(c)
4/mmm ~
0(2) 2(g)
4mm
0 0 0.0867
1
0.2149
0(3) 4(i)
2mm . 0
1
0.3562
0(4) 4(i)
2mm . 0
1 1
0(5) 2(e)
mmm . 0 ~
0.32
a
Prepared at 1.8 GPa.
b Multiphase refinement; sample also contained 20% Hg-1256 and 2% CaO.
Compound a (A) c (A) T c (K) Ref.
A1Sr2Ca4Cu5013
a
3.845 20.87 83 1
HgBa2Ca4CusO 12.32 b 3.85151 22.1049 101 2
Hgo.623Pbo.a44BaECa4CusO12.52 c
3.8529 22.172 116 3
Hgo.7Auo.3Ba2Ca4Cu5012+6 d
3.849 22.061 110 4
B Sr2Ca4Cu5013 e
3.837 20.22 85 5
a
Prepared at 5.5 GPa; nominal composition.
bprepared at 1.8 GPa.
c Prepared at 1 GPa.
d Prepared at 1.8 GPa; nominal composition.
e Prepared at 6 GPa; nominal composition.
References: 1, Isobe
et al.
(1994); 2, Huang
et aL
(1994a); 3, Schwer
et al.
(1995b); 4, Bordet
et al.
(1996); 5, Kawashima
et al.
(1995).
A critical temperature of 112 K was reached for Hg-1245 after optimization
(Loureiro
et al.,
1994). For Hgo.623Pbo.344Ba2Ca4CusO12.52, two distinct cation
sites were refined in the
additional
layer (Hg in l(a)0 0 0 and Pb in
8(/)) 0.044 0.087 0, occupancy 0.579 and 0.043) (Schwer
et al.,
1995b). Extra
Hg and Ba sites with low occupancy were introduced into the structural model to
take into account an intergrowth with 1234 and 1256.
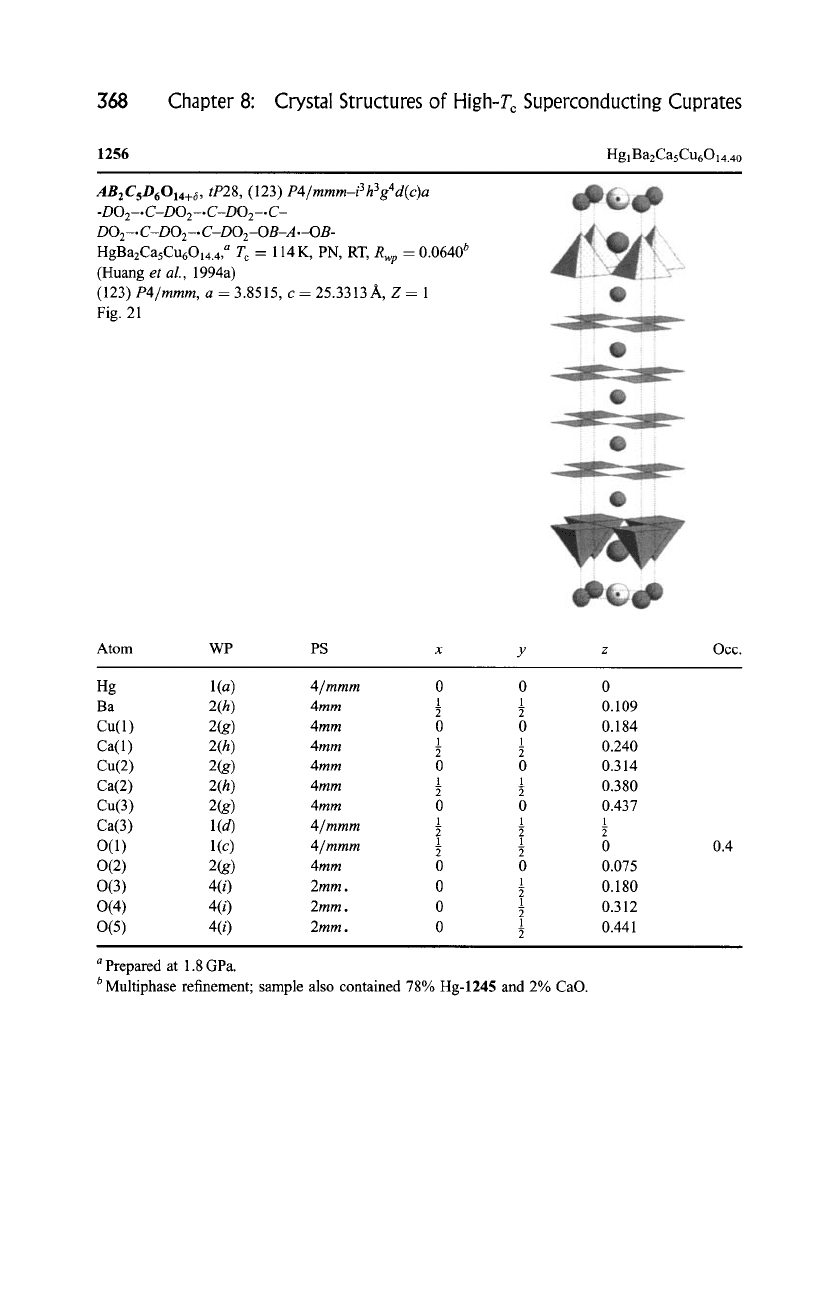
368
Chapter 8: Crystal Structures of High-T~ Superconducting Cuprates
1256
Hg
1Ba2CasCu6014.40
AB 2
C5D6014+6 , tP28,
(123) P4/mmm-i
3 h 3 g4 d(c)a
-DOE-.C-DO2-.C-DOE-.C-
DO2-.
C-DO2-. C-DOz-OB-A .-OB-
HgBazCasCu6014.4, a T c = 114 K, PN, RT,
Rwp
-- 0.0640 b
(Huang
et al.,
1994a)
(123)
P4/mmm,
a = 3.8515, c = 25.3313/~, Z = 1
Fig. 21
Atom WP PS x y
Occ.
Hg l(a)
4/mmm 0 0
1 1
Ba 2(h)
4mm ~
Cu(1) 2(g)
4mm 0 0
1 1
Ca(l) 2(h)
4mm ~
Cu(2) 2(g)
4mm 0 0
1 1
Ca(2) 2(h)
4mm ~
Cu(3) 2(g)
4mm 0 0
1 1
Ca(3) l(d)
4 / mmm ~ -~
1 1
O(1) l(c)
4/mmm ~
0(2) 2(g)
4mm 0 0
1
0(3) 4(i)
2mm . 0
1
0(4) 4(i)
2mm . 0
1
0(5) 4(i)
2mm . 0
0
0.109
0.184
0.240
0.314
0.380
0.437
1
_
2
0
0.075
0.180
0.312
0.441
0.4
a
Prepared at 1.8 GPa.
b Multiphase refinement; sample also contained 78% Hg-1245 and 2% CaO.
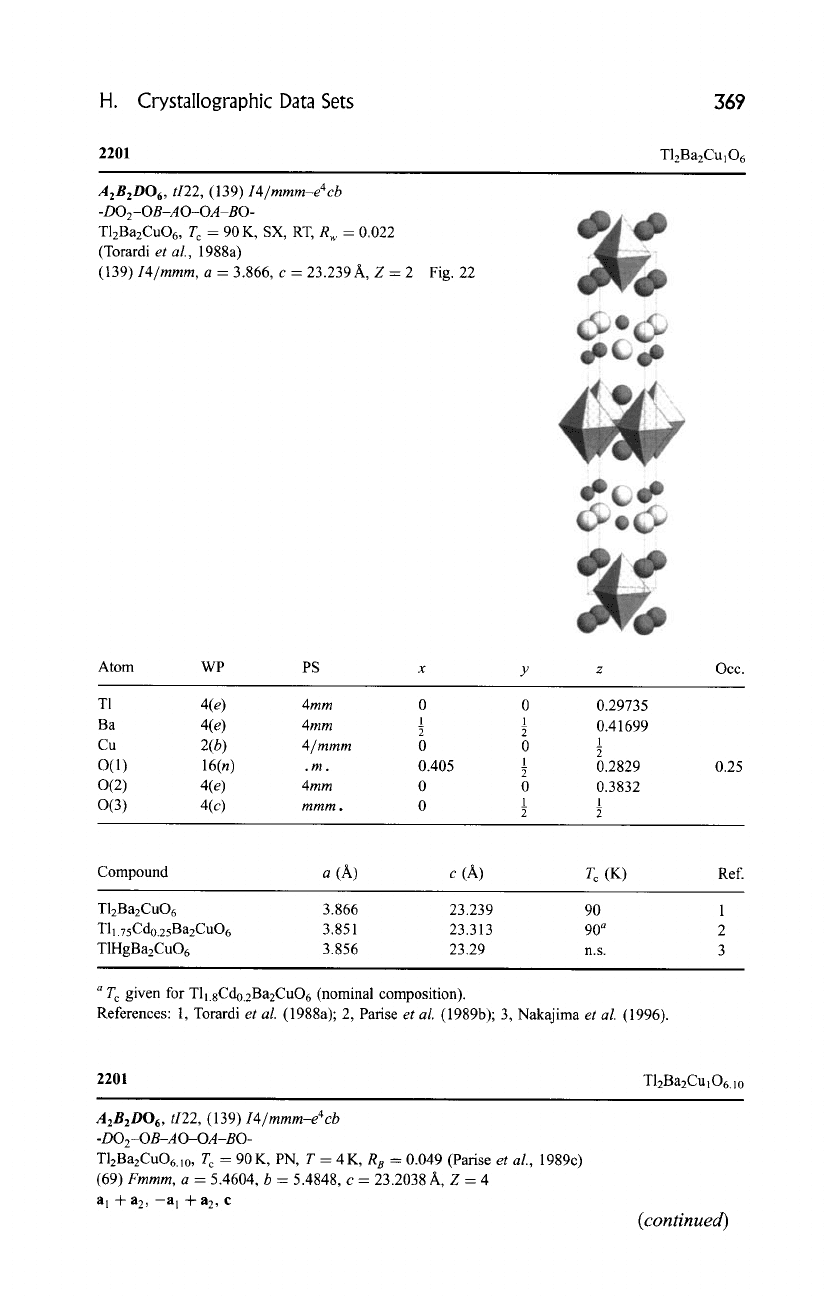
H. Crystallographic Data Sets
369
2201
T12Ba2Cu]O6
A2B2D06,
ti22, (139) I4/mmm-e4cb
-DO2-OB-A O-OA-BO-
TlzBa2CuO6, T c = 90 K, SX, RT, R w -- 0.022
(Torardi
et al., 1988a)
(139)
I4/mmm, a = 3.866, c = 23.239 A, Z = 2 Fig. 22
Atom WP PS x y z
Occ.
T1 4(e)
4mm 0 0 0.29735
1 1 0.41699
Ba 4(e)
4mm ~
1
Cu 2(b) 4/mmm 0 0
1
0.2829 O(1) 16(n) .m. 0.405
0(2) 4(e) 4mm 0 0 0.3832
1 1
0(3) 4(c) mmm . 0 ~
0.25
Compound a (A) c (A) T c (K)
Ref.
T12Ba2CuO6
3.866 23.239 90
T1].75Cdo.25Ba2CuO6
3.851 23.313 90 a
T1HgBazCuO6 3.856 23.29 n.s.
aT c
given for
Tll.8Cdo.2Ba2CuO6
(nominal composition).
References: 1, Torardi et al. (1988a); 2, Parise et al. (1989b); 3, Nakajima et al. (1996).
2201
T12Ba2Cu]O6.1o
A2B2D06,
ti22, (139) I4/mmm-e4cb
-DO2-OB-A O-OA-BO-
TlzBa2CuO6.1o, T c -- 90 K,
PN, T = 4 K, R B -- 0.049 (Parise et aL, 1989c)
(69) Fmmm, a = 5.4604, b = 5.4848, c = 23.2038/~, Z = 4
a I +a2, --a 1 +a2, c
(continued)
370
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
Atom WP PS x y z Occ.
T1 16(m) m.. 0 -0.025 0.2976 0.5
1 0 0.4172
Ba 8(i)
mm2
1
Cu 4(b)
mmm 0 0
1 1 1
0.07
O(1) a 8(f) 222 a a
1
--0.059 0.2895 0.49
0(2) 16(m) m..
0(3) 8(i)
mm2 0 0
0.3837
1 1 1
0(4) 8(e)
. .2/m ~ ~
a
Extra O site located between the T10 layers.
2201
Bi2Sr2CulO6
A2B2D06,
ti22, (139)
I4/mmm-e4 cb
-DO2-OB-A O-OA-BO-
BiaSr2CuO6, T c = 9 K, SX, RT,
R w
= 0.130 (Torardi
et al.,
1988a)
(66)
Amaa (Cccm),
a = 5.362, b = 5.374, c = 24.622 A, a Z = 4
al + a2, -al + a:, e; origin shift 0 1
Atom WP PS x y z
Occ.
Bi 8(/) m.. 0 0.2758 0.0660
1 0.2479 0.1790
Sr 8(/) m..
1 1
Cu 4(e)
2/m . . 0 ~
1 0.334 0.064
O(1) 8(/) m..
0(2) 8(/) m.. 0 0.226 0.145
1 0 0.254
0(3) 8(h) . .2
a
Average structure, additional reflections indicate superstructure (5a, b, 3c).
Compound a (A) b (A) c (A) T c (K) Ref.
Bi2 Sr2CuO6 a 5.375 5.378 24.377 b 9.5 1
Bi2Srl.6Lao.4CuO6.3o 5.388 5.398 24.399 29.5 c 2
a Refined composition Bil.86Srl.56Cuo.83Os.98.
b Space group
A2aa.
C From resistivity measurements (midpoint); T c = 30.9K for sample annealed under reducing
conditions.
References: 1, Rajagopal
et al.
(1993); 2, Schlrgl
et al.
(1993).
2201
(B io.9oPbo.
10)2(Sro.85B
io.o6Pbo.o
1 )2 Cu 10 6
A2B2D06, ti22,
(139)
I4/mmm-eacb
-DOE-OB-A O-OA-BO-
Bil.924Pbo.216Srl.7ooCuO6, a T c --- 18 K, SX, T = 293 K,
R w
= 0.053 (Torardi
et al.,
1991)
(52)
Pnan (Pnna),
a = 5.2757, b = 5.3797, c = 24.558 A, Z = 4
a l+a 2 -a l+a 2, e; origin shift 0 1 1
,
~
(continued)
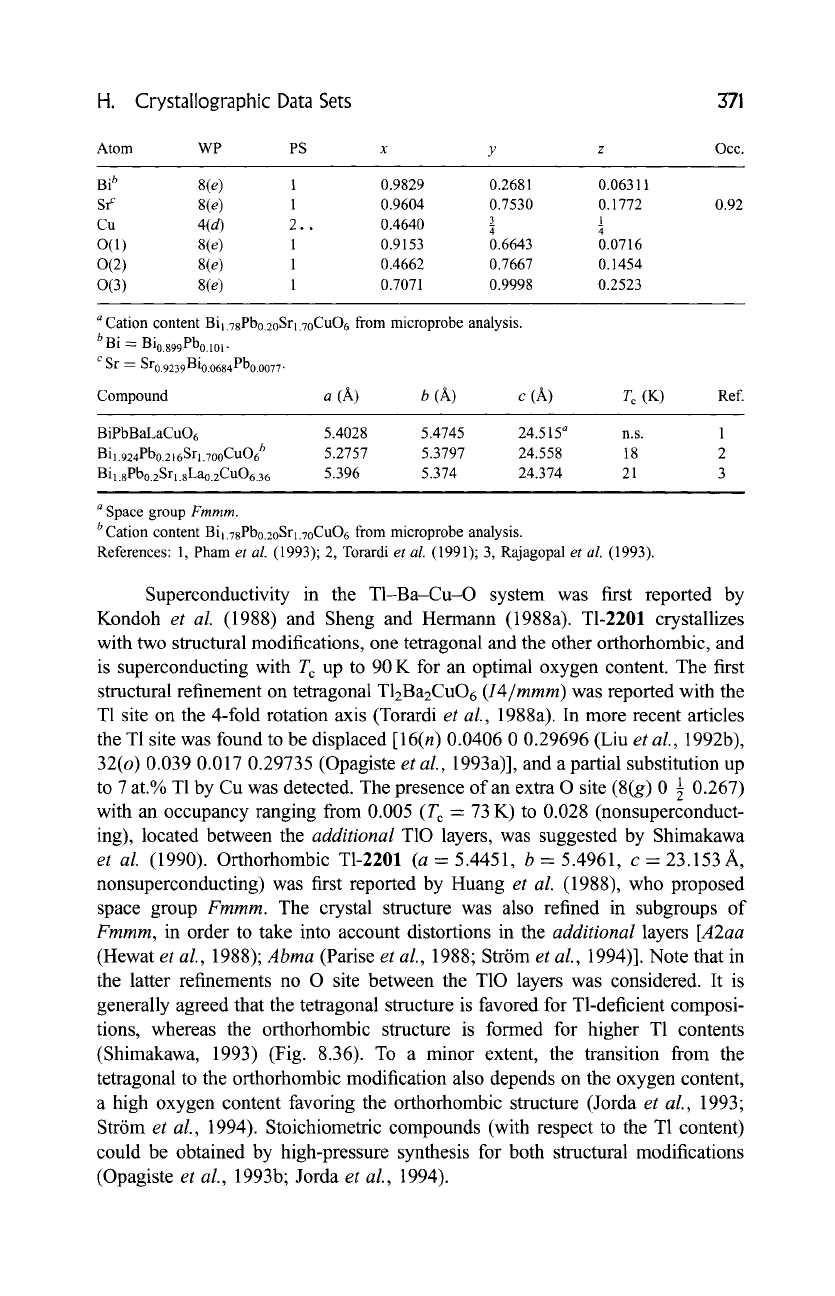
H. Crystallographic Data Sets 371
Atom WP PS x y
Occ.
Bi b 8(e) 1 0.9829 0.2681
Sr c 8(e) 1 0.9604 0.7530
3
Cu 4(d) 2.. 0.4640
O(1) 8(e) 1 0.9153 0.6643
O(2) 8(e) 1 0.4662 0.7667
0(3) 8(e) 1 0.7071 0.9998
0.06311
0.1772
1
4
0.0716
0.1454
0.2523
0.92
a
Cation content Bi1.78Pbo.2oSrl.70CuO 6 from microprobe analysis.
b Bi = Bio.899Pbo.lo 1.
c Sr -- Sro.9239Bio.0684Pbo.o077.
Compound a (A) b (A) c (A) T c (K) Ref.
BiPbBaLaCuO6 5.4028 5.4745 24.515
a n.s.
Bil.924Pbo.216Srl.7ooCuO6 b 5.2757 5.3797 24.558 18
Bi1.8Pbo.2Srl.8Lao.2CuO6.36 5.396 5.374 24.374 21
....
a Space group
Fmmm.
b Cation content Bil.78Pbo.2oSr1.7oCuO6 from microprobe analysis.
References: 1, Pham
et al.
(1993); 2, Torardi
et al.
(1991); 3, Rajagopal
et al.
(1993).
Superconductivity in the T1-Ba-Cu-O system was first reported by
Kondoh
et al.
(1988) and Sheng and Hermann (1988a). T1-2201 crystallizes
with two structural modifications, one tetragonal and the other orthorhombic, and
is superconducting with T c up to 90 K for an optimal oxygen content. The first
structural refinement on tetragonal TlzBazCuO6
(I4/mmm)
was reported with the
T1 site on the 4-fold rotation axis (Torardi
et al.,
1988a). In more recent articles
the T1 site was found to be displaced [16(n) 0.0406 0 0.29696 (Liu
et al.,
1992b),
32(0) 0.039 0.017 0.29735 (Opagiste
et al.,
1993a)], and a partial substitution up
to 7 at.% T1 by Cu was detected. The presence of an extra O site (8(g) 0 1 0.267)
with an occupancy ranging from 0.005 (T c = 73 K) to 0.028 (nonsuperconduct-
ing), located between the
additional
T10 layers, was suggested by Shimakawa
et al.
(1990). Orthorhombic T1-2201 (a -- 5.4451, b = 5.4961, c-- 23.153 A,
nonsuperconducting) was first reported by Huang
et al.
(1988), who proposed
space group
Fmmm.
The crystal structure was also refined in subgroups of
Fmmm,
in order to take into account distortions in the
additional
layers
[A2aa
(Hewat
et al.,
1988);
Abma
(Parise
et al.,
1988; Str6m
et al.,
1994)]. Note that in
the latter refinements no O site between the T10 layers was considered. It is
generally agreed that the tetragonal structure is favored for Tl-deficient composi-
tions, whereas the orthorhombic structure is formed for higher T1 contents
(Shimakawa, 1993) (Fig. 8.36). To a minor extent, the transition from the
tetragonal to the orthorhombic modification also depends on the oxygen content,
a high oxygen content favoring the orthorhombic structure (Jorda
et al.,
1993;
Str6m
et al.,
1994). Stoichiometric compounds (with respect to the T1 content)
could be obtained by high-pressure synthesis for both structural modifications
(Opagiste
et al.,
1993b; Jorda
et al.,
1994).
372
Chapter 8: Crystal Structures of High-To Superconducting Cuprates
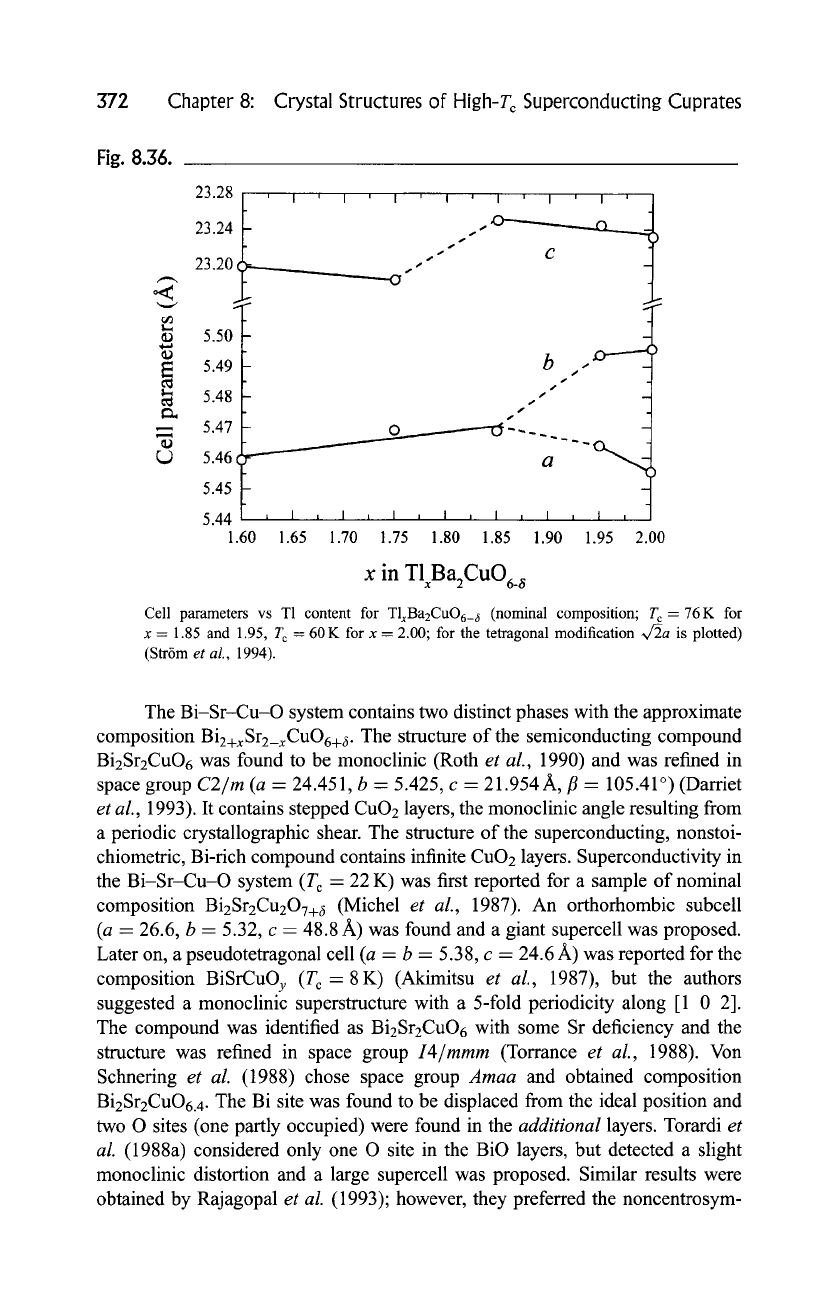
Fig. 8.36.
~<
23.28
23.24
23.20
5.50
5.49
5.48
5.47
5.46 (
5.45
5.44
' I ' I ' I ' I '
s
s
I ' I ' I"
C
- b
d
J
/
, d
I j
~ a
, I , I , I , I , I , I i I i
1.60 1.65 1.70 1.75 1.80 1.85 1.90 1.95 2.00
x in
TlxBa2CuO6. a
Cell parameters vs T1 content for TlxBa2CuO6_ ~ (nominal composition; T c = 76K for
x - 1.85 and 1.95, T c - 60 K for x - 2.00; for the tetragonal modification ~/2a is plotted)
(Str6m
et al.,
1994).
The Bi-Sr-Cu-O system contains two distinct phases with the approximate
composition Biz+xSr2_xCuO6+ 6. The structure of the semiconducting compound
BizSr2CuO6 was found to be monoclinic (Roth
et al.,
1990) and was refined in
space group
C2/m
(a = 24.451, b = 5.425, c = 21.954 A, fl = 105.41 ~ (Darriet
et aL,
1993). It contains stepped CuO2 layers, the monoclinic angle resulting from
a periodic crystallographic shear. The structure of the superconducting, nonstoi-
chiometric, Bi-rich compound contains infinite CuO2 layers. Superconductivity in
the Bi-Sr-Cu-O system (T c = 22 K) was first reported for a sample of nominal
composition
BizSr2Cu207+ 6
(Michel
et aL,
1987). An orthorhombic subcell
(a = 26.6, b = 5.32, c = 48.8 A) was found and a giant supercell was proposed.
Later on, a pseudotetragonal cell (a = b = 5.38, c = 24.6 A) was reported for the
composition BiSrCuOy (T c = 8K) (Akimitsu
et aL,
1987), but the authors
suggested a monoclinic superstructure with a 5-fold periodicity along [1 0 2].
The compound was identified as Bi2SrzCuO6 with some Sr deficiency and the
structure was refined in space group
I4/mmm
(Torrance
et al.,
1988). Von
Schnering
et al.
(1988) chose space group
Amaa
and obtained composition
BizSr2CuO6. 4.
The Bi site was found to be displaced from the ideal position and
two O sites (one partly occupied) were found in the
additional
layers. Torardi
et
aL
(1988a) considered only one O site in the BiO layers, but detected a slight
monoclinic distortion and a large supercell was proposed. Similar results were
obtained by Rajagopal
et al.
(1993); however, they preferred the noncentrosym-
H. Crystallographic Data Sets 373
metric space group
A2aa,
also used for the structural refinement on Ca-containing
Bi-2201 (Imai
et al.,
1988). A refinement in the monoclinic space group A2
(a- 26.908, b- 5.380, c-26.856A, t3-113.55 ~ gave the composition
Bi10SrloCusO29, because of oxygen vacancies in the
additional
layers (Onoda
and Sato, 1988). The same authors also reported Sr deficiency, based on
microprobe analysis. Zandbergen
et al.
(1988a, 1990b) explained the modulation
in the structures of Bi-based superconducting cuprates by the difference between
the translation periods of the BiO and CuO2 layers, which is reduced by
cooperative displacements of the atoms from the ideal positions and by the
incorporation of extra oxygen atoms into the former layers. A refinement of the
incommensurate structure in superspace group pA_2/~ (a- 5.3791, b- 5.3811,
c - 24.589 A, t3 - 89.93 ~ q - 0.2030a* + 0.467c*) was carried out by Leligny
et al.
(1992). Beskrovnyi
et al.
(1994) used space group
A2/a
to refine the
average structure, the 5-fold superstructure being described in A2.
Studies on the substitution of Sr by La in BizSr2_xLaxCuO6+ 6 showed that
single-phase samples can be prepared for 0.3 < x _< 1 (Schl6gl
et al.,
1993)
(0.2 < x _< 0.8; Khasanova and Antipov, 1995). An increase of the La content
causes a reduction of the c-parameter, accompanied by an expansion of the two
short cell parameters (Fig. 8.37), the structure remaining incommensurately
modulated. The oxygen content increases linearly with x (6- 0.5 for x- 1),
but the superconducting properties are suppressed at x > 0.7 (SchlSgl
et al.,
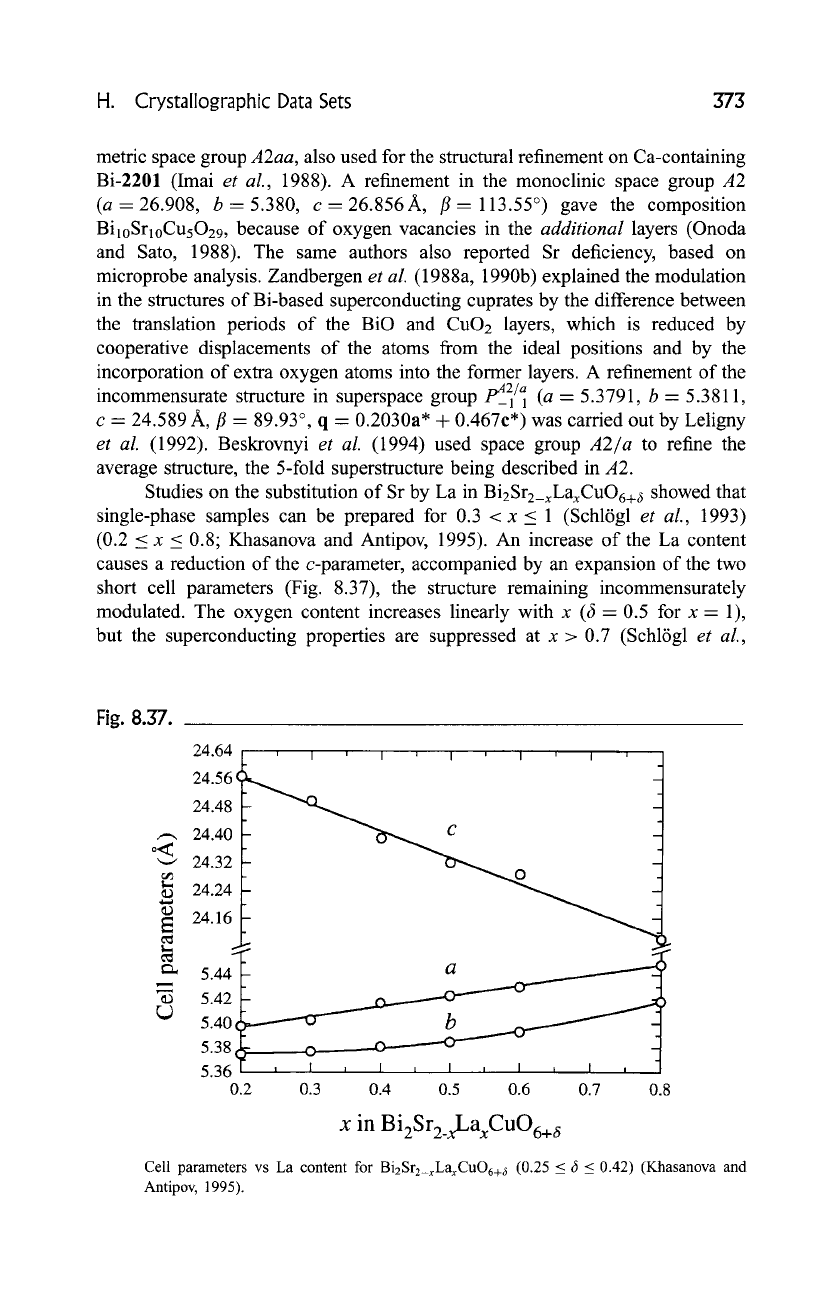
Fig. 8.37.
24.64
24.56 (
24.48
,--, 24.40
o<
24.32
ra~
r 24.24
~. ' I ' I ' 1 ' 1 ' I '
24.16 -
a
-
5.38 - ..... . . 1
5 36
0.2 0.3 0.4 0.5 0.6 0.7 0.8
x in Bi2Sr2_xLaxCuO6+6
Cell parameters vs La content for Bi2Sr2_xLaxCuO6+ 6 (0.25 _< 6 _< 0.42) (Khasanova and
Antipov, 1995).
374
Chapter 8: Crystal Structures of
High-Tc
Superconducting Cuprates
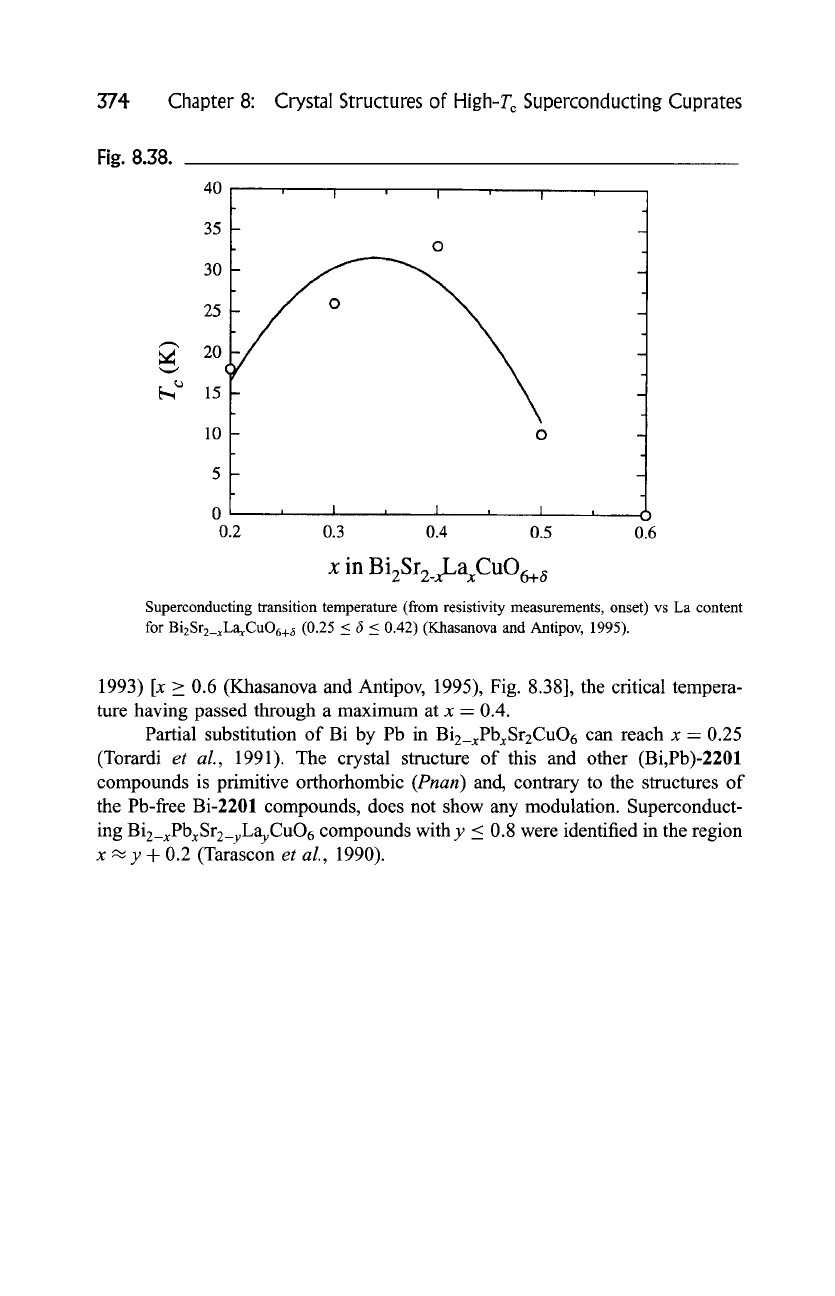
Fig. 8.38.
40 ' I ' i , i ,
35
30
25
20
15
O
10-
5-
0
0.2
i
I J I
n
I ,
0.3 0.4 0.5 0.6
x in
Bi2Sr2_xLaxCuO6+ 6
Superconducting transition temperature (from resistivity measurements, onset) vs La content
for BizSrz_xLaxCuO6+ ~ (0.25 < fi < 0.42) (Khasanova and Antipov, 1995).
1993) [x _> 0.6 (Khasanova and Antipov, 1995), Fig. 8.38], the critical tempera-
ture having passed through a maximum at x = 0.4.
Partial substitution of Bi by Pb in Biz_xPbxSrzCuO 6 can reach x = 0.25
(Torardi
et aL,
1991). The crystal structure of this and other (Bi,Pb)-2201
compounds is primitive orthorhombic
(Pnan)
and, contrary to the structures of
the Pb-free Bi-2201 compounds, does not show any modulation. Superconduct-
ing
Biz_xPbxSrz_yLayCuO 6
compounds with y _< 0.8 were identified in the region
x ~ y + 0.2 (Tarascon
et aL,
1990).
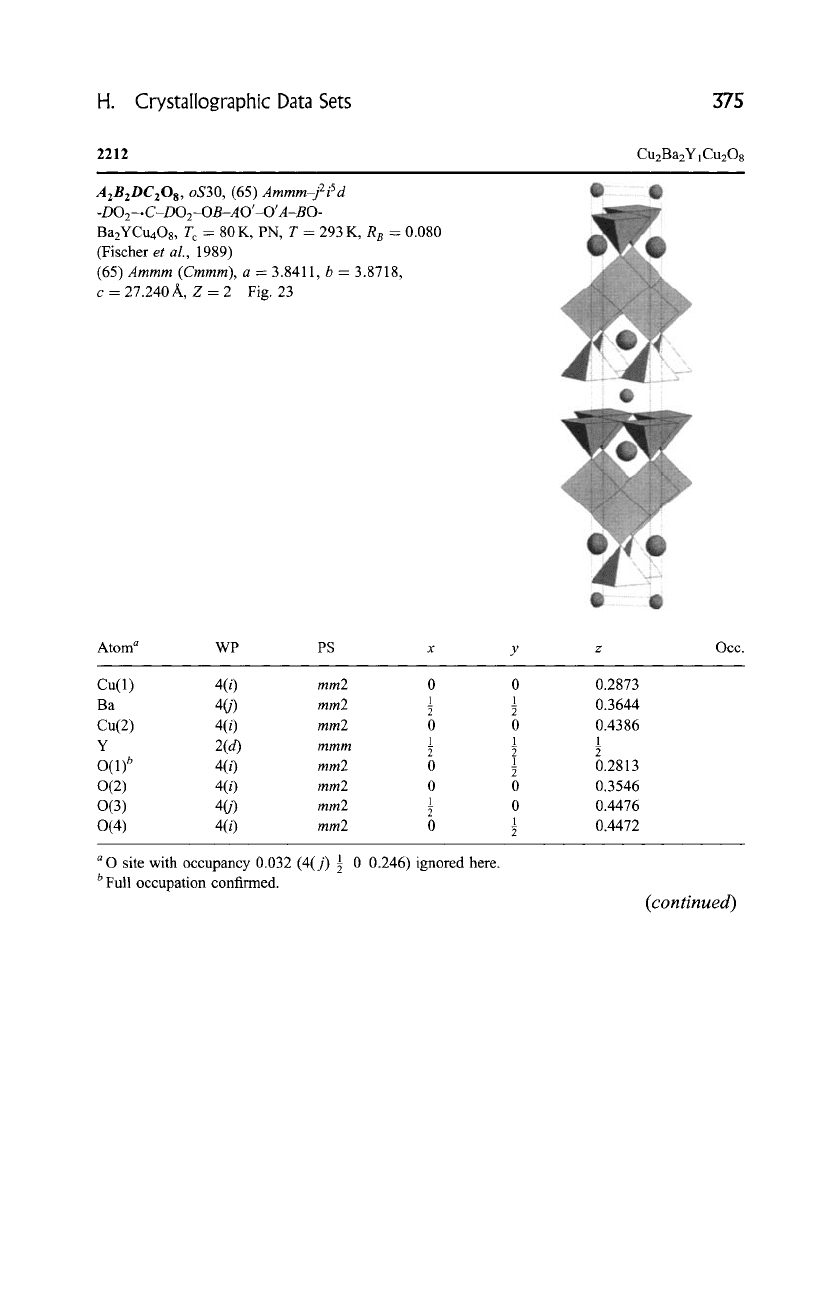
H. Crystallographic Data Sets 375
2212
Cu2Ba2YiCu208
A2B2DC208, oS30, (65) Ammm-j2iSd
-D02-.
C-DO2-OB-A O'-O' A-BO-
Ba2YCu4Os, Tc = 80 K, PN, T -- 293 K, R e = 0.080
(Fischer
et al., 1989)
(65)
Ammm (Cmmm), a = 3.8411, b = 3.8718,
c = 27.240 A, Z -- 2 Fig. 23
Atom a WP PS x
Occ.
Cu(1) 4(i)
mm2 0
1
Ba 4(/') mm2
Cu(2) 4(i) mm2 0
1
Y 2(d) mmm
O(1)b 4(i) mm2 0
O(2) 4(i) mm2 0
1
0(3) 4(]) mm2
0(4) 4(/) mm2 0
0.2873
0.3644
0.4386
1
2
0.2813
0.3546
0.4476
0.4472
a o site with occupancy 0.032 (4(j) 1 0 0.246) ignored here.
b Full occupation confirmed.
(continued)
