Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.

Human and
Rat
Liver Cytochromes P450
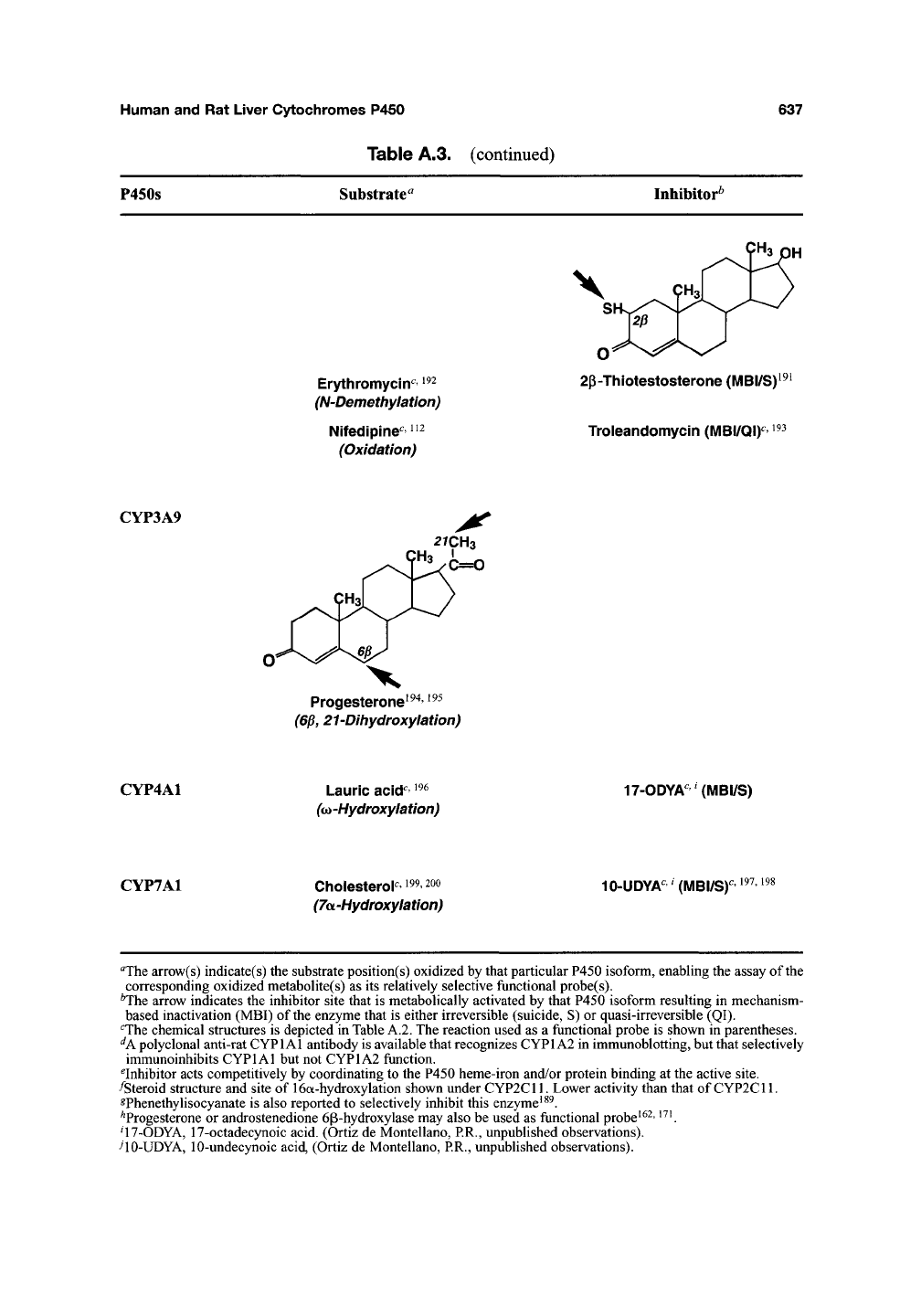
Table A.3. (continued)
637
P450s Substrate^
Inhibitor^
Erythromycin^'
^^^
(N-Demethylation)
Nifedipines^
^2
(Oxidation)
2p-Thiotestosterone (MBI/S)
Troleandomycin {MB\/Q\y
191
CYP3A9
2rcH3
Progesterone^^^,
195
(6p, 21-Dihydroxylation)
CYP4A1
Laurie acid^ ^^^
^(o -Hydroxylation)
17-ODYA"'(MBI/S)
CYP7A1
Cholesterol^ 199,200
(7oL-Hydroxylation)
10-UDYA"'(MBI/S)
,c,
197,
198
^The arrow(s) indicate(s) the substrate position(s) oxidized by that particular P450 isoform, enabUng the assay of the
corresponding oxidized metaboHte(s)
as its
relatively selective functional probe(s).
*The arrow indicates
the
inhibitor site that
is
metabolically activated
by
that P450 isoform resulting
in
mechanism-
based inactivation (MBI)
of
the
enzyme that
is
either irreversible (suicide,
S) or
quasi-irreversible (QI).
'^The chemical structures
is
depicted in Table A.2. The reaction used
as a
functional probe
is
shown
in
parentheses.
^A polyclonal anti-rat CYPl Al antibody is available that recognizes CYPl A2
in
immunoblotting, but that selectively
immunoinhibits CYPlAl but not CYP1A2 function.
^Inhibitor acts competitively
by
coordinating
to the
P450 heme-iron and/or protein binding
at
the active site.
^Steroid structure
and
site
of
16a-hydroxylation shown under CYP2C11. Lower activity than that
of
CYP2C11.
^Phenethylisocyanate
is
also reported to selectively inhibit this enzyme^
^9.
^Progesterone
or
androstenedione 6p-hydroxylase may also
be
used
as
functional probe^^^,
ni
'17-ODYA, 17-octadecynoic acid. (Ortiz
de
Montellano, PR., unpublished observations).
^10-UDYA, 10-undecynoic acid, (Ortiz
de
Montellano, PR., unpublished observations).
638 M.A. Correia
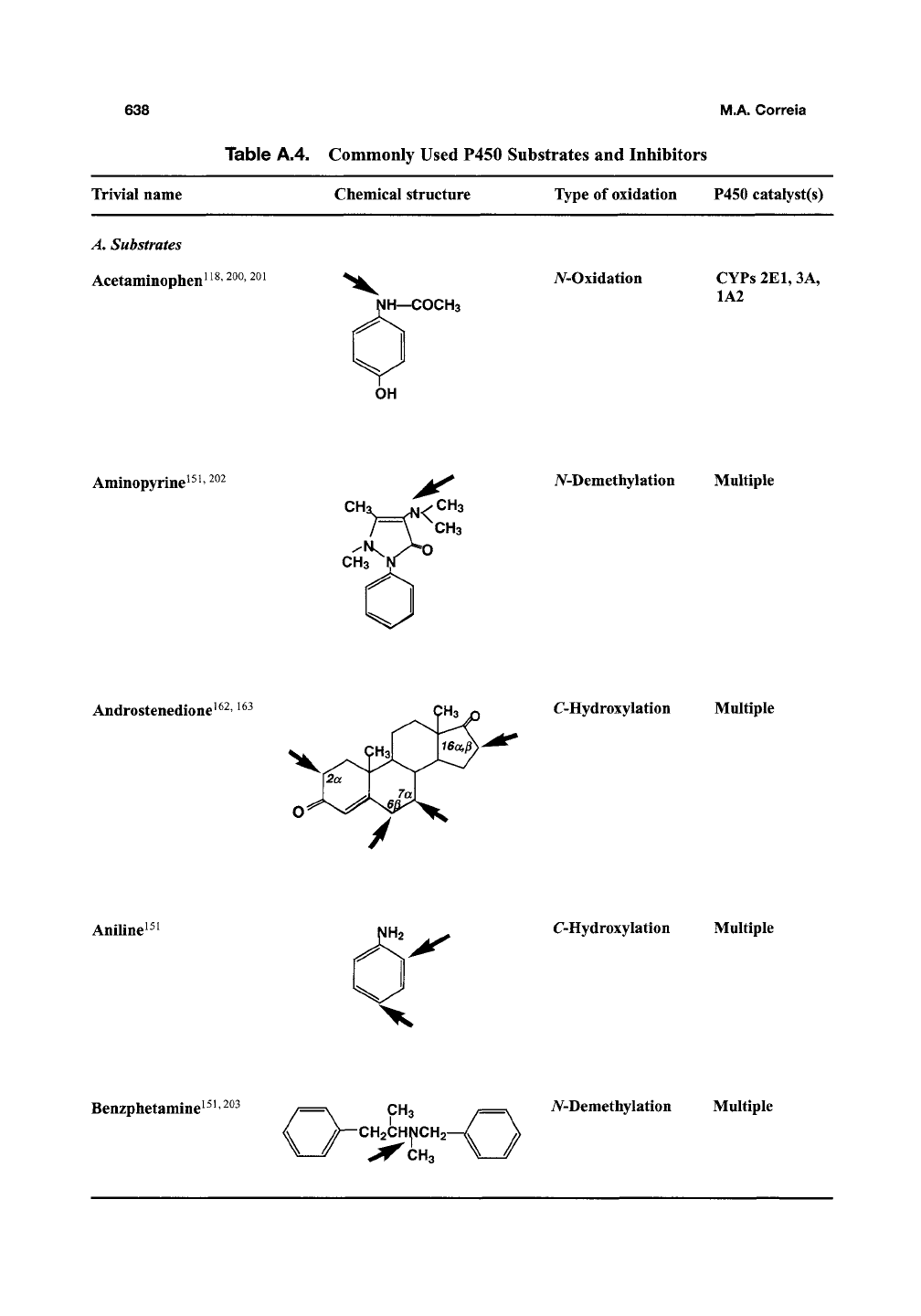
Table A.4. Commonly Used P450 Substrates and Inhibitors
Trivial name
A.
Substrates
Acetaminophen!^^ 200,201
Chemical structure
NH—COCH3
Type of oxidation
iV-Oxidation
P450 catalyst(s)
CYPs 2E1,3A,
1A2
OH
Aminopyrine^^!'
^^^
CHa JS1/CH3
CH3
CHa
A^-Demethylation Multiple
Androstenedione^^^, i63
C-Hydroxylation
Multiple
Aniline!
C-Hydroxylation
Multiple
Benzphetamine^^! 203 /=^=\ CH3 /=\ iV-Demethylation Multiple
\j-'ytt^
Human and Rat Liver Cytochromes P450
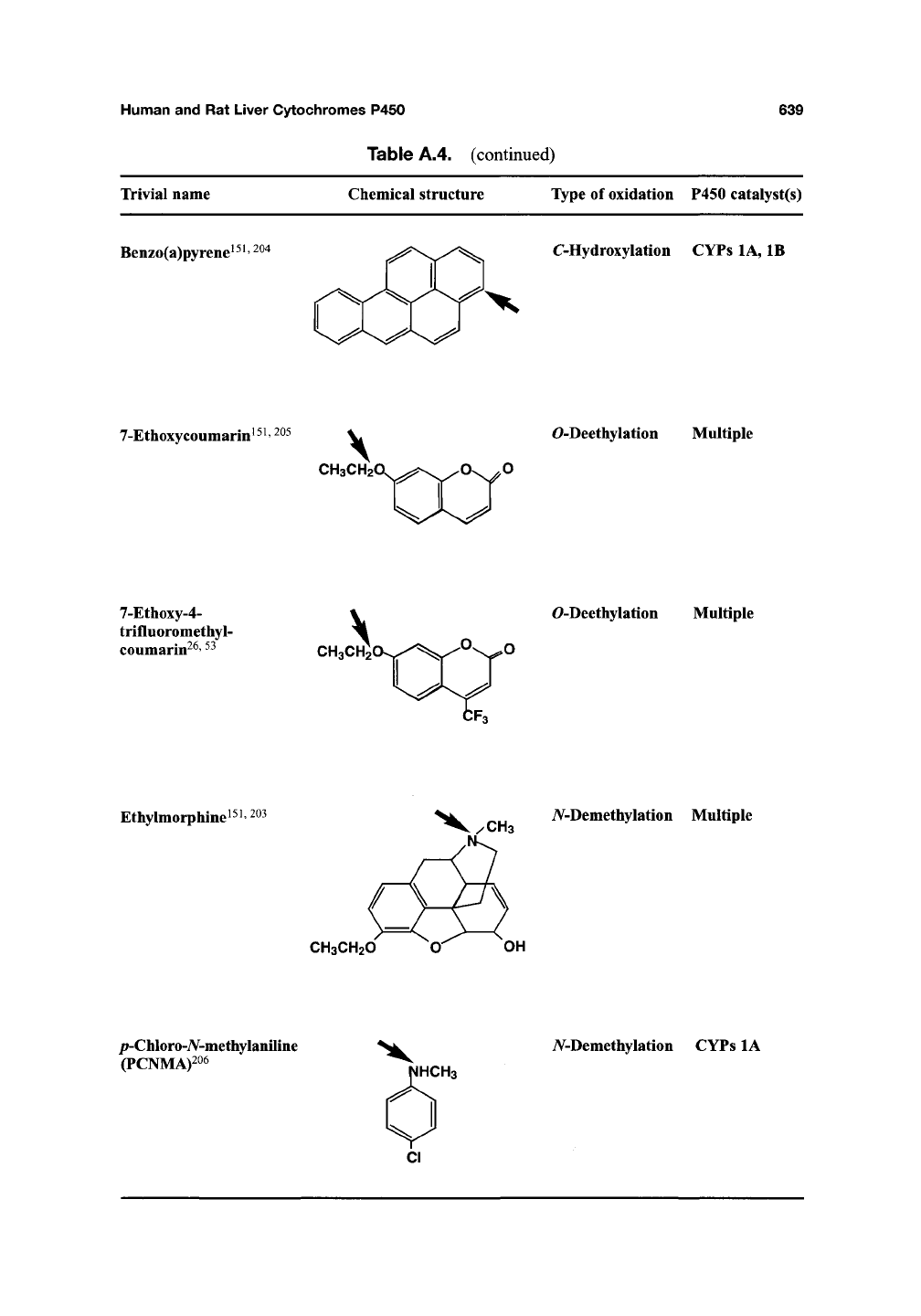
Table A.4. (continued)
639
Trivial name
Chemical structure Type of oxidation P450 catalyst(s)
Benzo(a)pyrene^^^'
^^"^
C-Hydroxylation
CYPs lA, IB
7-Ethoxycoumarin i ^ i,
205
^
CH3CH20.,^^;s>ss^O\^0
O-Deethylation
Multiple
^^^^^^^
7-Ethoxy-4-
trifluoromethyl-
coumarm
26,53
CH3CH2'
O-Deethylation
Multiple
Ethylmorphine ^
CH3
iV-Demethylation Multiple
CH3CH2O O OH
/j-Chloro-A'-methylaniline
(PCNMA)206
iV-Demethylation CYPs lA
HCH3
CI
640
M.A. Correia
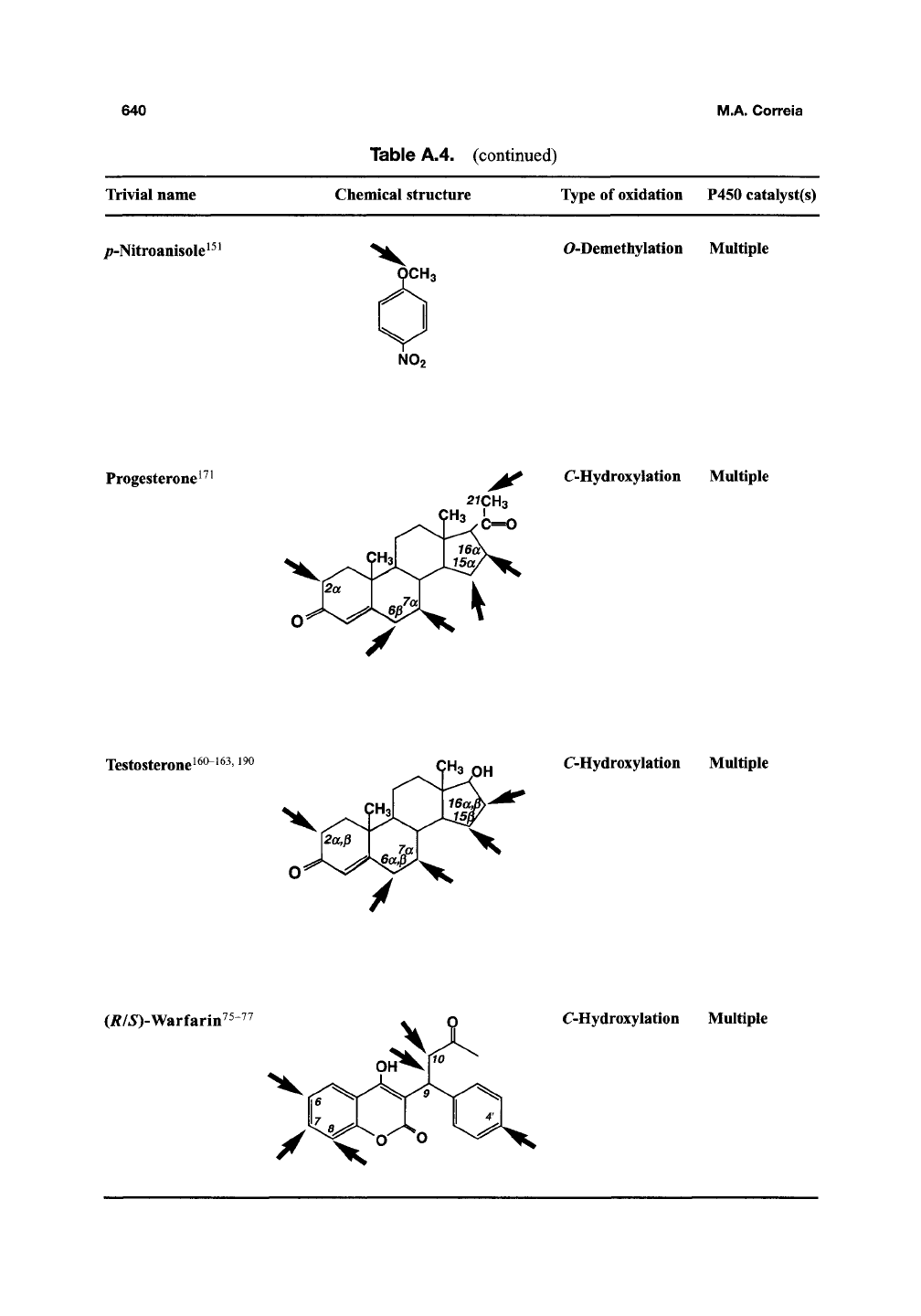
Table A.4. (continued)
Trivial name Chemical structure
lype of oxidation P450 catalyst(s)
/;-Nitroanisole^^^
CH3
NO2
O-Demethylation
Multiple
Progesterone^
^^
C-Hydroxylation
Multiple
2rCH3
^ c=o
Testosteronei60-i63, i90
'3 OH
C-Hydroxylation
Multiple
(i?/5)-Warfarin^
/ X
C-Hydroxylation
Multiple
Human
and Rat
Liver Cytochromes P450
Table A.4. (continued)
641
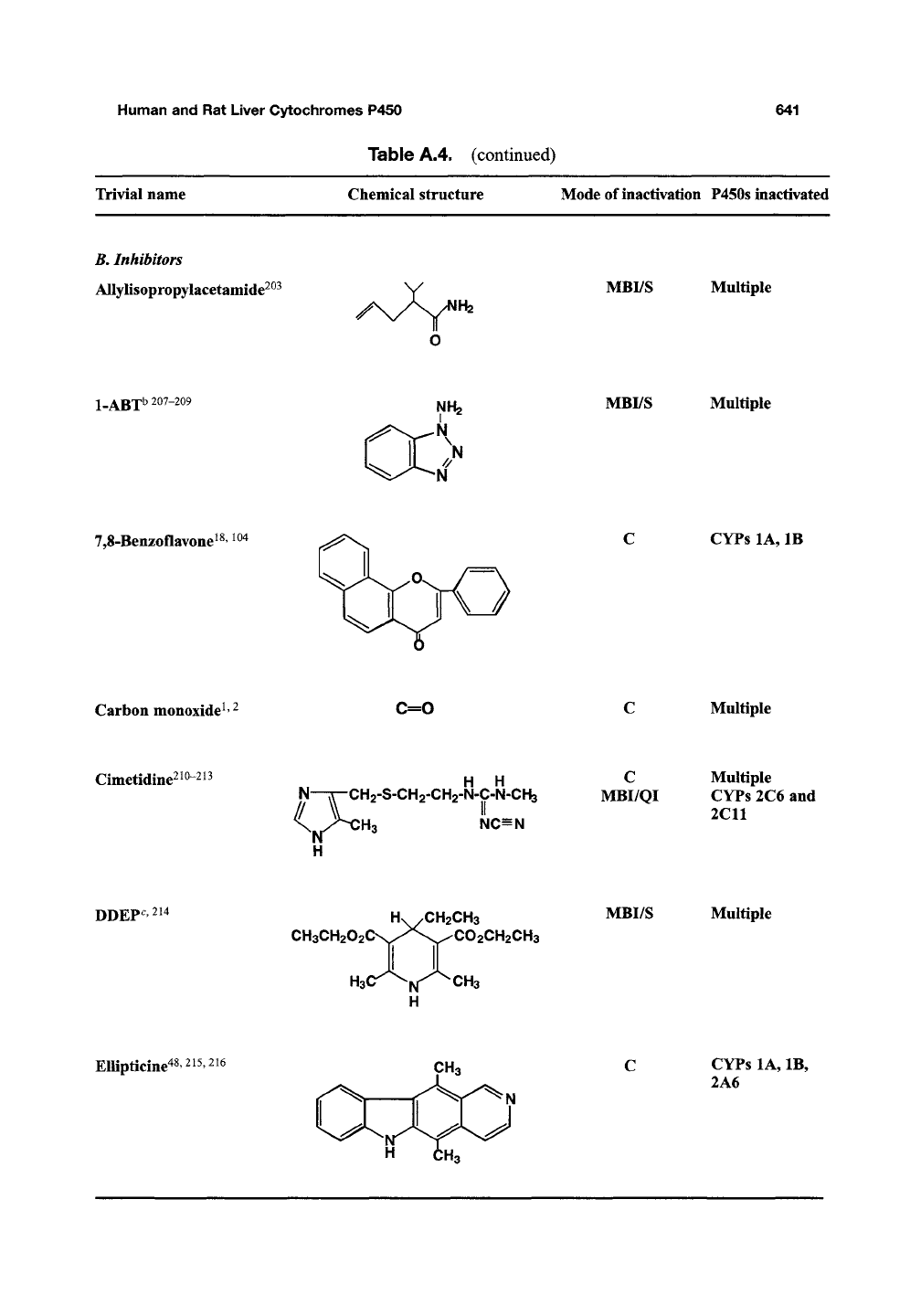
Trivial name Chemical structure Modeof inactivation P450s inactivated
B.
Inhibitors
Allylisopropylacetamide^^^
/
H2
MBI/S Multiple
1-ABT
b 207-209
NH2
N
>
N
MBI/S Multiple
7,8-Benzoflavone^
CYPs
lA, IB
Carbon monoxide^'
^
C=0
Multiple
Cimetidine^
,210-213
H
H
N—TT-CHz-S-CHa-CHg-N-C-N-CHa
/
\
NC=N
C
MBI/QI
Multiple
CYPs
2C6 and
2C11
DDEP^
HV/CH2CH3 MBI/S Multiple
CH3CH202CN,^^^\S-^C02CH2CH3
H3U
l^ CH3
H
Ellipticine^
CYPs
lA, IB,
2A6
642 M.A. Correia
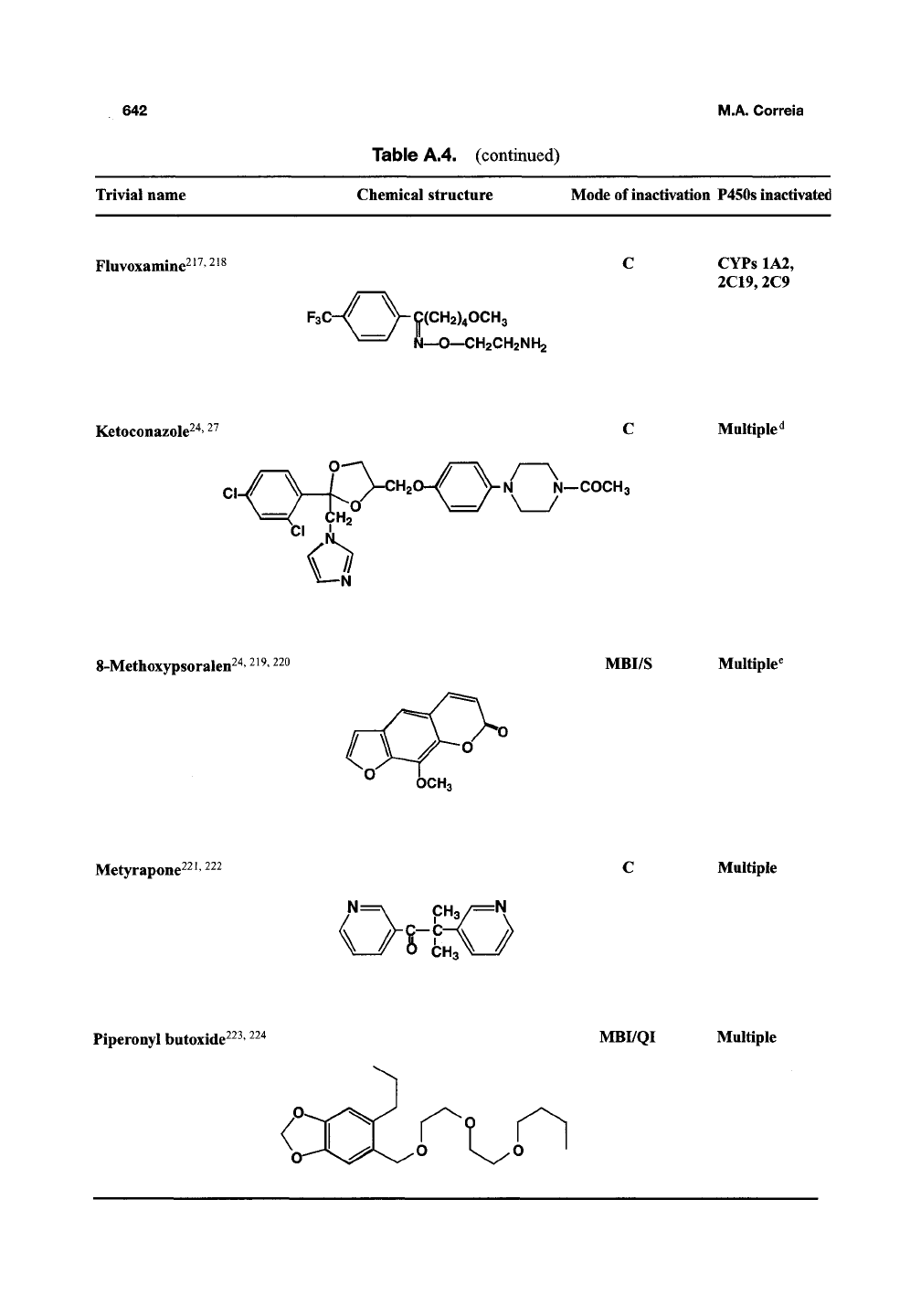
Table A.4. (continued)
Trivial name
Chemical structure Mode of inactivation P450s inactivated
Fluvoxamine^^^'^^^
F3C-/
V9(CH2)40CH3
N—O—CH2CH2NH2
CYPs 1A2,
2C19,2C9
Ketoconazole^
"6°
I—COCH3
Multiple^
8-Metlioxypsoralen^
MBI/S Multiple^
Metyrapone^^^'
^^^
N=
V_/S-EO
Multiple
Piperonyl butoxide^^^'
^^"^
MBI/QI Multiple
Human and Rat Liver Cytochromes P450 643
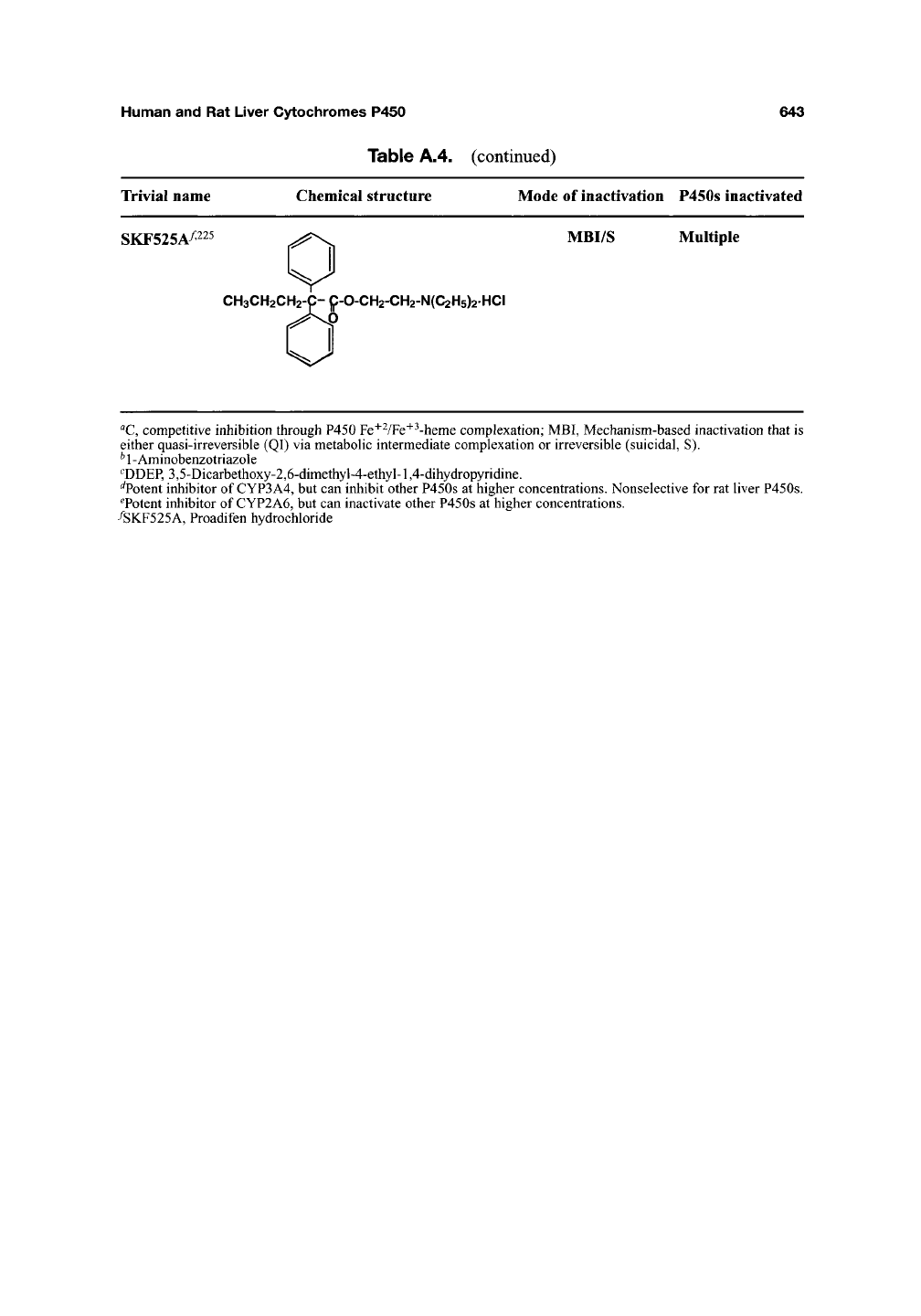
Table A.4. (continued)
Trivial name Chemical structure Mode of inactivation P450s inactivated
SKF525A^225 .^^^^ MBI/S Multiple
CH3CH2CH2-C-C-0-CH2-CH2-N(C2H5)2HCI
"C,
competitive inhibition through P450 Fe'''VFe"'"^-heme complexation; MBI, Mechanism-based inactivation that is
either quasi-irreversible (QI) via metabohc intermediate complexation or irreversible (suicidal, S).
* 1 - Aminobenzotriazole
^DDEP, 3,5-Dicarbethoxy-2,6-dimethyl-4-ethyl-1,4-dihydropyridine.
^Potent inhibitor of CYP3A4, but can inhibit other P450s at higher concentrations. Nonselective for rat liver P450s.
^Potent inhibitor of CYP2A6, but can inactivate other P450s at higher concentrations.
/SKF525A, Proadifen hydrochloride
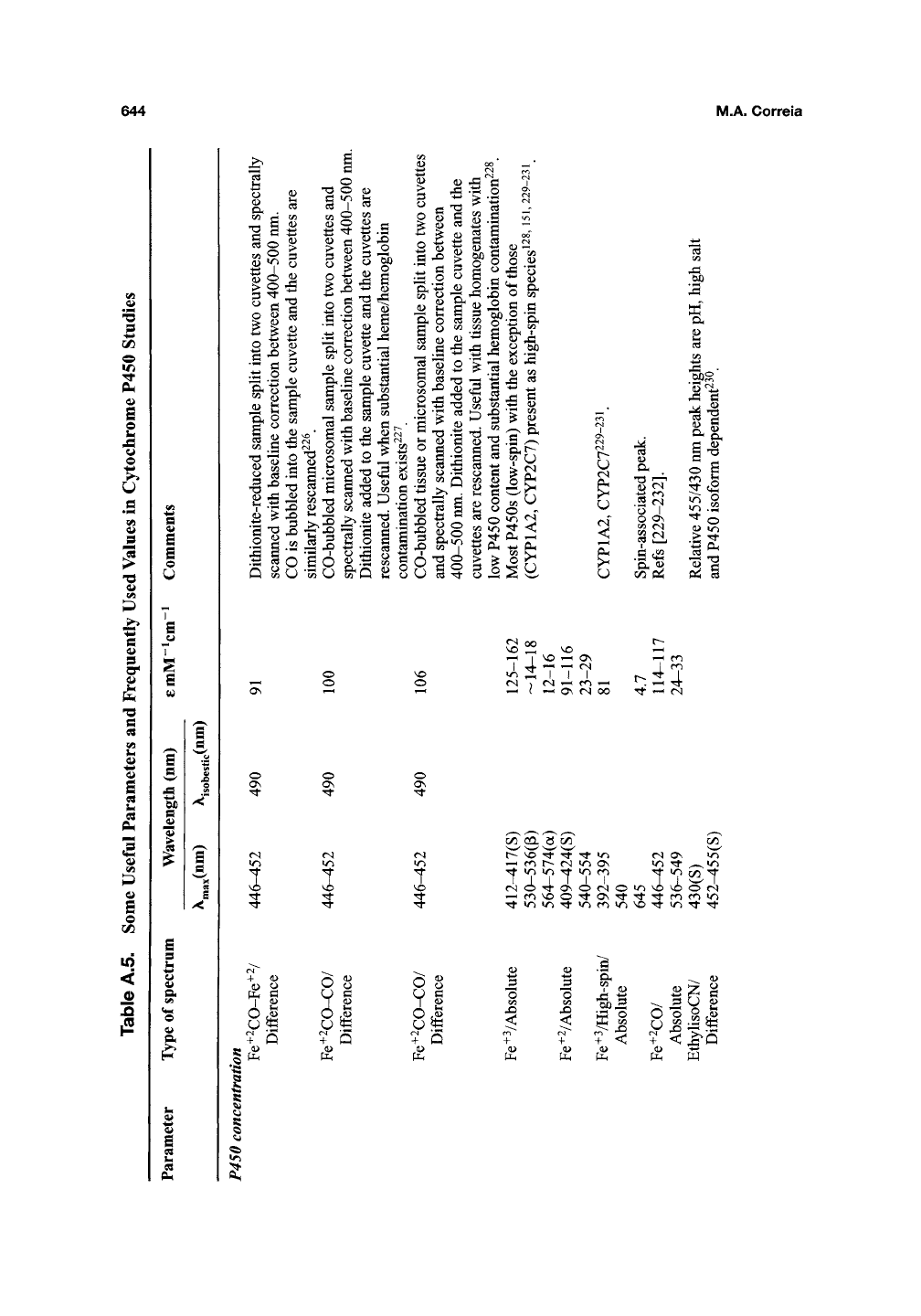
644
M.A. Correia
^^
o
"^
PH
s
£
O
>^
U
0^
E
s
o
<
.(0
s
s
o
1
a
fs"
IN
4>
t>
s
A
k^
CQ
PLH
.1
•«
«
V.
•««*
s:
Q
;j
^
«^
>
0^
<u.
11
i "«
o .S ^
^ JD O
P Of) O
^ .2 (u eg
O
111
^ . So
"^ T3 .^
I i!
"T
^
^ S I
^H
<L>
O
c/5
U
<^ o ^'^ ^
00 lO ^ <
a>
rj- OH ^
>
^ O »^
U
^ I
<N
.^
e«
0)
T3
>—r
5 <N
O
OS
X
<^
5S
^
CQ L_J
^
W)
'A
ffi
CU
<D
^
xn
^ ^•
•
SP^
<D
"^
45 «
^1
-1
PH
<L)
g 73
O B
5l
^ o
<D
»r^
•^;?
4_> p.
s irt
o
o
'—1
^
o
^^
<N 00
^ 1 vo ^ 0>
r>4 ) rvj '-H m ^^
^ ( '^ OS (N OO
Tf ^ <N
O
OS
o
OS
1 1
^
^
o
OS
c/3 CO. d c/5
r-
^ -^ '^ Tf »r>
^
CO
r^ <N u^ OS
"^ u-^ u-^ Tt lo m
rsi o Ti- OS o <N o
^ m ^ o Tf OS Tt
Tf u-i »r> Tt «ri CO u^
CN OS »^
u-^
"1-
^^'/^
lo vo vo o^rsi
rt
TT
CO CO
»0
VO rj- lo Tf Tt
t:^ 9^
? S ? S
O ,i>
? 5
<
+
^5
<u
^
'o
t/5
<
O
U
+
•\ <i^
sz S
S U J>
^ -^ T2
<^Q
w
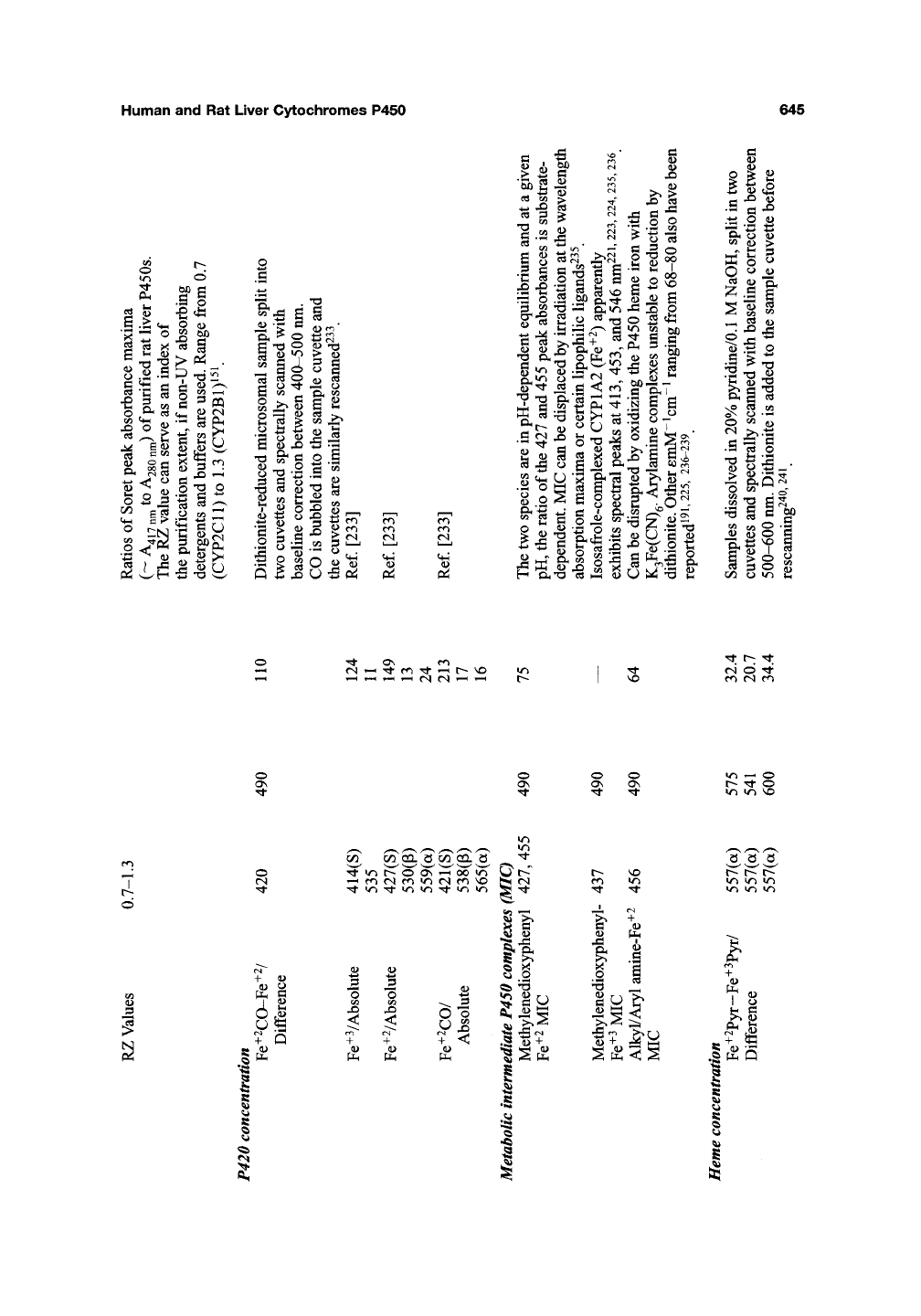
Human and Rat Liver Cytochromes P450 645
ys
•e*
o a>
> ^
o ,
x^
t3<:
o a
o -g o
&~
U
-SB
o
5SJ
CO
P(^
PilH
<N O '^t
CO fS ro
O
ON
o
ON
^
o
OS
^
o
OS
Tf
U^ '—1 O
r- Tt o
to u^ ^
I
o
lyj
GO.
d c/3 oQ. d
O OS ^H 00 iTi
CO «o <N en vo
to u-^ Ti" in »o
*r> *o »o
m »o to
§^
CD
+ 0)
^ (D
6
I
I
I
S
J
CO
+
I
S
1
^
s
I
&
S
;^ pL, < ;^
i
I
I
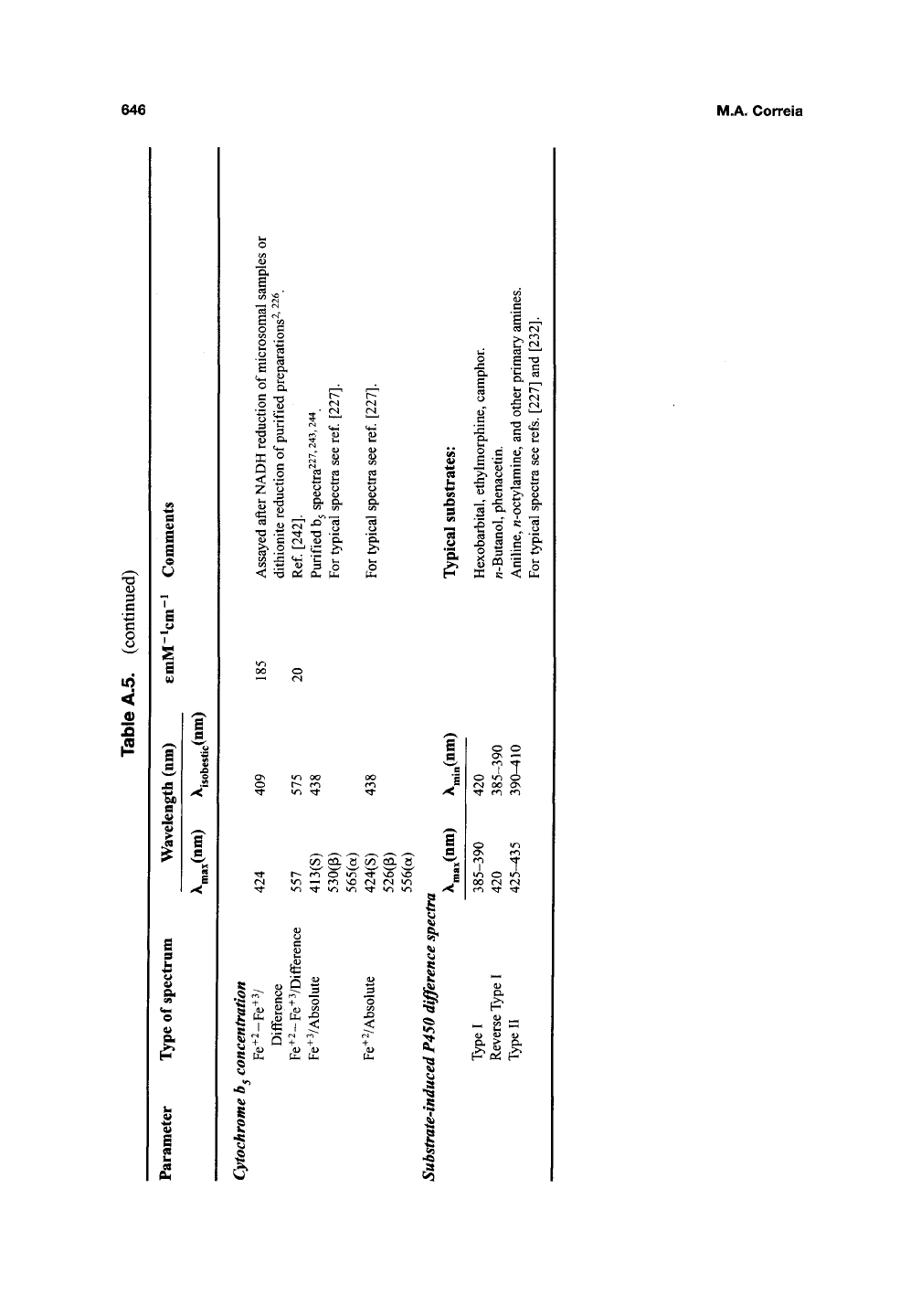
646
M.A.
Correia
g
O
<
o
S
g
o
U
s
s
—
w
OH
(U
Ok
S S
fi
(N
"^
£1
Di^
<u
OH
(73
JP
TS
•g
PH
(L>
(X
on
1
o
fe
4>
-w
CS
is
CA
,fi
s
CA
&
o
B
"B
43
_^
B
^
e3
X>
O
X
.c
"S
o
CO
(U
J3
a.
"o
1
OQ
a
'B
M
"^
o
o
K
'S
<
(Z>
c«
o
(U
ex
1/3
13
r-
ro
O
u-^
Tf
vo
^
»n
^^
m
vo
<N
rN
«o
yn
-rf
in
IT)
'Tt *r) *r)
s
2
§
i
I
^
+
to
I
5+
:
I
I
«
I
•I
.5
I
3
yn
o
00
<N
CO
Tf
(U a>
(u
