Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

USE OF LIPASES FOR THE PRODUCTION OF BIODIESEL
319
the presence of a high content of free fatty acids an acid-catalysed process is used
(Fig. 1d). This employs strong acids such as H
2
SO
4
, as well as high temperatures
and pressures, and requires longer reaction times than the alkaline process. Industrial
plants where both alkaline and acid processes are performed require that reactors
and accessories are resistant to these aggressive agents; moreover, high safety
standards are needed. Due to these drawbacks, alternative and more environmentally
sustainable routes for biodiesel production are being investigated.
2. BIODIESEL PRODUCTION USING LIPASES
Biodiesel production using lipases was first described by Mittlebach (1990) who
showed that the lipase from Pseudomonas fluorescens was superior to those from
Candida sp. and Mucor miehei for sunflower oil alcoholysis. The alcoholysis was
carried out both in the presence of solvent (petroleum ether) and in solvent free
conditions, and using five homologous alcohols with or without the addition of
water. Since then, subsequent studies have focused on different lipases, different
triglyceride feedstocks, different alcohols and different experimental conditions
(temperature, water content, stoichiometric ratio between reagents, enzyme concen-
tration, solvent use etc).
2.1. Lipases in Non-aqueous Media
It is now well established that enzymes can work with high activities in water-poor
environments usually called non-conventional media (Vermue and Tramper, 1995;
Salis et al., 2005a). The interest in using enzymes in non-aqueous media (organic
solvents, supercritical fluids, solvent-free systems, gaseous media and ionic liquids)
arises from the possibility to perform unusual reactions. Like other hydrolytic
enzymes, lipases can function differently in such media, and instead of triglyceride
hydrolysis they can catalyse transesterification reactions such as the alcoholysis
involved in biodiesel production when suitable reagents and only limited amounts
of water are present.
2.2. Sources of Lipases
Lipases used in biotechnology are normally of microbial origin (Jaeger and Eggert,
2002) and are produced by fermentative processes. A number of commercial lipases
are available for applied biocatalysis (Pandey et al., 1999). Table 1 lists those
most often utilised for biodiesel production. Whilst some are employed as free
powders the majority are used as immobilized preparations. Some of the latter
are commercially available, and in a number of cases the enzymes have been
immobilized on different supports. References given in Table 1 cite first use of a
lipase or its best performance.

320 SALIS ET AL.
Table 1. Source of free and immobilised lipases used for biodiesel production
Lipase source Commercial name Supplier Support Reference
Candida antarctica SP435 Novo Acrylic resin
a
Nelson et al., 1996
Novozym 435 Novo Acrylic resin
a
Shimada et al., 1999
Chirazyme L-2 Roche None (Lee et al., 2002)
Candida cylindracea OF Meito Sangyo None Lara and Park 2004
Candida rugosa - Meito Sangyo None Kaieda et al., 2001
Chromobacterium viscoum - Asahi Celite-545
b
Shah et al., 2004
Cryptococcus spp. S-2 Lipase produced in the researchers’ laboratory None Kamini and Iefuji 2001
Porcine pancreatic - Sigma Anion exchange resin
a
Yesiloglu, 2004
Pseudomonas cepacia PS Amano Sol-gel matrix
b
Noureddini et al., 2005
PS Amano None Kaieda et al., 2001
PS-30 Amano None Abigor et al., 2000
PS-30 Amano Pyllosilicate sol-gel matrix
b
Hsu et al., 2002
PS-D Amano Diatomaceous earth
a
Salis et al., 2005b
Pseudomonas fluorescens - Rhöm GmbH None Mittlebach, 1990
AK Amano None Kaieda et al., 2001
AK Amano Porous kaolinite
b
Iso et al., 2001
AK Amano Polypropylene EP100
b
Soumanou and Bornscheuer, 2003b
Mucor Miehei Lipozyme IM60 Novo Anion exchange resin
a
Nelson et al., 1996
Rhizopus oryzae F-AP15 Amano None Kaieda et al., 1999
Thermomyces lanuginosa Lipozyme TL IM Novo Acrylic resin
a
Du et al., 2003
- Novo Pyllosilicate sol-gel matrix
b
Hsu et al., 2004b
a
: Commercially available immobilised lipases.
b
: Lipases immobilised by researchers in their own laboratories.
USE OF LIPASES FOR THE PRODUCTION OF BIODIESEL
321
2.3. Use of Immobilized Lipases
The use of immobilized enzymes confers two important advantages: i) the ability
to recycle the catalyst and ii) the ability to perform continuous processes. Several
reviews on this topic have been published (Fukuda et al., 2001; Shimada et al.,
2002; Shah et al., 2003). A number of methods for the immobilisation of lipases
on solid supports have been reported (Adlercreutz et al., 1996; Pedersen and
Christensen, 2000). Among these, the best seem to be based on entrapment of
the enzyme in hydrophobic sol-gel matrices (Reetz, 1997) or its adsorption onto
hydrophobic supports such as polypropylene (Bosley and Peilow, 1997; Salis et al.,
2003a). Commercially available lipases are supplied both as lyophilised powders,
which contain other components in addition to the lipase (Salis et al., 2005c), and
immobilied preparations. The immobilized lipase most frequently used for biodiesel
production is lipase B from Candida antarctica (Nelson et al., 1996; Shimada
et al., 1999; Samukawa et al., 2000; Watanabe et al., 2000; Watanabe et al., 2001;
Bélafi-Bakó et al., 2002; Köse et al., 2002; Watanabe et al., 2002; Chen and
Wu, 2003; De Oliveira et al., 2004; Du et al., 2004b; Tuter et al., 2004;
Chang et al., 2005; Lai et al., 2005). This is supplied by Novozymes under
the commercial name Novozym 435 (previously called SP435) and is immobi-
lized on an acrylic resin. The Mucor miehei commercial lipase (Lipozyme
IM60 - Novozymes) immobilized on a macroporous anionic exchange resin has
also been extensively used for the same purpose (Mittlebach, 1990; Nelson
et al., 1996; Selmi and Thomas, 1998; Dossat et al., 1999; Shieh et al.,
2003; De Oliveira et al., 2004). Although commercially immobilized prepara-
tions may find immediate application, the development of new supports is of
considerable interest.
Pseudomonas fluorescens lipase immobilized on porous kaolinite (Toyonite
200-M) gave high conversion ratios for propyl oleate and butyl oleate compared
to those obtained with the lipases from Pseudomonas cepacia, Mucor javanicus,
Candida rugosa and Rhizopus niveus. The Pseudomonas cepacia lipase (PS-30)
immobilized on a phyllosilicate sol-gel matrix was found to be more active than the
lipases of Candida antarctica and Thermomyces lanuginosa immobilized on granu-
lated silica. It was suggested that the higher ester yields of lipase PS-30 may be due
to entrapment of the lipase within the clay sol-gel matrix and its protection from
methanol inactivation. Granulated lipase preparations do not protect the enzymes
from inactivation by polar substrates (i.e. methanol) since they are adsorbed onto
the support (Hsu et al., 2002). Thermomyces lanuginosa and Pseudomonas cepacia
lipases immobilized on a phyllosilicate sol-gel matrix were shown to catalyse ester
formation (80–90% yield) from greases containing a range of free fatty acids from
2.6 to 36% (Hsu et al., 2004b). Porcine pancreatic lipase immobilized by ionic
linkage to a macroporous anion exchange resin was used for the ethanolysis of
sunflower oil in a solvent-free system. High substrate conversion was obtained by
performing the reaction with an oil:alcohol molar ratio of 1:3, at a temperature of
45
C, 0% of added water and 10% wt of lipase based on the weight of the substrate
(Yesiloglu, 2004). The choice of support seems to influence the methanolysis of
322 SALIS ET AL.
triolein in n-hexane. Although not described in detail, it has been reported that
Pseudomonas fluorescens lipase was significantly more active when immobilized
on polypropylene EP100 compared to celite. A conversion of 72.4% was achieved
in the former case but only 1.5% in the latter (Soumanou and Bornscheuer, 2003b).
It should be pointed out that these two supports have very different morphological
features in terms of surface area, pore size distribution and chemical nature (Bosley
and Peilow, 1997; Barros et al., 1998). These parameters strongly influence enzyme
performance but this interesting subject has not been further investigated. Shah
et al. (2004) immobilized Chromobacterium viscosum lipase on Celite 545 for the
ethanolysis of Jatropha oil and found that this procedure increased ester yield from
62 %, obtained with the free lipase, to 71%.
A procedure for the immobilisation of Pseudomonas cepacia lipase was recently
proposed by Noureddini et al. (2005). The lipase was gel-entrapped by polycon-
densation of hydrolysed tetramethoxysilane and iso-butyltrimethoxysilane. The
immobilized lipase catalysed full triglyceride conversion in a very short time
(30 min), it was very stable and lost little activity when subjected to repeated use.
3. SUBSTRATES USED FOR BIODIESEL PRODUCTION
3.1. Use of Different Oil/Fat Sources
The use of a triglyceride feedstock for biodiesel production depends on regional
availability and economics. Rapeseed oil is the most widely used feedstock in
Europe; soybean is mainly used in the United States, and palm oil is used in tropical
regions (i.e. Malaysia). The main difference between these oils is their fatty acid
composition, and this strongly affects some important features of the final biodiesel
mixture. Table 2 details the compositions of the most common oils suitable for
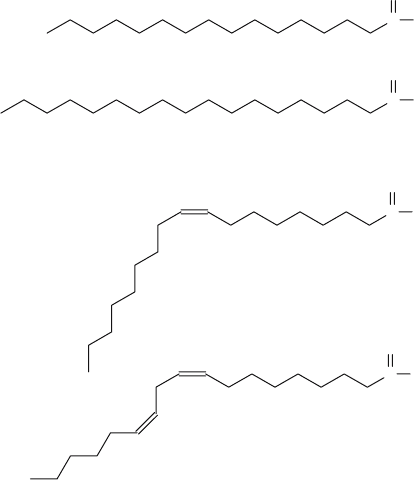
biodiesel production. The most abundant fatty acids are palmitic, stearic, oleic and
linoleic acids. The main physical and chemical properties of an oil/fat depend on
the chemical structures of its fatty acids (Fig. 2). In this regard, a frequent problem
with biodiesel is its stability to oxidation. In linseed, sunflower and soybean oils
the high contents of linoleic acid confers low stability to oxidation as a result of
the presence of two double bonds. Indeed, the oxidation of unsaturated compounds
Table 2. Main fatty acids constituent of the most common oil/fat sources for biodiesel production
Oil/fat Palmitic acid Stearic acid Oleic acid Linoleic acid Problems
source (C16:0) (C18:0) (C18:1) (C18:2)
Soy bean oil 8 4 28 53 Oxidation stability
Palm oil 42 5 41 10 Low temperature
Rape seed oil 4 1 60 20 –
Sun flower oil 6 4 28 61 Oxidation stability
Beef tallow 26 18 37 10 Low temperature
Jatropha oil 13 7 45 34 Low temperature
USE OF LIPASES FOR THE PRODUCTION OF BIODIESEL
323
C OH
O
C OH
O
C OH
O
Linoleic acid (C18:2)
C OH
O
Palmitic acid (C16:0)
Stearic acid (C18:0)
Oleic acid (C18:1)
Figure 2. Chemical structure of the most common fatty acids occurring in oils and fats
proceeds at different rates depending on the numbers and positions of the double
bonds. The CH
2
groups in allylic positions relative to the double bonds in the fatty
acid chains are those susceptible to oxidation (Knothe, 2005b). By comparison,
palm oil and animal fats contain high percentages of saturated fatty acids that are
responsible for the poor low-temperature properties (i.e. high cloud point and pour
point values) of biodiesel fuel. This constitutes a problem in cold regions during
winter. From these considerations and the data in Table 2, it can be concluded that
rapeseed oil is one of the most suitable sources for biodiesel production. Clearly,
triglyceride source affects the properties of the biodiesel blends. Indeed, it was
found that palm kernel oil ethyl esters have a viscosity of 933mm
2
/s, a cloud point
of 12
C and a pour point of 8
C, whereas coconut 1-butyl esters have a viscosity
of 734 mm
2
/s, a cloud point of 5
C and a pour point of −8
C. The properties of
these fuels are likely to be dependent on both the oil and the alcohol used in the
transesterification (Abigor et al., 2000).
3.2. Vegetable Oils
The vegetable oils most studied for use in biodiesel production by biocatalysis
(see Table 3) originate from: soy bean (Nelson et al., 1996; Kaieda et al., 1999;
Kaieda et al., 2001; Shieh et al., 2003), sunflower (Mittlebach, 1990; Bélafi-Bakó
324 SALIS ET AL.
et al., 2002; Soumanou and Bornscheuer, 2003b) and rapeseed (Nelson et al., 1996).
However, some other oleaginous, non-edible species should be mentioned: Jatropha
(euphorbiaceae) is a plant that grows in harsh soils and its seed kernel is 40–60 %
(w/w) oil. This plant cannot be used for edible purposes since its oil contains some
toxic substances, i.e. phorbol esters, that render the oil unsuitable for use in cooking
(Shah et al., 2004). Other triglyceride sources have been explored including the
Nigerian lauric oils palm kernel oil and coconut oil (Abigor et al., 2000), rice
bran oil (Kamini and Iefuji, 2001; Lai et al., 2005), refined cotton seed oil (Köse
et al., 2002), peanut palmolein oil (Soumanou and Bornscheuer, 2003b) and castor
oil (De Oliveira et al., 2004). Regarding biocatalytic processes, almost all sources
of triglycerides can be considered equivalent as enzyme substrates. The different
conversion percentages obtained from the transesterification of palm kernel oil and
coconut oil with lipase PS30 are likely to be due to the different alcohols used
(ethanol and butanol respectively) (Abigor et al., 2000).
3.3. Low Value Triglyceride Feedstocks
The main hurdle in the commercialisation of biodiesel is the cost of the raw
material. Biodiesel – produced by base catalysis - cost more than 0.50 US$/dm
3
in 2001 as compared with 0.35 US$/dm
3
for petroleum-based diesel (Zhang et al.,
2003). It has been reported that 60–75% of the price of biodiesel derives from
the cost of the feedstock oil (Krawczyk, 1996). For this reason, low value triglyc-
eride feedstocks are interesting alternatives for biodiesel production. The principle
problem associated with their use is the necessity for preliminary treatments to
render the oil/fat suitable for the transesterification process. Some of these can be
performed by lipases. Attention has also been paid to the use of low-value triglyc-
erides such as those from restaurant grease (Hsu et al., 2002), waste edible oil
(Watanabe et al., 2001) and animal fats, i.e. tallow (Nelson et al., 1996).
Waste bleaching earths from crude vegetable oil refining processes contain
approximately 40% oil by weight. Efficient methanolysis of oils recovered by
organic solvent extraction - identified as originating from soybean, palm and
rapeseed – has been reported for Rhizopus oryzae lipase in the presence of a high
water content and a single injection of methanol (Lara and Park, 2003). In a follow-
up study the same authors found that Candida cylindracea lipase was the most
active enzyme in methanolysis of oil from waste activated bleaching earths when
n-hexane was used as the solvent (Lara and Park, 2004).
Sunflower acid oils mainly consist of 55.6% free fatty acids and 24.7% triacyl-
glycerols. They are the main by-product of the alkali refining process of crude
vegetable oils to produce edible oils, and are obtained by acidification of soapstocks.
This waste oil was transformed into FAME (65% yield) by means of immobilized
Candida antarctica lipase B (15% based on acid oil weight) at 40
C after 1.5 h and
using n-hexane as the solvent (Tuter et al., 2004).
As already mentioned, animal fat produces a biodiesel with poor low-temperature
properties. In order to improve cold temperature resistant biodiesel several strategies

USE OF LIPASES FOR THE PRODUCTION OF BIODIESEL 325
Table 3. Biodiesel production through different triglyceride feedstocks, alcohols, solvents, reactor types
Oil/fat source Alcohol Lipase source Solvent Type of reactor Conversion (c) or
yield (y)
(mol or wt%)
Reference
Sunflower oil Ethanol Pseudomonas
fluorescens
Petroleum
ether
Batch 82 (y) Mittlebach, 1990
Methanol Pseudomonas
fluorescens
Solvent free 3-step batch > 90 Soumanou and
Bornscheuer, 2003b
Methanol Candida antarctica Solvent free Membrane
reactor
97 (c) Bélafi-Bakó et al.,
2002
Ethanol Mucor miehei Solvent free Batch
(4 cycles)
83 (y) Selmi and Thomas,
1998
Ethanol Porcine pancreatic Solvent free Batch 81 (y) Yesiloglu, 2004
Methanol Rhizomucor miehei Solvent free 3-step batch
(8 cycles)
> 80 (c) Soumanou and
Bornscheuer, 2003a
High oleic sunflower
oil
Butanol Rhizomucor miehei n-Hexane Packed bed
reactor
> 80 (c) Dossat et al., 1999
Sunflower acid oil Methanol Candida antarctica n-Hexane Batch 63.6 (y) Tuter et al., 2004
Soybean oil Methanol Pseudomonas cepacia Solvent free Batch ∼ 60(y) Kaieda et al., 2001
Methanol Candida antarctica Solvent free 3-step batch
(20 cycles)
97 (y) Samukawa et al.,
2000
Methanol Thermomyces
lanuginosa
Solvent free Continuous batch 80–90 (y) Du et al., 2003
Methanol Thermomyces
lanuginosa
Solvent free 3-step batch
(15 cycles)
94 (y) Xu et al., 2004
Methanol –
ethanol
Pseudomonas cepacia Solvent free Batch (12 cycles) 67 (y) Noureddini et al.,
2005
(Continued)

326 SALIS ET AL.
Table 3. (Continued)
Oil/fat source Alcohol Lipase source Solvent Type of
reactor
Conversion (c) or
yield (y)
(mol or wt%)
Reference
Methanol Rhizopus oryzae Solvent free Batch 80 (y) Kaieda et al., 1999
Degummed soybean
oil
Methanol Candida antarctica Solvent free 3-step batch
(25 cycles)
93.8 (c) Watanabe et al.,
2002
Soybean and
rapeseed oil mixture
Methanol Candida antarctica Solvent free 3-step batch
50 cycles
98.4 (c) Shimada et al., 1999
Methanol Candida antarctica Solvent free 3-packed-bed
reactors (100
days)
93 (y) Watanabe et al.,
2000
Triolein – safflower
oil
1-propanol
1-propanol
Pseudomonas
fluorescens
1,4-dioxane
Solvent free
Batch
Batch (10 cycles)
Iso et al., 2001
Triolein Fusel oil-like
mixture
Pseudomonas
cepacia
Solvent free Batch 100 (c) Salis et al., 2005b
Nigerian lauric
oils (palm kernel
and coconut)
Ethanol
1-butanol
Pseudomonas
cepacia
Solvent free Batch 72 (c) Abigor et al., 2000
Pseudomonas
cepacia
Solvent free Batch 40 (c)
Castor oil Ethanol Rhizomucor miehei n-Hexane Batch 98 (c) De Oliveira et al.,
2004
Cotton seed-oil Primary and
secondary
alcohols
Candida antarctica Solvent free Batch 91.5 (methanol) Köse et al., 2002
Methanol Rhizomucor miehei Solvent free Batch
(8 cycles)
> 90 (c) Soumanou and
Bornscheuer, 2003b

USE OF LIPASES FOR THE PRODUCTION OF BIODIESEL
327
Rice bran oil Methanol Cryptococcus spp.
S-2
Solvent free Batch 80.2 (y) Kamini and Iefuji,
2001
Methanol Candida antarctica Solvent free Batch 98 (c) Lai et al., 2005
Jatropha oil Ethanol Chromobacterium
viscoum
Solvent free Batch 92 (y) Shah et al., 2004
Waste activated
bleaching earths
(ABE) oil
Methanol Candida cylindracea Diesel fuel Batch ∼ 100 (y) Kojima et al., 2004
Methanol Rhizopus oryzae Water Batch 55 (y) Lara Pizarro and
Park, 2003
Waste edible-oil Methanol Candida antarctica Solvent free Packed-bed
reactor (100
days)
90 (y) Watanabe et al.,
2001
Tallow (other oils) Primary alcohols Mucor Miehei n-Hexane Batch > 90 (c) Nelson et al., 1996
Restaurant grease Methanol Pseudomonas
cepacia
Solvent free Batch 98 (y) Hsu et al., 2002
Methanol Candida antarctica Solvent free Batch 96 (c) Lee et al., 2002
Ethanol Burkholderia
cepacia
Solvent free Packed bed
reactor
> 96 (y) Hsu et al., 2004a
Fractionated lard Methanol Candida antarctica Solvent free Batch 58 (c) Lee et al., 2002
328 SALIS ET AL.
can be followed. Lee et al. (2002) decreased the content of saturated fatty acids
present in lard and restaurant grease by performing an acetone fractionation step
followed by methanolysis catalysed by Chirazyme L-2 (Candida antarctica lipase).
Methanolysis of rice bran oil having a free fatty acid content greater than
18% gave conversions < 68%. A two-step lipase-catalysed (Candida antarctica)
methanolysis of rice bran oil was developed for the efficient conversion of both free
fatty acids and acylglycerides to FAME. More than 98% conversion can be obtained
in 4–6 h, depending on the relative proportion of free fatty acids and acylglycerides
present (Lai et al., 2005).
3.4. Alcohols
As already discussed, for cost reasons methanol is the reagent most frequently used
for triglyceride transesterification. Nevertheless, other alcohols are also used. In
Brazil biodiesel is produced by ethanolysis of triglycerides since ethanol is obtained
cheaply by the fermentation of sucrose from sugarcane. The use of different alcohols
gives different results. Alcoholysis of Nigerian lauric oils catalysed by lipase PS-30
gave different oil conversions with methanol, ethanol, 1-propanol, iso-propanol,
1-butanol and iso-butanol (Abigor et al., 2000). However, this was not only related
to the alcohol since the conversion trend was different for palm kernel compared
to coconut oil. It is worth noting that methanol gave the lowest conversions in both
these cases.
Nelson et al. (1996) used linear and branched alcohols for the biocatalytic trans-
esterification of tallow using hexane as solvent. They found that Candida antarctica
lipase was the most efficient in the transesterification with secondary alcohols,
whereas lipase from Mucor miehei was the most efficient with primary alcohols.
The use of C
3
-C
5
linear and branched alcohols from fusel oil, a low-value residue
from ethanol distillation, might constitute an interesting and cheap alternative to
methanol. Salis et al. (2005b) carried out the biocatalytic alcoholysis of triolein
with a fusel-oil like mixture. On a molar basis, fusel oil mainly comprises: isoamyl
alcohol (64.4%), 2-butanol (27.6%), 2-methyl-1-propanol (12.3%), 1-propanol
(5.6%) and 1-butanol (1.3%). These alcohols are not enzyme denaturing and
their esters, mainly the branched ones, improve the low-temperature properties of
biodiesel blends (Dunn, 2005). It should be remarked that the absence of methanol
makes the whole process more environmentally friendly. A different result was
obtained by Kaieda et al. (1999). In their case the ester content decreased in
the series methanol > iso-butanol > ethanol > butanol > propanol in catalytic
alcoholysis of rice bran oil using crude Cryptococcus spp. S-2 lipase. A methyl
ester content of 80.2% was obtained in the presence of a high content of water
(80% of substrate weight).
Conversion of cottonseed oil in a 24 h reaction at 40
C in a solvent free
system has been performed with various alcohols. Low conversions (10%) were
observed with short chain alcohols especially with Thermomyces lanuginosus lipase.
Higher conversions were obtained with Rhizomucor miehei lipase (about 30%)
