Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.

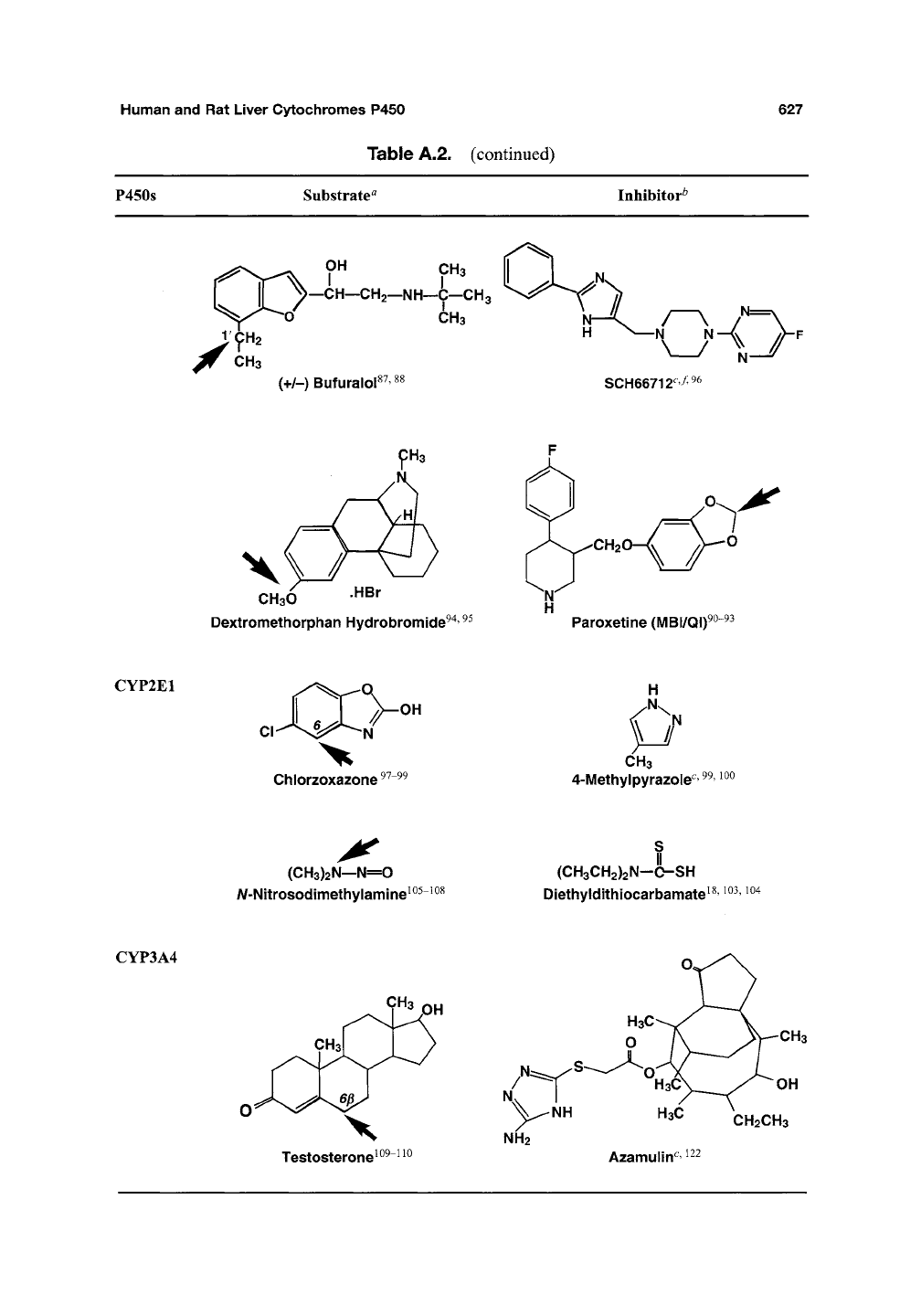
Human
and Rat
Liver Cytochromes P450
Table
A.2.
(continued)
627
P450S
Substrate"
Inhibitor^
\>—CH—CH2—NH—C—CHg
CH3
(+/-)
Bufuralor
SCH66712^^96
CH3O
-^^^
Dextromethorphan
Hydrobromide^"^ ^^
c„.o^J>-o
Paroxetine (l\/IBI/Qlf
CYP2E1
Chlorzoxazone
97-99
H
1>
CH3
4-l\/lethylpyrazole^^9^^^
(CH3)2N—N=0
yV-Nitrosodimethylamine^^^^°^
(CH3CH2)2N-C-SH
Diethyldithiocarbamate^^
^^^ ^^^
CYP3A4
CH3
X
Testosterone^o^^^^
CH3
Azamulin"^
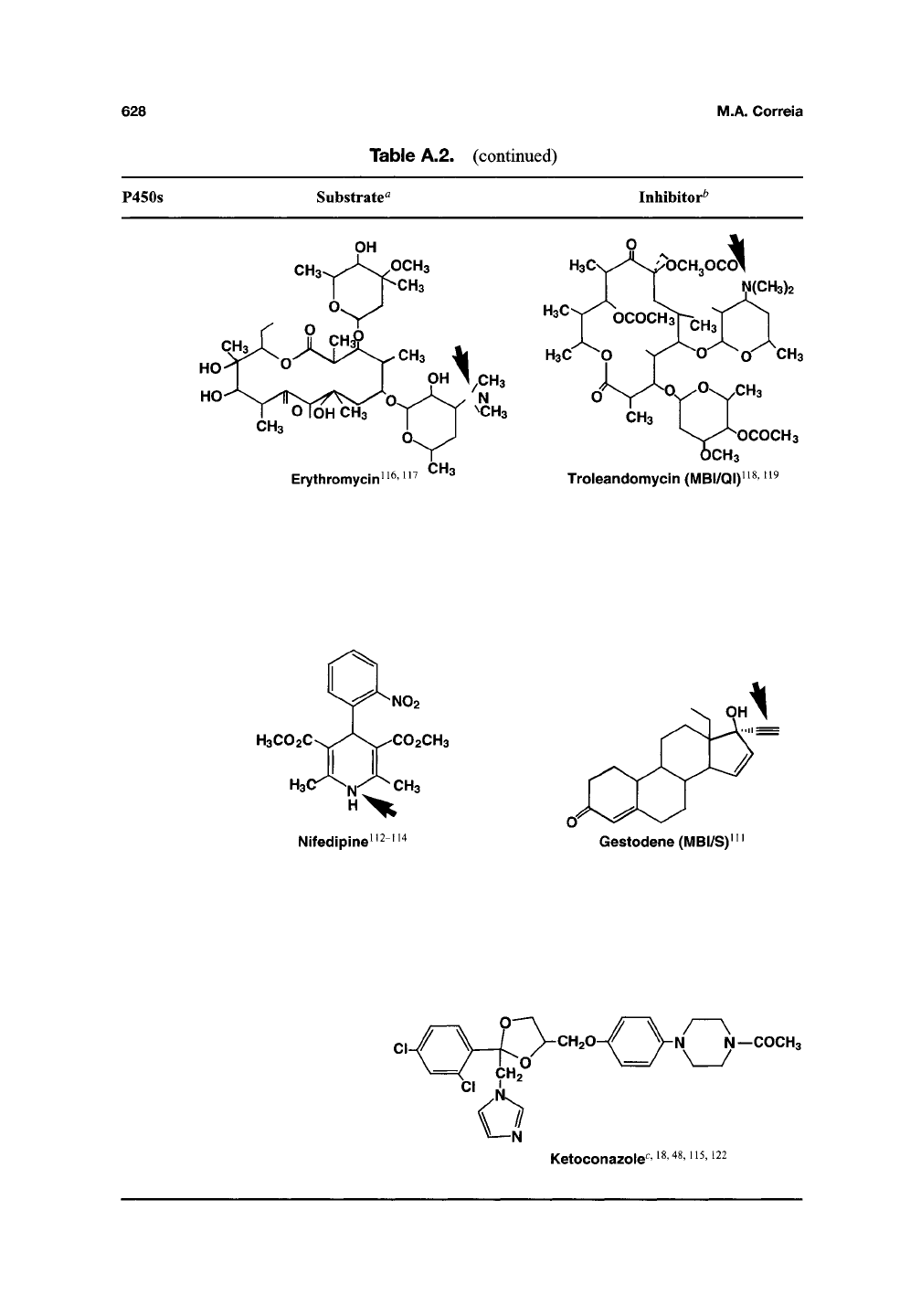
628 M.A.
Correia
Table A.2. (continued)
P450s
Substrate^
Inhibitor^
OH
CH3^.^-^^OCH3
I >CH3
OHCH3
Erythromycin
116,117
^^3
H3C
CH3
^ H3C' ^O
^
OH I/CH3
\^^^0C0CH3
6CH3
Troleandomycin
(MBI/QO^i^ ^^^
H3CO2C
Nifedipine'
Gestodene (MBI/S)
Ketoconazole'
A18,48,
115, 122
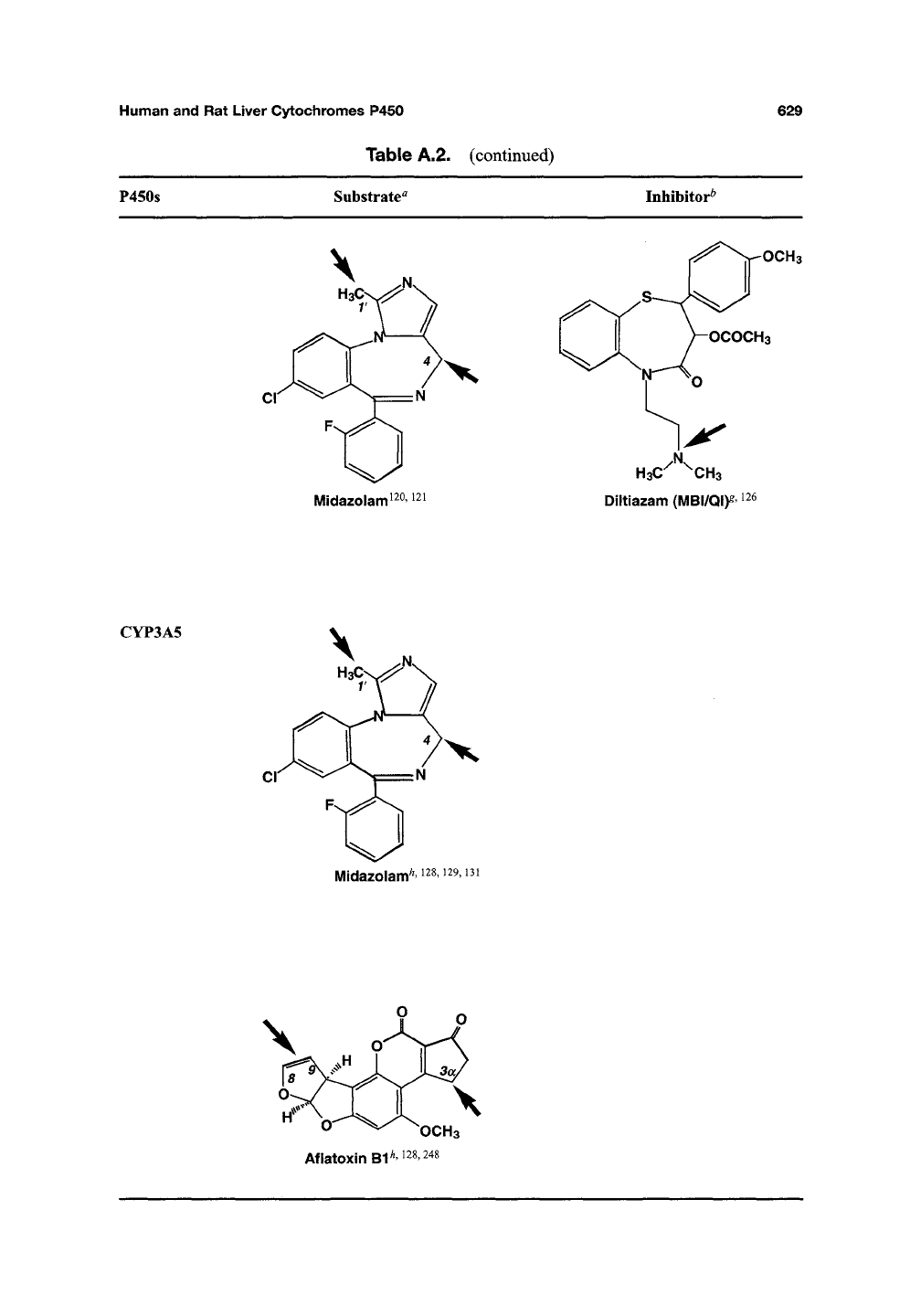
Human and
Rat
Liver Cytochromes P450
Table A.2. (continued)
629
P450s
Substrate^
Inhibitor^
f<::^=^^^^N-0CH3
V-OCOCHa
Midazolam''
H3C
CH3
Diltiazam (MBI/QI)^
^^e
CYP3A5
%^
Midazolam^ 128,129,131
OCH3
Aflatoxin
BV
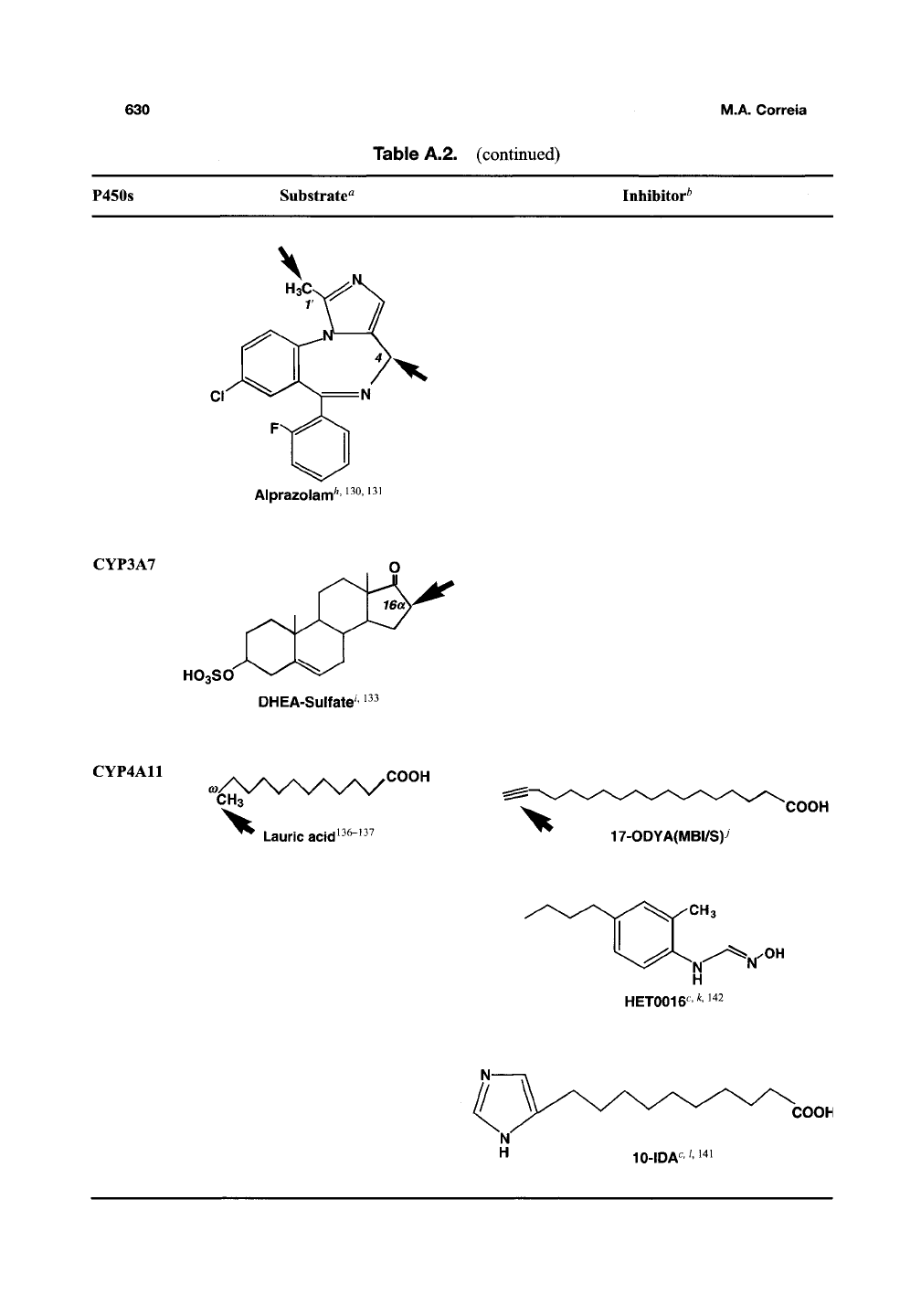
630
M.A.
Correia
Table
A.2.
(continued)
P450S
Substrate^ Inhibitor^
CYP3A7
HO3SO
DHEA-Sulfate'
CYP4A11
COOH
Laurie acid
^
17-0DYA(MBI/S)>
COOH
^^N^^N'°"
~\-
\
H
HET0016^^^^^2
10-IDA^^
141
COOH
Human and
Rat
Liver Cytochromes P450
Table A.2. (continued)
631
P450S
Substrate^
Inhibitor^
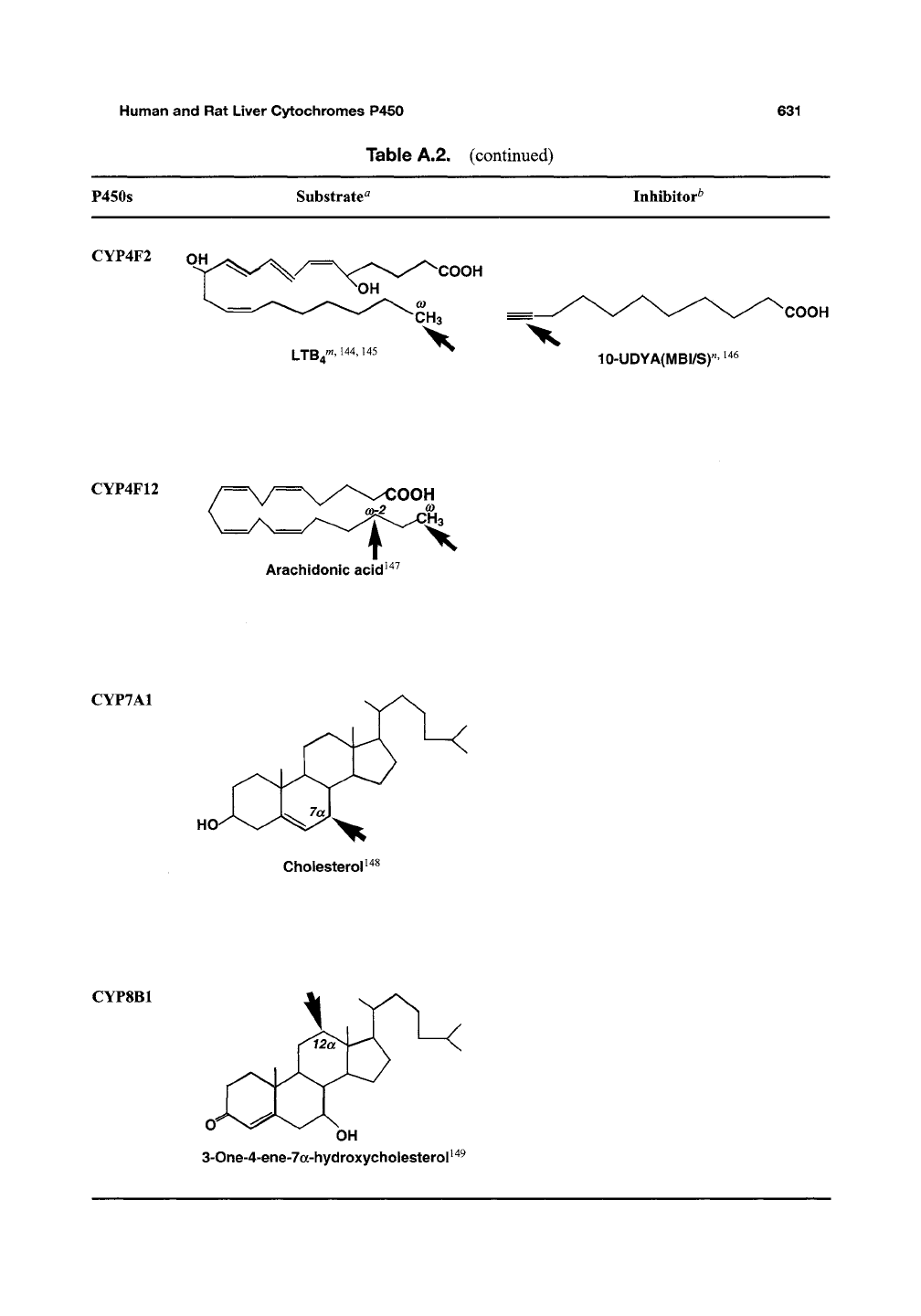
CYP4F2
OH
COOH
10-UDYA(MBI/Sr
CYP4F12
Arachidonic acid^"^^
OOH
CYP7A1
Cholesterol^
CYP8B1
3-One-4-ene-7a-hydroxycholesterol
632 M.A. Correia
Table A.2. (continued)
Additional literature references are listed in Table A.2.
"Arrow(s) indicate(s) the substrate position(s) oxidized by that particular P450 isoform, enabling the assay of the
corresponding oxidized metabolite(s) as its relatively selective functional probe(s).
*The arrow indicates the inhibitor site that is metabolically activated by that P450 isoform resulting in mechanism-
based inactivation (MBI) of the enzyme that is either irreversible (suicide, S) or quasi-irreversible (QI).
^Inhibitor acts competitively by coordinating to the P450 heme-iron and/or ligation to the protein at the active site.
^2-PMADA, 2-isopropenyl-2-methyladamantane.
^3-PMDIA, 3-isopropenyl-3-methyldiadamantane.
^SCH66712, 5-fluoro-2-[4-[(2-phenyl-1 H-imidazol-5-yl)methyl]-1 -piperazinyl]pyrimidine.
^A metabolic intermediate complex (MIC) observed only with CYP3A4 but not CYPs 3A5 and 3A7.
^Given their —89% sequence similarity, CYP3A4 and CYP3A5 have similar functional and inhibitory profiles.
However, CYP3A5 may be distinguished from CYP3A4 by its higher metabolic ratio of midazolam
1
'-/4-hydroxyla-
tion, aflatoxin Bl 8,9-epoxidation to 3a-hydroxylation, and alprazolam 4-/1'-hydroxylation, as well as by its inabil-
ity to form a diltiazam-MIC. Mifepristone has also been found to distinguish between the two CYP3A isoforms.
'DHEA-sulfate, dehydroepiandrosterone sulfate.
^17-ODYA, 17-octadecynoic acid. (Ortiz de Montellano, personal communication)
^ETOOl
6,
A^-Hydroxy-A'^'(4-butyl-2-methylphenyl)-formamidine.
^10-IDA, 10-imidazolyldecanoic acid.
'"LTB4, leukotriene B4.
"10-UDYA, 10-undecynoic acid. (Ortiz de Montellano, personal communication)
Table A.3. Rat Liver P450s: Chemical Structures of Diagnostic
Substrate and Inhibitor Probes
P450s Substrate^ Inhibitor^
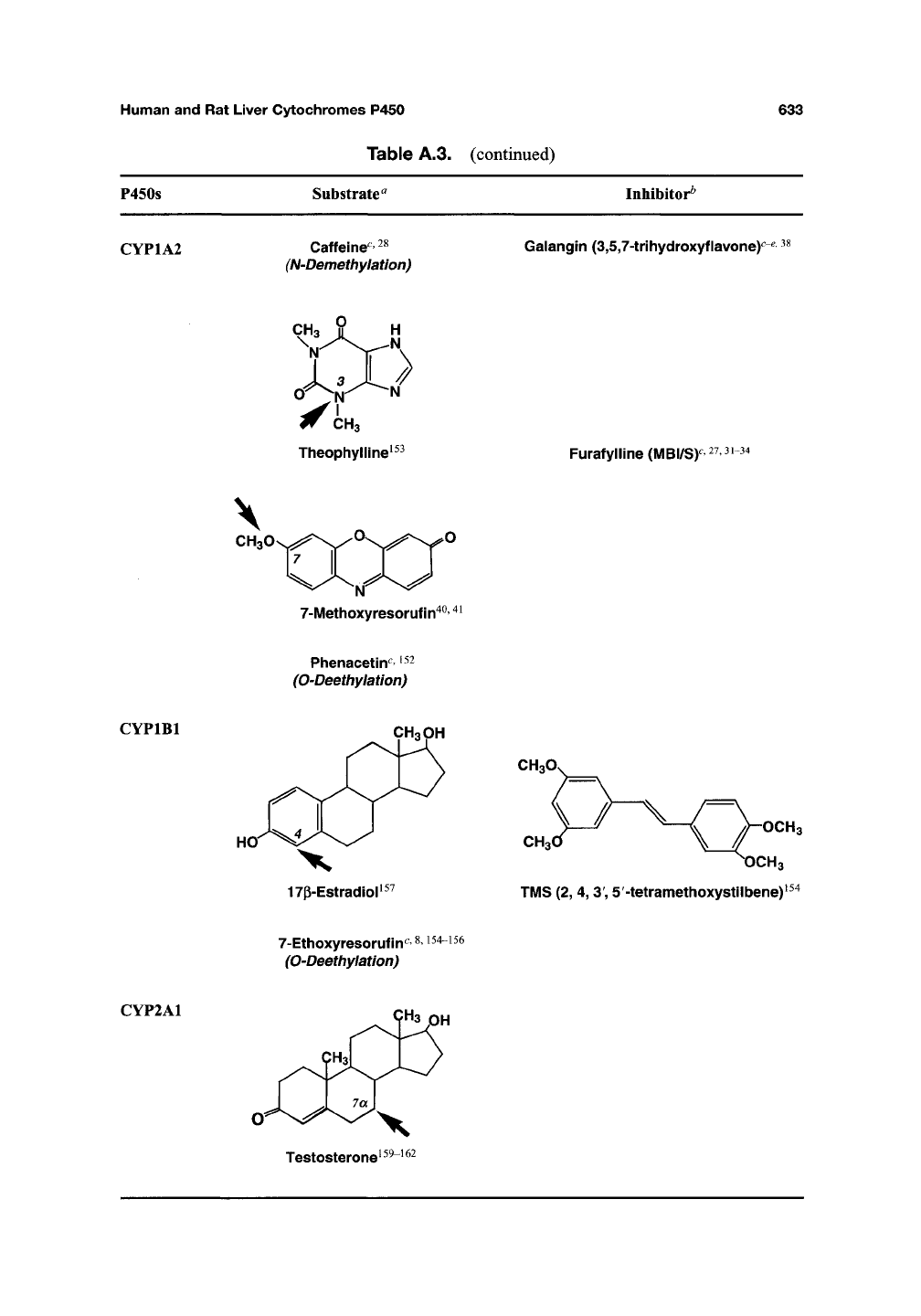
<^YP1A1 NH-COCH3 H0\
Acetanilide^^^ Rhapontigenin(MBI/S)^^^
(R)-Warfarin^5^6^^i
7-Ethoxyresorufin^ 40,41
(O-Deethylation)
Human and
Rat
Liver Cytochromes P450
Table A.3. (continued)
633
P450S
Substrate^
Inhibitor^
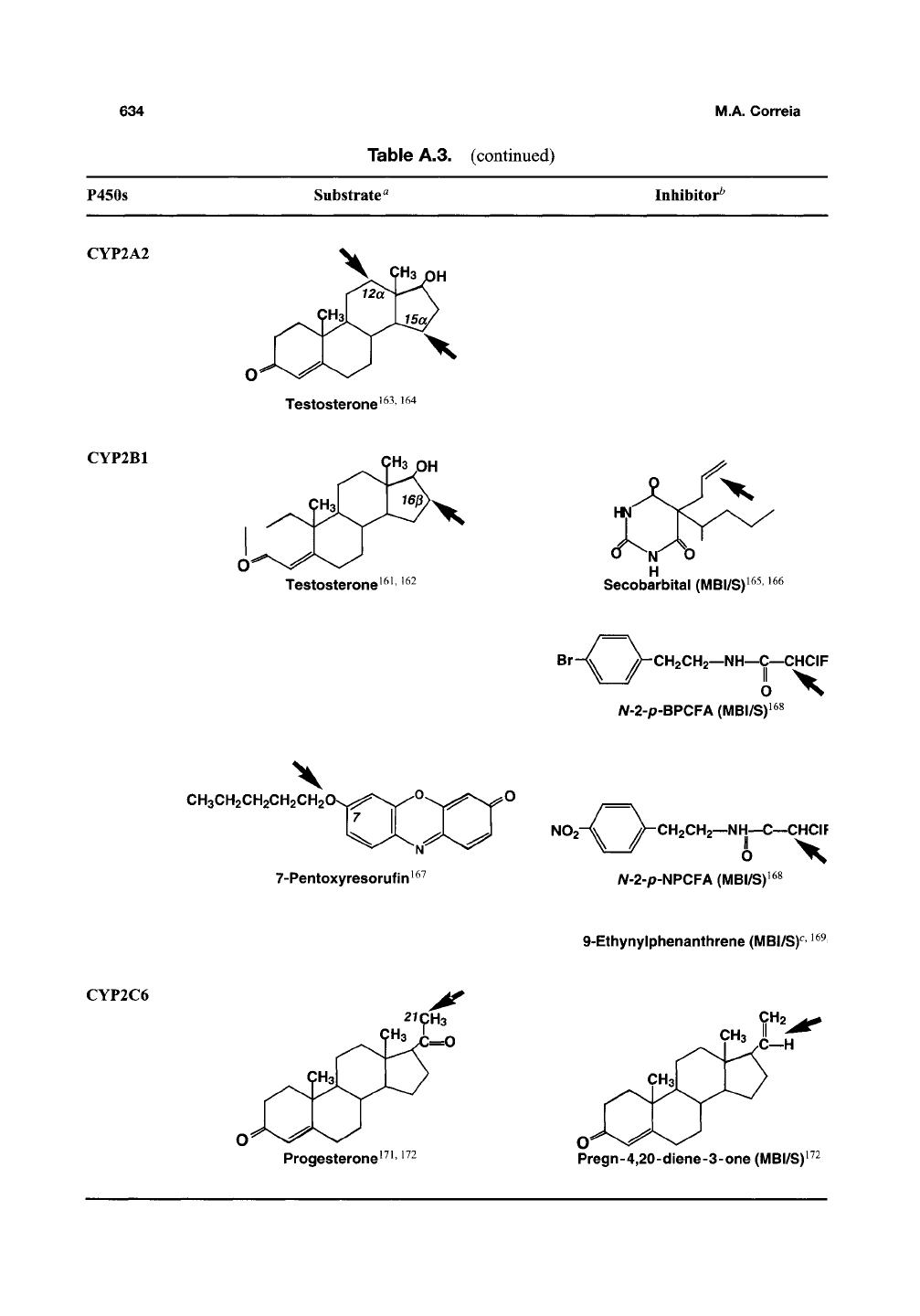
CYP1A2
Caffeine^'
^^
(N'Demethylation)
Galangin (3,5,7-trihydroxyflavoney
f-e,
38
Theophylline^
\
CH3O
7-Methoxyresorufin
in40,4i
Furafylline (MBI/S)^
Phenacetin"^ ^^2
(O'Deethylation)
CYPIBI
CH3OH
CH3O
-OCH3
"OCH3
TMS (2,
4,
3', 5'-tetramethoxystilbene)i^4
7-Ethoxyresorufin^
^'
i54^i56
(O-Deethylation)
CYP2A1
9^3 PH
Testosterone^
634
M.A. Correia
Table A.3. (continued)
P450S Substrate^
Inhibitor^
CYP2A2
Testosterone'^^
'^^
CYP2B1
9^3
OH
Testosterone'* Secobarbital (MBI/S)'^^
'^^
BK
y
\ /"CH2CH2—NH—C—CHCIF
O
^
A/-2-P-BPCFA (MBI/S)'68
\
CH3CH2CH2Cn2CH2
7-Pentoxyresoruf
in'
^^2
\
y-CH2CH2—NH—C—CHCIF
O
"X
A/-2-P-NPCFA (IVIBI/S)'68
9-Ethynylphenanthrene (l\/IBI/Sy
,169.
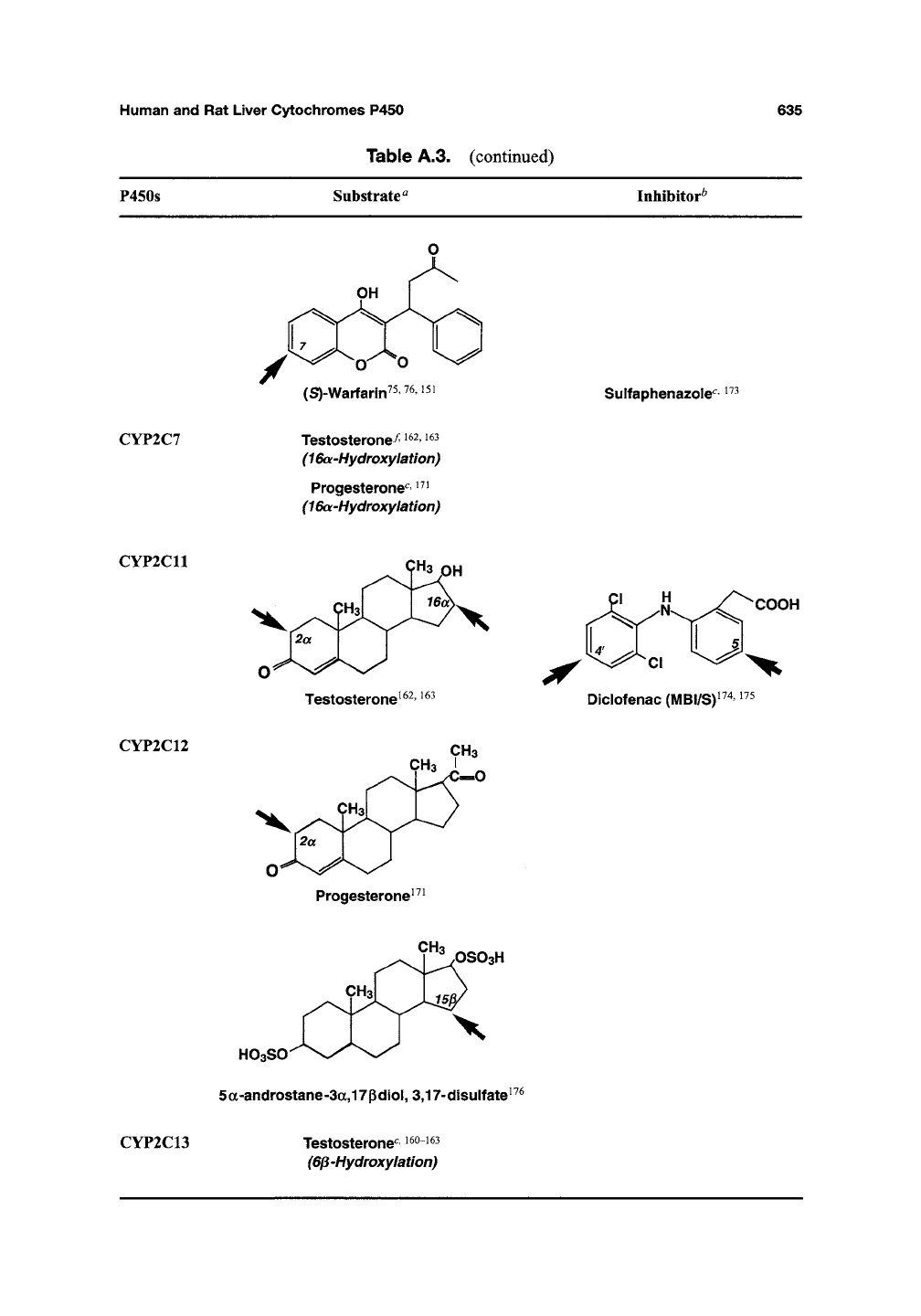
CYP2C6
2ICH3
Progesterone
171,172
CH2
C—H
Pregn-4,20-diene-3-one (l\/IBI/S)'
Human
and
Rat
Liver Cytochromes P450
Table A.3. (continued)
635
P450S
Substrate^
Inhibitor^
(S)-Warfarin^5^6^5i
CYP2C7
Testosterone^
1^2,
i63
(16a-Hydroxylation)
Progesterone^'
^^^
(16a-Hydroxylation)
Sulfaphenazole"^
CYP2C11
CYP2C12
9"3 PH
Testosterone^^^'
^^^
CH3
^"^C-0
Progesterone^^^
COOH
Diclofenac
(l\1BI/S)^
CYP2C13
OSO3H
HOaSO-^
5a-androstane-3a,17pdiol,
3,17-disulfate^^^
Testosterone"^
^^°^^^
(6p'Hydroxylatlon)
636
M.A.
Correia
Table
A.3.
(continued)
P450S
Substrate^
Inhibitor^
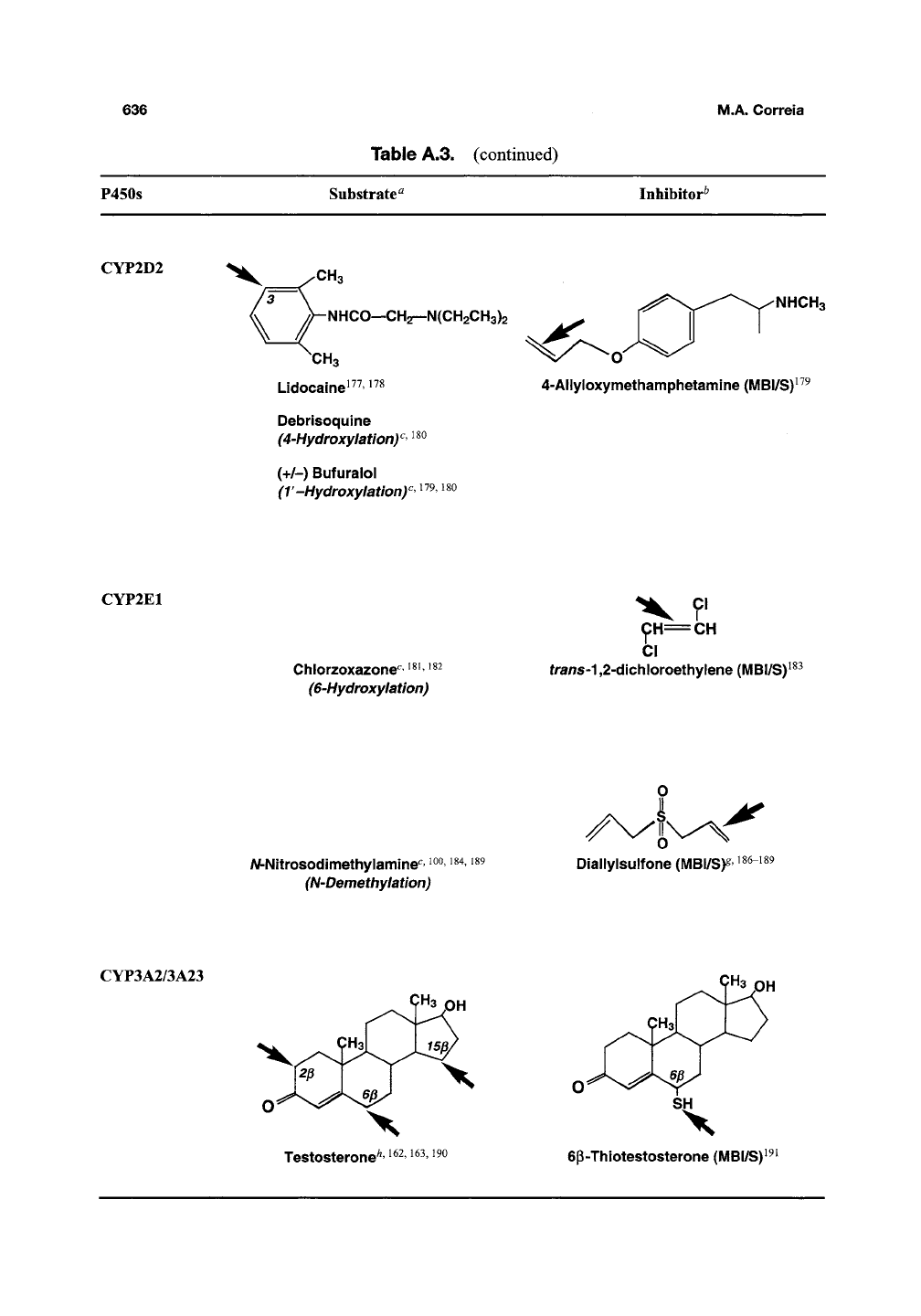
CYP2D2
CH3
NHCO—CH^-N(CH2CH3)2
Debrisoquine
(4-Hydroxylationy ^^^
(+/-)
Bufuralol
(T-Hydroxylationy
^^9,
iso
NHCH3
4-Allyloxymethamphetamine(MBI/S)^^^
CYP2E1
Chlorzoxazone"^
^^^
^^2
(6-Hydroxylation)
^H=CH
CI
trans'^
,2-dichloroethylene
(MBl/S)^^^
/V-Nltrosodlmethylamine^
100,
i84,
i89
(N-Demethylation)
DIallylsulfone
(MBI/S)^
186-189
CYP3A2/3A23
Testosterone^'
i^^,
i63,190
CH3
ep-Thiotestosterone
(MBI/S)^-
