Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.


DNA POLYMERASES FOR PCR APPLICATIONS 381
Table 1. DNA polymerases in Escherichia coli, Saccharomyces cerevisiae and Humans
∗
Family Name Bacterial
gene
Human gene Yeast gene Mol. Wt.
(kDa)
a
3’Exo
activity
Other activities
A Ec Pol I Pol A 103 + 5’ Exonuclease
(gamma) POLG MIPI 140 + dRP lyase
(theta) POLQ - 290 - ATPase, helicase
(nu) POLN - 100 -
B Ec Pol II Pol B 89 +
(alpha) POLA POL1(CDC17) 165 - Primase
(delta) POLD1 POL3 (CDC3) 125 +
(epsilon) POLE POL2 225 +
(zeta) POLZ (REV3) REV3 353 -
C Ec Pol III dnaE 130 (separate
subunit)
X (beta) POLB - 39 - dRP lyase,
AP lyase
(lambda) POLL POL 4 (POLX) 66 - DRP lyase, TdT
(mu) POLM - 55 - TdT
TdT TdT 56 -
(sigma) POLS (TRF4-1) TRF4 60 -
Y Ec Pol IV dinB 40 -
Ec Pol V umuC 46
(eta) POLH RAD30 78 -
(RAD30A, XPV)
(iota) POLI (RAD30B) - 80 - dRP lyase
(kappa) POLK 9DINB) -76-
Rev1 REV1 REV1 138 -
∗
From Bebenek and Kunkel, 2004, p 138.
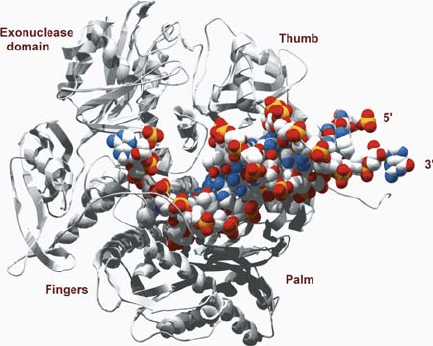
382 DROUIN ET AL.
Figure 1. Structure of a replicative DNA polymerase (Franklin et al., 2001, p 660)
Six highly conserved regions termed I–VI have been identified among eukaryotic,
prokaryotic and viral polymerases. Region IV is the most N-terminally located,
followed by regions II, VI, III, I and V. Region I is located in the palm and contains
two conserved aspartic acid residues. The other invariant aspartic acid occurs in
region II and is located at the tip of a -sheet that is part of the palm subdomain.
Included in this region is the highly conserved SLYPS-II region, which is important
for deoxynucleoside triphosphate (dNTP) binding. Other residues important for
dNTP binding are in region III. Region IV is located at the N-terminus that is part of
the 3' to 5' exonuclease active site. The other two conserved regions, V and VI, are
located in the thumb and finger subdomains, respectively (Hubscher et al., 2002).
The phosphoryl transfer that takes place during polymerisation is catalysed by
a two-metal-ion mechanism which also plays an important role in the exonuclease
activity (Sawaya et al., 1994; Steitz, 1998). Two Mg
2+
ions form a pentacoordinated
transition state with the phosphate groups of the incoming nucleotide via interactions
with conserved carboxylate residues in regions I and II. In addition to this feature,
the finger subdomain rotates toward the palm to move from an 'open' to a 'closed'
conformation forming the binding groove for the incoming dNTP. The thumb
domain also rotates toward the palm. The resulting closed conformation allows
interactions between the conserved residues of the fingers with the dNTP binding
site and the exonuclease domain (Franklin et al., 2001; Lewin, 2004).
Polymerases , , and (family B) and (family A) are well characterized in
eukaryotes. They are heteromultimers and are composed of a large subunit and a
variety of smaller subunits. The latter have been implicated in the stabilization of the
catalytic subunit, establishment of protein-protein interactions, cell-cycle regulation
and also checkpoint function (Hubscher et al., 2002).
DNA POLYMERASES FOR PCR APPLICATIONS 383
Polymerase is a heterotetrameric enzyme. The N-terminal domain seems to
be dispensable for catalytic activity and the assembly of the tetrameric complex.
A central domain contains all the conserved regions responsible for DNA binding,
dNTP binding and phosphoryl transfer. The C-terminal domain is not essential for
catalysis but is necessary for the interaction with the other subunits. Pol might
be involved in cell regulatory functions (Hubscher et al., 2002).
Polymerase is the smallest eukaryotic polymerase and consists of two domains.
The N-terminal domain performs the 5'-deoxyribose phosphatase activity (to remove
the 5'-deoxyribose phosphate) and single-stranded DNA binding, whereas the large
domain carries out the polymerase activity. Polymerase is able to fill short gaps in
a distributive way and these gaps contain a 5'-phosphate (Beard and Wilson, 2006).
The N-terminal part of polymerase contains three regions of high homology:
a nuclear targeting signal and nuclear targeting regions 1 and 2. The C-terminal
part has three highly similar regions termed CT-1 to 3 and a zinc-finger
domain. Polymerase might be involved in double-strand break repair (Hubscher
et al., 2002).
Polymerase is a heterodimeric protein composed of a large subunit, which is
responsible for the catalytic activities (DNA polymerase and both the 5' to 3' and
3' to 5' exonuclease activities - Graves et al., 1998), and a small accessory subunit.
The small subunit dimerizes with the large one and binds DNA. Pol is one of the
key players in mitochondrial DNA repair (Vanderstraeten et al., 1998).
The C-terminal region of polymerase contains a highly acidic region and a
zinc-finger domain. Polymerase is implicated in DNA replication in budding yeast
and DNA repair in human cells (D'Urso and Nurse, 1997; Pospiech et al., 1999).
3. CATALYTIC PROPERTIES OF DNA POLYMERASES
DNA polymerases have different enzymatic activities:
5' to 3' DNA polymerase activity: DNA polymerases catalyse the linkage of
dATP, dCTP, dGTP and dTTP in a specific order, using single-stranded DNA
as a template such that the newly polymerised molecule is complementary to the
template. DNA polymerases synthesize DNA in the 5' to 3' direction. They are
unable to begin a new chain de novo and require a pre-existing 3'-OH group in the
form of a primer to which the first nucleotide of a new chain is added. Primers are
RNA in most organisms but can be DNA in some; in the case of certain viruses
the primer is a protein the presents a nucleotide to the polymerase (Lewin, 2004).
To complete DNA strand synthesis RNA is removed from the fragments, a DNA
polymerase fills the gaps and the nicks remaining are ligated.
3' to 5' exonuclease, proofreading activity: error correction by proofreading
is a property of some DNA polymerases. This process corrects mistakes in newly
synthesized DNA. When an incorrect base pair is recognized DNA polymerase
reverses its direction by one or more base pairs of DNA. The 3' to 5' exonu-
clease activity of the enzyme allows the incorrect base to be excised. Following
proofreading, the polymerase re-inserts the correct base and replication continues.
384 DROUIN ET AL.
Generally, DNA polymerases lacking 3' to 5' exonuclease activity have higher error
rates than the polymerases that possess it (Table 2) and tend to pause after inserting
incorrect bases.
5' to 3' exonuclease, nick translation activity: this enzymatic activity plays an
essential role in DNA replication by removing RNA primers.
Terminal transferase activity: causes the addition of a single nucleotide
(generally adenine) to the 3' end of PCR products.
4. TYPES OF DNA POLYMERASES COMMERCIALLY
AVAILABLE
DNA polymerase I. This is a bacterial enzyme that plays an important role in
DNA repair and a secondary role in replication (Camps and Loeb, 2004). The
commercial form is extracted from E. coli. Its 5' to 3' DNA polymerase activity
requires a template; it also has 3' to 5' and 5' to 3' exonuclease activity. The
DNA synthesized is strictly complementary to the template. This enzyme is used to
synthesize DNA from a single-strand DNA template at 37
C. Its major applications
are: the determination of a DNA sequence using an enzymatic sequencing method,
the synthesis of radioactive probes, transformation of a staggered end into a double-
stranded blunt end, and the construction of vectors from a single DNA strand.
The Klenow Fragment of DNA polymerase I. The Klenow fragment is a fragment
of DNA polymerase I obtained by limited proteolysis. The 5' to 3' exonuclease
activity is removed while preserving the 5' to 3' polymerase and the 3' to 5'
exonuclease activities (Sanger et al., 1977).
T4 DNA polymerase. Isolated from bacteriophage T4 of E. coli. It has the same
enzymatic activities as the Klenow fragment and the same biological uses (Kaplan
and Delpech, 1994).
Taq DNA Polymerase. This is a thermostable enzyme isolated from Thermus
aquaticus which is used for PCR amplification of DNA fragments up to 5 kb in
length, as well as DNA labelling and sequencing. It is ideal for TA (T for 'T
vector' (thymidine); A for adenosine) cloning (Zhou and Gomez-Sanchez, 2000).
This procedure exploits the enzyme’s terminal transferase activity. Taq polymerase
has a non-template dependent activity which adds a single adenosine to the 3' ends
of double-stranded DNA molecules. Thus most molecules PCR amplified by Taq
polymerase possess single 3'-A overhangs. The use of a linearized 'T-vector' which
has single 3'-T overhangs allows direct, high-efficiency cloning of PCR products
facilitated by the complementarities between the PCR product 3'-A overhangs and
the 3'-T overhangs of the vector (Zhou and Gomez-Sanchez, 2000).
Terminal transferase. This is a mammalian enzyme expressed in lymphocytes.
The enzyme purchased commercially is usually produced by expression of the
bovine gene DNTT in E. coli. It catalyses deoxynucleotide addition to a free
3'-OH end without the need for a template. The choice of deoxynucleotide added
is made randomly. The base composition of the synthesized polydeoxynucleotide
depends on the base concentrations in the incubation medium. Terminal transferase

DNA POLYMERASES FOR PCR APPLICATIONS 385
Table 2. DNA polymerases characteristics
DNA
polymerase
Error rate
(error/bp)
3' to 5'
exonuclease
Terminal
transferase
activity
Half-life Price per
U
∗
in
US$
Applications Companies
Taq 11 ×10
−4
- + 60 min at 94
C 0.46$ ¬ TA cloning
¬ DNA labelling
¬ Sequencing
QIAGEN
Deep Vent
®
45×10
−5
+ - 23hat95
C
8 h at 100
C
0.42$ ¬ High-fidelity PCR
¬ Primer-extension
¬ Cloning
NEW
ENGLAND
BioLabs
Pfu DNA
polymerase
16×10
−6
+ - 19hat95
C 1.74$ ¬ High-fidelity PCR
¬ Primer-extension
¬ Cloning
¬ Blunt-end
amplification product
generation
STRATAGENE
Herculase
®
Enhanced
28 ×10
−6
+ + NF 1.28$ ¬ GC rich fragment STRATAGENE
Phusion
™
44 ×10
−7
+-> 6 h at 96
C 1.45$ ¬ High-fidelity PCR
¬ Fast long amplicon
FINNZYMES
T4 DNA
polymerase
1×10
−6
+ - NF 0.38$ ¬ Form blunt ends
¬ Probe labelling
NEW
ENGLAND
BioLabs
Klenow
Fragment
18×10
−6
+ NF Heat
inactivated:
70
C for 10
minutes
0.27$ ¬ Labelling recessed 3'
end of double
stranded DNA
¬ Random-priming
DNA labelling
FERMENTAS
(Continued)

386 DROUIN ET AL.
Table 2.(Continued)
DNA polymerase Error rate
(error/bp)
3' to 5'
exonuclease
Terminal
transferase
activity
Half-life Price per
U
∗
in US$
Applications Companies
Terminal
transferase
NA NA + Heat
inactivated:
75
C for 20
minutes
0.11$ ¬ Tailing
¬ Labelling the DNA
3-OH ends
NEW
ENGLAND
BioLabs
Sequenase
™
version
2.0
34×10
−5
- + NF 0.70$ ¬ Sequencing USB
DNA polymerase I NF + NF Heat
inactivated:
75
C for 10
minutes
0.15$ ¬ Sequencing
¬ Transforming a single
blunt end into a
double stranded blunt
end
¬ Vector construction
USB
rTth DNA
polymerase XL
+ NF NF 0.58$ ¬ Amplify long
fragments
> 40 kb
Applied
Biosystems
Isis proofreading
DNA polymerase
066 ×10
−6
+ - 18hat95
C
and 5 h at
100
C
1.40$ ¬ GC rich fragments, or
contain secondary
structure
¬ Cloning
¬ Characterization of
rare mutations in
tissue
Krackeler
Scientific Inc
rBst DNA
polymerase
NF + NF Optimal
activity at
65
C
0.50$ ¬ GC rich fragments, or
contain secondary
structure
EPICENTRE
Biotech-
nologies

DNA POLYMERASES FOR PCR APPLICATIONS 387
phi29 DNA
polymerase
NF + NF Heat
inactivated:
65
C for 10
minutes
0.23$ ¬ Rolling circle
replication
¬ Multiple displacement
amplification
¬ Unbiased amplification
of whole genome
¬ Amplify long
fragments
> 70 kb
NEW
ENGLAND
BioLabs
SurePRIME
™
DNA
polymerase
NF NF NF > 40 min at
95
C
0.80$ ¬ High-fidelity PCR,
even when using
non-optimised primers
¬ Hot Start PCR
Krackeler
Scientific,
Inc.
BioTHERM
™
DNA
polymerase
NF NF + NF 0.13$ ¬ PCR with
low-abundance
template
¬ TA cloning
GENECRAFT
SpeedSTAR
™
HS DNA
polymerase
NF - + NF 0.87$ ¬ High speed
amplification
Takara
MTP
™
Taq
DNA
polymerase
NF - NF NF 0.90$ ¬ Detecting and
identifying bacterial
DNA
SIGMA-
ALDRICH
KOD “Hot
Start” DNA
Polymerase
NF + - NF 0.90$ ¬ High speed
amplification
¬ Hot Start PCR
¬ High-fidelity PCR
Novagen
∗
U: One unit is defined as the amount of enzyme required to incorporate 10 nmoles of dNTPs into acid precipitable form after 30 minutes at 70
C
NF: Not Found
NA: Non applicable
388 DROUIN ET AL.
is an example of a DNA polymerase that does not require a primer. Cobalt is a
necessary cofactor for activity of this enzyme. The enzyme lacks 3' to 5' and 5' to
3' exonuclease activities. It is used to generate DNA blunt ends and for labelling of
DNA 3' ends (Chang and Bollum, 1986).
Pfu DNA polymerase. This polymerase derives from the hyperthermophilic
archae Pyrococcus furiosus. It exhibits a long half-life and proofreading properties
comparable to those of other thermostable polymerases. It has 3' to 5' exonuclease
activity and a high proofreading efficiency and lacks 5' to 3' exonuclease and
terminal transferase activities. It is used for high-fidelity PCR and primer-extension
reactions (Angers et al., 2001), PCR cloning and the generation of blunt-end
amplification products.
Sequenase. The sequenases are a family of polymerases derived from the DNA
polymerase of bacteriophage T7. They are dimers of the gene 5 protein of the
phage and a protein produced by its host cell (thioredoxin). They are the fastest of
all DNA polymerases and are used in sequencing with dideoxyribonucleotides (the
Sanger method).
Deep Vent. Isolated from Thermococcus litoralis; also known as Tli polymerase
(Jannasch et al., 1992). Used for high-fidelity PCR and primer-extension reactions.
Herculase Enhanced. A mixture of the high fidelity Pfu polymerase, Taq
polymerase and ArchaeMaxx enhancing factor. Used to amplify long and GC-rich
DNA fragments.
Phusion. A novel enzyme with extreme fidelity and high speed, used for PCR
cloning. It allows high product yields with minimal enzyme concentrations.
rTth DNA polymerase. A recombinant enzyme from Thermus thermophilus used
to amplify DNA fragments of more than 40 kb (Fromenty et al., 2000).
rBst DNA polymerase. The product of the DNA pol I gene of the thermophilic
bacterium Bacillus stearothermophilus (Bst) produced in E. coli. It can synthesize
DNA regions of high GC content where other non-thermostable DNA polymerases
may fail. It is used for replicating difficult templates such as those that contain
hairpin structures (Ye and Hong, 1987).
phi29 DNA polymerase. Obtained from Bacillus subtilis phage phi29. This
enzyme acts preferentially on single-stranded DNA and can synthesize stretches
of more than 70 kb. However, its half-life is only 10 minutes at 65
C (Blanco
et al., 1989). It is a very accurate polymerase and can yield large amounts of
amplified DNA even from small amounts of template. It is suitable for rolling circle
amplification, multiple displacement amplification, unbiased whole genome ampli-
fication, protein-primed DNA amplification and in situ genotyping with padlock
probes.
Isis proofreading DNA polymerase. From Pyrococcus abyssi. One of the most
thermostable proofreading polymerases available, permitting highly accurate DNA
synthesis (Gueguen et al., 2001).
SurePRIME
TM
DNA polymerase. This enzyme is a highly purified form of
recombinant Taq polymerase that has been chemically modified by the addition
of heat-labile blocking groups to specific amino acid residues. Prior to the PCR
DNA POLYMERASES FOR PCR APPLICATIONS 389
reaction, the enzyme is in an inactive state. It is incapable of extending primer-
dimers or mis-annealed primer-template species that form below the specific
annealing temperature. Primer-dimers can occur when two primers, or parts of
them, are complementary and hybridise to each other. The 95
C incubation step
therefore serves to activate the enzyme and also ensure a completely 'clean' initial
PCR cycle. This procedure is called 'Hot Start' PCR.
BioTherm
tm
DNA polymerase. A thermostable DNA polymerase purified from
Thermus aquaticus. It is used for PCR primed off a low-abundance template (less
than 100 DNA molecules).
SpeedSTAR
TM
HS DNA polymerase. Allows very fast extension.
MTP
TM
Taq DNA Polymerase. A recombinant Taq DNA polymerase specifically
useful for applications involving the detection and identification of bacterial DNA.
KOD DNA Polymerase. Isolated from the extreme thermophile Thermococcus
kodakaraensis KOD1. It is commercially available in three different versions:
KOD HiFi is able to amplify targets up to 6 kb. KOD Hot Start is KOD HiFi
DNA polymerase premixed with two monoclonal antibodies that inhibit the DNA
polymerase and 3' to 5' exonuclease activities at ambient temperatures. It is able
to amplify longer targets than KOD HiFi alone (up to 21 kb with plasmid DNA
template), in addition to having the advantage of room temperature set up and
fewer mis-priming problems. It can synthesize DNA in regions containing particular
secondary structures or a high GC content and generates blunt-ended PCR products
suitable for cloning. KOD XL DNA polymerase is an optimised blend of KOD
HiFi DNA polymerase and a mutant form of KOD HiFi that is deficient in 3' to 5'
exonuclease activity. It is designed for the amplification of longer (up to 30 kb) and
more complex GC rich targets. KOD XL DNA polymerase generates a mixture of
PCR products with blunt and 3'-dA overhangs.
5. THE PCR TECHNIQUE
DNA amplification by PCR requires a DNA template which contains the targeted
region. Best results are obtained by using well-purified DNA lacking contamination
by RNA or protein. Two primers that determine the beginning and end of the region
to be amplified are required. The choice of primers is critical, and several factors
must be carefully considered, e.g. the melting temperature (Tm) which is defined
as the temperature at which half of the primer binding sites are occupied, GC
content, the presence of secondary structures. The sequence of the primers should
be such as not to permit the formation of hairpins or hybrids between them, and
programs are available to help in their design e.g. Oligo and Gene Jockey. The
choice of DNA polymerase depends on the specific application (see section 4).
Deoxynucleotide triphosphates (dATP, dCTP, dGTP, dTTP) are the monomers from
which the DNA polymerase synthesizes the new DNA. The buffer used for a PCR
reaction maintains the pH at the optimal level for the polymerase. Bivalent cations
(Mg
2+
) and monovalent cations (K
+
,Na
+
or NH
4
+
) are necessary to neutralize the
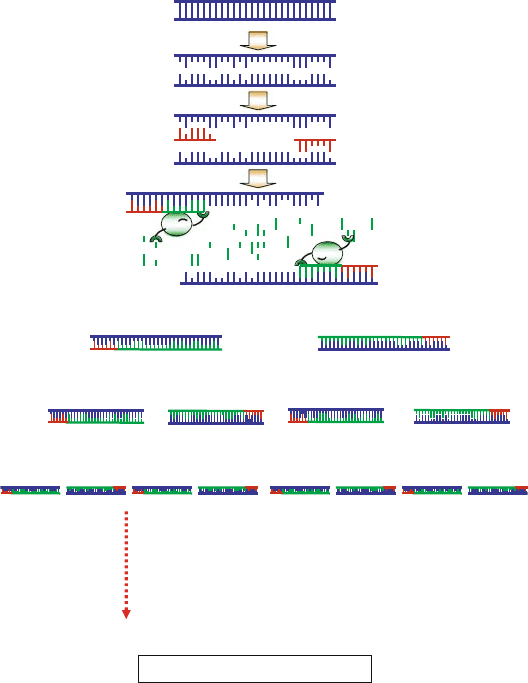
390 DROUIN ET AL.
negative charges of the phosphate groups of the DNA and to stabilize DNA/DNA
hybrids. The PCR reaction is carried out in a thermal cycler which successively
heats and cools the reaction tubes to the precise temperatures and for the specific
periods required for each step of the reaction. The PCR technique usually consists
of three principal steps - denaturation, primer annealing and extension - which are
repeated for 20 to 30 cycles (Fig. 2).
PCR is a powerful tool but errors and mistakes can easily occur. The polymerase
reaction is very sensitive to different variables. Divalent cations, especially Mg
2+
,
play an important role in nucleotide stability and affect the polymerisation activity
5’
5’3’
5’
5’3’
5’
5’3’
5’
3’
3’
5’
Exponential amplification
35
th
cycle
2
3
= 8 copies
2
2
= 4 copies
2
35
= 34 billion copies
3
rd
cycle
2
nd
cycle
1
st
cycle
Denaturation
Annealing
Extension
3’
3’
3’
Figure 2. Schematic illustration of exponential amplification. First step: denaturation at 94–96
C. Second
step: annealing at (e.g.)65
C. Third step: elongation at 72
C. Fourth step: the first cycle is complete.
The two resulting DNA strands make up the template DNA for the next cycle, thus doubling the amount
of DNA duplicated for each new cycle
