Pati S.K., Enoki T., Rao C.N. R. (eds.) Graphene and Its Fascinating Attributes
Подождите немного. Документ загружается.

x Contents
3. Results and Discussion 36
3.1. Electrochemical detection of dopamine
using graphene-alloy nanocomposites 38
3.2. Composites of exfoliated graphene oxide- and
Co
3
O
4
or RuO
x
40
4. Summary 41
Acknowledgments 42
References 42
Chapter 3 Singlet Open-Shell Character of Polyperiacenes 45
A. Shimizu, A. Konishi, Y. Hirao and T. Kubo
1. Introduction 45
2. Theoretical Consideration on Open-Shell Character 46
2.1. Clar’s aromatic sextet valence bond model 46
2.2. Quantum chemical method 49
2.3. Aromaticity of each ring 50
2.4. More extended ring system 51
3. Experimental Elucidation of the Smallest
Polyperiacene 52
3.1. Geometrical consideration 52
3.2. Physical properties 53
4. Conclusion 54
Acknowledgments 55
References 55
Chapter 4 Doping of Graphene: A Computational Study 59
A. K. Manna and S. K. Pati
1. Introduction 60
2. Computational Details 62
3. Metal Nanoclusters Graphene Complexes 63
4. Molecule-Graphene Complexes 67
5. Summary 72
Acknowledgments 73
References 73
Contents xi
Chapter 5 Vibrations and Buckling of Uni-Axially
Strained Graphene and BN-Monolayer: A
First-Principles Study 77
K. P. S. S. Hembram and U. V. Waghmare
1. Introduction 77
2. Methods 78
3. Results 79
3.1. Structure 79
3.2. Phonons 80
3.3. Electronic structure 86
4. Conclusion 88
Acknowledgment 88
References 88
Chapter 6 Raman Spectroscopy of Graphene Edges 91
R. Saito
1. Introduction 91
2. Method 94
3. Calculated Raman Spectra 95
4. Discussion and Summary 98
Acknowledgments 99
References 99
Chapter 7 Probing Single and Bilayer Graphene Field Effect
Transistors by Raman Spectroscopy 105
A. Das, B. Chakraborty and A. K. Sood
1. Introduction 105
2. Vibrational Properties of Graphene 107
3. Raman Spectra of Graphene 108
4. Tuning the Fermi Energy by Field Effect Gating 112
4.1. Single layer top gating 113
4.2. Bilayer top gating 117
4.2.1. Conversion of V
TG
into E
F
117
xii Contents
4.3. Theoretical calculations 120
4.3.1. Comparison between the experiment
and theory (Bilayer) 127
4.3.2. Physical interpretation 127
5. Conclusions 130
Acknowledgments 130
References 131
Chapter 8 Phonons and Electron-Phonon Interaction in
Graphene and Nanotube 135
T. Ando
1. Introduction 135
2. Monolayer Graphene and Nanotube 135
3. Acoustic Phonon 136
4. Optical Phonon 138
5. Zone-Boundary Phonon 142
6. Spontaneous Lattice Distortion 144
7. Bilayer Graphene 144
Acknowledgments 147
References 148
Chapter 9 Magnetic Structures of Edge-State Spins in
Nanographene and a Network of Nanographene Sheets 151
T. Enoki, V. L. J. Joly and K. Takai
1. Introduction 151
2. Electronic Structure of Edge State and STM/STS
Observations 153
3. Magnetic Properties of Nanographene and
Nanographite 156
3.1. Effect of electron localization on the
magnetism of the edge-state spins 157
3.2. Spin glass state in the nanographite network 162
4. Summary 164
Acknowledgments 165
References 165
Contents xiii
Chapter 10 Electronic and Transport Properties of Graphene
Nanoribbons 167
K. Wakabayashi
1. Introduction 167
2. Electronic States of Graphene Nanoribbons 168
3. Electronic Transport Properties 169
3.1. One-way excess channel system 170
3.2. Perfectly conducting channel 173
3.3. Graphene nanoribbons with generic edge
structures 174
4. Transport Properties through Graphene Junction 176
5. Summary 178
References 178
Chapter 11 Gate-Voltage Modulation in Graphene 179
K. Tsukagoshi, H. Miyazaki, S.-L. Li, A. Kumatani,
H. Hiura and A. Kanda
1. Introduction 179
2. Experimental 180
2.1. Quick and precise judgment method for
number of layers 180
2.2. Device fabrication process 182
2.3. Top gate capacitance 184
2.4. Conductance control in graphene by dual
gate voltages 185
Acknowledgments 186
References 186
Chapter 12 Kondo Physics in Graphene 189
K. Sengupta
1. Introduction 189
2. Analysis of the Kondo Model 190
3. Experiments 196
4. Conclusion 197
References 197
xiv Contents
Chapter 13 Noise in Graphene Transistors 199
A. N. Pal and A. Ghosh
1. Introduction 199
2. The Correlated Number and Mobility
Fluctuation Model 202
3. Experimental Section 206
3.1. Sample preparation and characterization 206
3.2. Noise characteristics in graphene based
FET devices 207
3.3. Noise in dual gated BLG device 209
4. Conclusion 211
Acknowledgments 211
References 211
Chapter 14 Spin Transport in Single- and Multi-Layer Graphene 215
M. Shiraishi, M. Ohishi, N. Mitoma, T. Takano,
K. Muramoto, R. Nouchi, T. Nozaki, T. Shinjo
and Y. Suzuki
1. Introduction 215
2. Experimental 216
3. Results and Discussion 218
Acknowledgments 224
References 224
Chapter 15 Quantum Complexity in Graphene 227
G. Baskaran
1. Introduction 227
1.1. Complexity — Classical & quantum 229
1.2. Novel phenomena in graphene 231
2. Spin-1 Collective Mode 234
3. Relativistic Type Effects 241
4. Possibility of Room Temperature Superconductivity
in Optimally Doped Graphene 248
5. Composite Fermi Sea 255
Contents xv
6. Two Channel Kondo Effect 263
7. Summary 266
Acknowledgment 266
References 266
1
Chapter 1
Graphene: Synthesis, Functionalization and Properties
C. N. R. Rao, K. S. Subrahmanyam, H. S. S. Ramakrishna Matte
and A. Govindaraj
Chemistry and Physics of Materials Unit,
International Centre for Materials Science,
New Chemistry Unit and CSIR Centre of Excellence in Chemistry,
Jawaharlal Nehru Centre for Advanced Scientific Research,
Jakkur P.O., Bangalore – 560 064, India
Graphenes with varying number of layers can be synthesized by different
strategies. Thus, single-layer graphene is obtained by the reduction of single layer
graphene oxide, CVD and other methods besides micromechanical cleavage.
Few-layer graphenes are prepared by the conversion of nanodiamond, arc-
discharge of graphite and other means. We briefly present the various methods of
synthesis and the nature of graphenes obtained. We then discuss the various
properties of graphenes. The remarkable property of graphene of quenching
fluorescence of aromatic molecules is shown to be associated with photo-induced
electron transfer, on the basis of fluorescence decay and time-resolved transient
absorption spectroscopic measurements. The interaction of electron donor and
acceptor molecules with few-layer graphene samples has been discussed.
Decoration of metal nano-particles on graphene sheets and the resulting changes
in electronic structure are examined. Few-layer graphenes exhibit ferromagnetic
features along with antiferromagnetic properties, independent of the method of
preparation. Graphene-like MoS
2
and WS
2
have been prepared by chemical
methods, and the materials are characterized by electron microscopy, atomic
force microscopy (AFM) and other methods. Boron nitride analogues of graphene
have been obtained by a simple chemical procedure starting with boric acid and
urea and have been characterized by various techniques.
1. Introduction
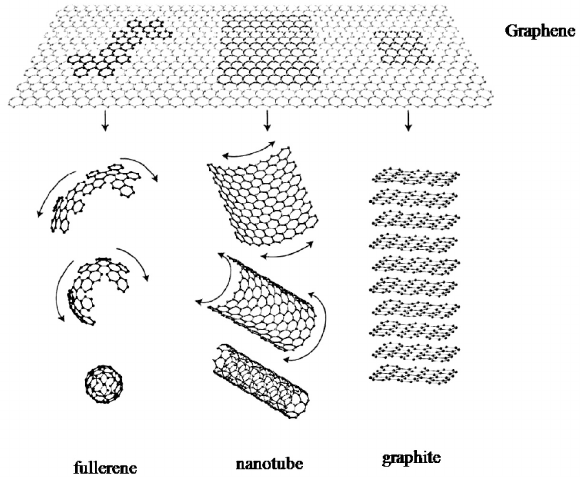
Graphene, the mother of all graphitic forms (Fig. 1), has become one of
the most exciting topics of research in the last 4 to 5 years [1-6]. This
2 C. N. R. Rao et al.
two-dimensional material constitutes a new nanocarbon comprising
layers of carbon atoms forming six-membered rings.
Fig. 1. Graphene: mother of all graphitic forms (From reference 1).
It is distinctly different from carbon nanotubes and fullerenes,
exhibiting unique properties which have fascinated the scientific
community. Typically important properties of graphene are fractional
quantum Hall effect at room temperature [7-9], an ambipolar electric
field effect along with ballistic conduction of charge carriers [10],
tunable band gap [11] and high elasticity [12]. Although graphene is
expected to be perfectly flat, ripples occur because of thermal
fluctuations [1]. Ideally graphene is a single-layer material, but graphene
samples with two or more layers are being investigated with equal
interest. One can define three different types of graphenes: single-layer
graphene (SG), bi-layer graphene (BG) and few-layer graphene (number
of layers ≤ 10). Although SG and BG were first obtained by micro-
mechanical cleavage [10], since then, graphenes containing different
Graphene: Synthesis, Functionalization and Properties 3
numbers of layers have been prepared using diverse strategies [4,5,13].
There are a few reports on some of the properties of few-layer graphenes,
but there are not many studies reporting changes in properties brought
about by the number of layers. Furthermore, we do not have well-defined
procedures for the synthesis of graphenes with the desired number of
layers.
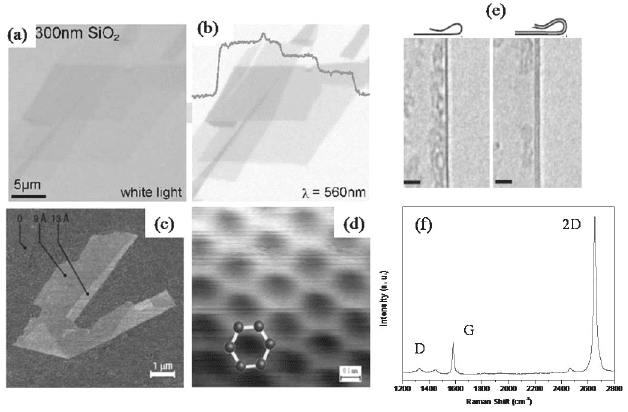
Fig. 2. Optical microscope images of grapheme crystallites on 300 nm SiO
2
imaged with
(a) white and (d) green light. (b) Shows step-like changes in the contrast for single, bi-
and tri-layer graphenes. (c) AFM image of single-layer graphene. The folded edge
exhibits a relative height of ≈ 4 Å indicating that it is single-layer. (d) High-resolution
STM image. (e) TEM images of folded edges of single and bi-layer graphenes (From
references 14, 15, 17 and 19). (f) Raman spectrum of single-layer graphene prepared by
micromechanical clevage.
Graphene has been characterized by a variety of microscopic and
other physical techniques including atomic force microscopy (AFM),
transmission electron microscopy (TEM), scanning tunneling microscopy
(STM) and Raman spectroscopy [1-5]. It is interesting that SG placed on
a Si wafer with a 300 nm thick layer of SiO
2
, becomes visible in an
optical microscope (Figs. 2(a) and 2(b)) [14-16]. While AFM directly
gives the number of layers (Fig. 2(c)) [14-15], STM (Fig. 2(d)) [17]
