Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.


Modern Industrial Microbiology and Biotechnology
SUGGESTED READINGS
Ahuja, S. 2000. Handbook of Bioseparations. Vol 2 Academic Press. San Diego, USA.
Dobie, M., Kruthiventi, A.K., Gaikar, V.G. 2004. Biotransformations and Bioprocesses. Marcel
Dekker, New York, USA.
Endo, I., Nagamune, T., Katoh, S., Yonemoto (eds) 1999. Bioseparation Engineering. Elsevier
Amsterdam, the Netherlands.
Garcia, A.A., Bonem, M.R., Ramirez-Vick, J., Saddaka, M., Vuppu, A. 1999. Bioseparation Process
Science. Blackwell Science, Massachussets, USA.
Harrison, R.G., Todd, P., Rudge, S.R., Petrides, D.P. 2003. Bioseparation Science and Engineering.
Oxford University Press, New York, USA.
Kalyanpur, M. 2000. Downstream Processing in Biotechnology In: Downstream Processing of
Proteins: Methods and Protocols. M. Desai, (ed) Humana. Totowa, NJ: USA. pp. 1–10.
Naglak, T.J., Hettwer, D.J., Wang, H.Y, 1990. Chemical Permeabilization of cells for intracellular
product release. In: Separation Processes In Biotechnology, Marcel Dekker, New York, USA.

In the microbiology laboratory, sterility is a most important consideration and ways of
achieving it form the earliest portions of the training of a microbiologist. In the
fermentation industry contamination by unwanted organisms could pose serious
problems because of the vastly increased scale of the operation in comparison with
laboratory work. If Pediococus streptococcus damnosus which causes sourness in beer were
to contaminate the fermentation tanks of a brewery then hundreds of thousand of liters of
beer may have to be discarded, with consequent loss in revenue to the brewery. The
situation would be similar if a penicillianase-producing Bacillus sp were to contaminate
a penicillin fermentation, or lytic phages an acetone-butanol mash.
11.1 THE BASIS OF LOSS BY CONTAMINANTS
Contaminations in industrial microbiology as seen above could lead to huge financial
losses to a fermentation firm. Losses due to contaminations may be explained in one or
more of the following ways:
(i) The contaminant may utilize the components of the fermentation broth to produce
unwanted end-products and therefore reduce yield. When slime-forming
Leuconostoc mesenteroides invades a sugar factory, it utilizes sucrose to form the
polysaccharide in its capsule which forms the slime. Similarly, in the beer industry
when lactic acid bacteria contaminate the fermentating wort, they utilize sugars
present therein to produce unwanted lactic acid which renders the beer sour.
(ii) The contaminant may alter the environmental conditions such as the pH or
oxidation-reduction potential of the fermentation and render it unsuitable for
maximum production of the required product. Thus, if E. coli which grows much
more rapidly than the highly aerobic Streptomyces griseus should contaminate a
streptomycin fermentation it may use up a large proportion of the oxygen thereby
reducing the yield of the antibiotic, because less than optimal amounts of oxygen
are available to the actinomycete.
(iii) Contamination by lytic organisms such as bacteriophages or Bdellovibrio could
lead to the entire destruction of the producing organism.
Sterility in Industrial
Microbiology
11
+0)26-4

Modern Industrial Microbiology and Biotechnology
(iv) Finally, it is conceivable that contaminants could even, if they did not reduce yield
in a product, produce by-products not removable in the extraction process already
established in the factory. The result could be losses in manpower time needed to
devise means of dealing with the product.
Although contaminants are generally undesirable, not all fermentation need to be
carried out under strict asepsis, depending on the selling price of the end-product. Thus
while the high cost of antibiotics justifies strict sterility during production, such sterility
is not called for in such bulk products as yeasts or industrial alcohol.
11.2 METHODS OF ACHIEVING STERILITY
The various methods for achieving sterility are well-known and include physical and
chemical methods.
11.2.1 Physical Methods
11.2.1.1 Asepsis
Asepsis involves general cleanliness and is a procedure routinely observed in many
microbiological, pharmaceutical and food industries. In such organizations, laboratory
coats, face masks, gloves, and other protective clothing are often worn to prevent the
transfer of organisms from the individual to the product. Hands are regularly washed;
pipes, utensils, fermentation vats, and floors are washed with water and disinfectants. In
some industries such as those concerned with parenteral (injection) material, or with
vaccines, even the incoming air must be sterile. The maintenance of asepsis does not
sterilize but it helps reduce the load of microorganisms and hence lessens the stringency
of the sterility measures employed. It also helps to remove foci of microbial growth such
as particles of food, or media which could be sources of future contaminations.
11.2.1.2 Filtration
Filtration is used in industry and in the laboratory to free fluids (i.e., gases and liquids) of
dust and other particles and microorganisms. If properly used, it is highly effective and
also relatively inexpensive. Large volumes of sterile air and other gases are sometimes
required for ‘sterile’ areas where in the pharmaceutical industries, injections and
vaccines are handled, and for aeration in most fermentations.
Two types of air filters are available, the so-called absolute filters which are usually
made of ceramic and are so called because their pores are not large enough to admit a
microorganism and hence, they should theoretically be highly efficient. Their
disadvantage is that they are suitable for only small volumes of the gas being sterilized.
The second group, fibrous filters, is made of fibers of wool, cotton, glass or mineral slag,
whose diameters are in the order of 0.5-15 m. Fibrous filters are not absolute; nevertheless
they are quite effective and hold back organisms of the diameter of about 1.0m or even
viruses. The factors which contribute to their removal of microorganisms include direct
interception by the fibers, settlement by gravity electrostatic attraction between fiber and
particles, Brownian movement and convection (Fig. 11.1).
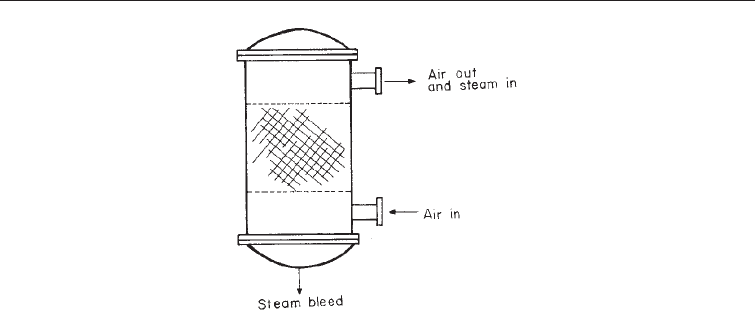
Sterility in Industrial Microbiology !
Note the fibers placed in the central portion of a steel casing.
Fig. 11.1 Fibrous Filter
Prefilters usually consist of discs of mats of asbestos of the type used in Seitz filters. They,
however, let in fine fibers, which are undesirable in injectable materials. The fine fibers
are removed in the final filter. Prefilters also absorb large amounts of the liquid, although
such absorbed liquid can be re-extracted by flushing the filter at the end of filtration with
sterile nitrogen. The filters may also be made of compressed paper pulp; filter paper
coated with Kieselghur may be placed between the filter pads.
Final filters which may be of unglazed porcelain are usually made in the form of
cylimerial candles over which the liquid to be filtered flows. The filtrate is drained from
the inside of the cylinder. This type of arrangement increases the surface area available
for filtration. The candles may be sterilized by autoclaving. Sintered glass is usually
made in form of discs and, like porcelain, they are fragile. Membrane (‘Millipore’) filters of
cellulose acetate may also be used as final filters. They can be autoclaved.
Sterilizing filters should have pores with maximum diameters of 0.2 m. They should be
themselves sterilized before being used. Membrane filters can be sterilized by chemical
sterilants (such as ethylene oxide, hydrogen peroxide in vapor form, propylene oxide,
formaldehyde, and glutaraldehyde), radiant energy sterilization (such as c-irradiation)
or steam sterilization. The most common method of sterilization is steam sterilization.
Steam sterilization of a membrane filter can be accomplished either by an autoclave or
by in situ steam sterilization.
11.2.1.3 Heat
Heat may be applied dry or moist:
Dry heat: Not only is dry heat used to sterilize glassware on a small scale in industry
associated laboratories, more importantly it is used on a large scale in industry for
sterilizing some types of air filters. Principally, however, it is used for sterilizing air by
compression. When air is compression the temperature rises in accordance with the gas
law, PV = RT where P is the pressure, V the volume, R the gas constant and T temperature.
If P and V are increased, T, the temperature would rise as shown in Table 11.1. However,
compression is expensive. Furthermore, heated air must be at a high temperature (at a
" Modern Industrial Microbiology and Biotechnology
much higher pressure than that at which it will be used) and for fairly long holding
periods. Although not a very practical method, compression could reduce the microbial
population of air.
Other methods which have been explored include direct or indirect heating with the
gases and also with electrical heating. In each case while the procedure was effective, it
was too expensive.
Moist heat: Moist heat can be employed in industry to kill microorganisms during boiling,
tyndallization, and autoclaving.
Tyndallization consists of boiling the material for one half hour on three consecutive days.
Vegetative cells are killed on the first day’s boiling. Spores are not but they germinate.
During the second day’s boiling, the vegetative spores resulting from the spores not killed
on the first day, are killed. Any spore still surviving after the second day will be killed
during boiling on the third day as the spores would have germinated. After the third
day’s boiling the medium is expected to be sterile. It is a method which can be used for
sterilizing heat-labile media where filtration is not possible for whatever reason,
including that the medium is too viscous for filtration.
Pasteurization is very widely used in the food industry. It is used for treating beer and
wine. It consists of exposing the food or material to a temperature for a sufficiently long
period to destroy pathogenic or spoilage organisms. Pasteurization can either be batch or
continuous. The low temperature long time (LTLT) technique usually involves heating at
about 60°C for one half hour and is used in batch pasteurization whereas the high
temperature short time (HTST) of flash method involves heating at about 70°C for about
15 seconds. The flash method is employed in continuous pasteurizing.
When batch pasteurization is used on a large scale the final temperature of
pasteurization is attained by gradual increases. Similarly, the temperature is lowered
gradually to cool it; for 600 ml bottles in many breweries batch pasteurization time is a
total of about 90 minutes divided equally between raising the temperature, holding at the
pasteurization temperature, and cooling. This prolonged time during which the material
is exposed to high temperature and which may give rise to a ‘burnt’ odor is the major
deficiency of batch in comparison with continuous pasteurization.
Steam under pressure: Steam is useful as a sterilizer for the following reasons:
(i) It has a high heat content and hence a high sterilizing ability per unit weight or
volume; this heat is rapidly released.
Table 11.1 Temperature of air after compression
Final pressure (p.s.i.g.) Temp. (
o
C)
20 78
40 117
60 140
80 169
100 189
150 229
200 261

Sterility in Industrial Microbiology #
(ii) Steam releases its heat at a readily controlled and constant temperature.
(iii) It can be fairly easily produced and distributed.
(iv) No obnoxious waste products result from its use and it is clean, odorless and
tasteless.
Its disadvantages are that it is not suitable for sterilizing anhydrous soils, greases,
powders, and its effectiveness, as will be seen later, may be limited in the presence of air.
Steam is widely used for the sterilization of equipment in the laboratory as well as in
industry. Pipes, fermentors and media are all sterilized with the steam. Steam used for
this purpose is under pressure because the higher the pressure the higher the
temperature. The relationship between steam temperature and pressure will be
discussed further later in this section.
There are three ‘types’ of steam.
Wet steam is steam in which sufficient heat is lacking to keep all the steam in the
gaseous vapor phase. The effect of this is that some liquid water is present in the steam.
In ‘saturated’ (or sometimes wrongly called dry saturated) steam, all the steam is in the
vapor phase; its heat content is such that there is an equilibrium between it and water at
any temperature and pressure. Saturated steam is water vapor in the condition in which
it is generated from the water with which it is in contact. Saturated steam cannot undergo
a reduction in temperature without a lowering of its pressure, nor can the temperature be
increased without expanding the pressure. When steam is saturated therefore, it can be
described either by its pressure or its temperature, with which the two characteristics are
linked. Wet steam has far less heat than saturated steam per unit weight of steam.
Furthermore, wet steam introduces a lot more water than necessary in the material being
sterilized; for example media in fermentors may become diluted. One major reason for the
occurrence of wet steam is the use of long poorly insulated pipes.
In superheated steam no liquid water is present, and the temperature is higher than that
of saturated steam at the same pressure. Superheated steam is produced by, for example,
passing it over heated surfaces or coils. For the purposes of sterilization, saturated steam
is the most dependable, efficient and effective of the three types of steam. Superheated
steam behaves more like a gas than vapor and takes up water avidly. Although it has a
higher temperature than saturated steam at the same pressure, it does not sterilize to the
extent of saturated steam. This is because it lacks moisture which enables heat to kill
micro-organisms at considerably lower temperatures than dry air. Superheated steam,
like dry air, would require that the organisms be exposed for periods as long as glassware
is exposed in a dry air oven. For transportation over long distances steam is transported
in the superheated form in pipes in order to reduce heat losses; it is returned to saturated
steam at the end of the transportation and at the point of use by the introduction of water.
The temperature of steam sterilization is 121°C for media both in industry and in the
laboratory, although other time-temperature combinations are equally satisfactory (Table
11.2). When industrial media are sterilized by heat, steam is forced into the medium
which is gently agitated; heating is supplemented when necessary by passing steam
through coils running along the fermentor wall. The dilution resulting from steam
injection is calculated from the quantity of steam introduced. In some instances the
medium may be autoclaved in a much larger version of a laboratory autoclave known as
a retort.
$ Modern Industrial Microbiology and Biotechnology
Table 11.2 Minimum time/temperature relationship arrangements
Time (min) Temp
o
C
30 116
18 118
12 121
8 121
2 132
The major difference between sterilization of media in industry and in the laboratory is
the much greater scale of the former. Due to the greater scale it takes a much longer time to
attain the sterilizing temperature and to cool down than would be the case in the
laboratory. In the laboratory a liter of medium would probably require ten minutes to
attain the sterilizing temperature. It would remain there for 15 minutes and cool down
gradually over another 10-15 minutes, making a total of 40-45 minutes. With a 10,000 liter
medium the equivalent periods may well take several hours for each of the three periods.
11.2.1.4 Radiations
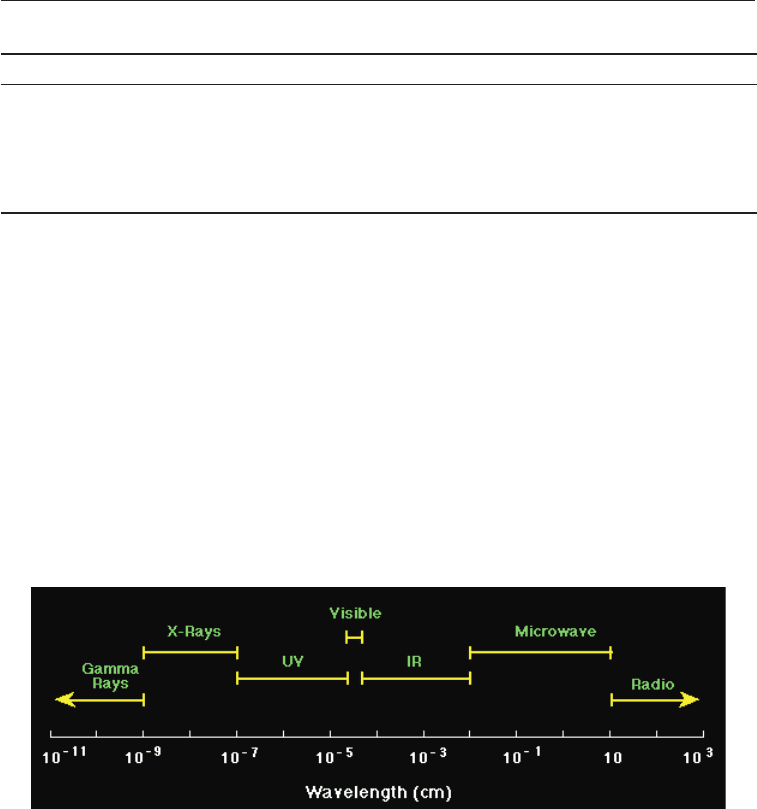
The electromagnetic spectrum is given in Fig. 11.2. The shorter the wavelength the more
powerful the radiation. Thus on the electromagnetic spectrum the most powerful
wavelengths are those of gamma rays, while the least powerful are radio waves. The
radiations used for sterilizing ultra violet light, x-rays and gamma rays.
Fig. 11.2 The Electromagnetic Spectrum
Ionizing radiations: These are extremely high frequency electromagnetic waves (X-rays
and gamma rays), which have enough photon energy to produce ionization (create
positive and negative electrically charged atoms or parts of molecules) by knocking off
the electrons on the outer orbits of atoms of the materials through which they pass. The
atoms knocked out are accepted by other atoms. The atoms losing the electrons and those
accepting them become ionized on account of the electron changes. It is this ability of x-
rays and gamma rays to create ions that has earned them the name ionizing radiations.
Gamma rays are generated from x-ray machines such as those used in hospitals to take x-
ray pictures. Gamma rays are also produced by the spontaneous decay of radioactive
metals such as cobalt 60 (Co
60
). Ionizing radiations can be used to sterilize plastic
syringes, rubber gloves, and other materials which are liable to damage by heat or
chemicals.

Sterility in Industrial Microbiology %
Ultraviolet light: Visible light falls between wavelengths of 400 and 700 nm. Ultraviolet
light (UV) ranges from 100 to 400 nm. Not all uv is germicidal. The ‘germicidal range’ is
approximately 200 – 300 nm, with a peak germicidal effectiveness at 254 nm. The process
of the killing of microbes by UV involves absorption of a UV photon by DNA chains. This
causes a disruption in the DNA chain by causing adjacent thymine bases to dimerize or
become linked. The organism’s metabolism is disrupted and it may eventually die.
Unfortunately, ultraviolet light does not penetrate, and acts mainly on the surface.
Therefore its use would be limited to laboratory work such as sterilizing the laboratory
air, for creating mutations in culture improvement. In industry it is used for sterilizing the
air in fermentation halls and other such large open spaces.
11.2.2 Chemical Methods
These can be divided into two groups: chemosterilants (which kill both vegetative cells as
well as spores of bacteria, fungi, viruses, and protozoa) and disinfectants which may no-
kill spores, or even some vegetative cells, but at least kill unwanted (pathogenic or
spoilage) organisms.
11.2.2.1 Chemosterilants
For a chemical to be useful as a sterilant it should have the following properties:
(i) It should be effective at low concentrations.
(ii) The components of the medium should not be affected, when used for media.
(iii) Any breakdown products resulting from its use should be easily removed or be
innocuous.
(iv) It should be effective under ambient conditions.
(v) It should act rapidly, be inexpensive and be readily available.
(vi) It should be non-flammable, non-explosive, and non-toxic.
The discussion on chemosterilants will focus on gaseous sterilants because they have
special advantages when parts of the materials to be sterilized are difficult to reach or
when they are of heat-labile.
11.2.2.2 Gaseous Sterilants
(i) Ethylene oxide: Ethylene oxide CH
2
– CH
2
has become accepted as a gaseous sterilant
and a lot of information about it has accumulated. It reacts with water, alcohol, ammonia,
amines, organic acids and mineral acids. Above 10.7
o
C it is gaseous. It is very penetrating
and is widely used in the food and pharmaceutical industries where it is capable of
killing all forms of microorganisms. Bacterial spores are however 3-10 more resistant
than vegetative cells.
Spores of some bacteria e.g. the thermophilic Bacillus stearothermophilus are in fact less
resistant than vegetative cells of some bacteria e.g. Staphylocous aureus, Micrococcus
radiodurans, and Streptococcus faecalis.
Relative humidity is very important in deciding the bacterial activity of ethylene oxide;
it is most effective in the range of 28-33% relative humidity. At humidities higher than
33% it is converted to ethylene glycol which has a weaker anti-bacterial activity. For

& Modern Industrial Microbiology and Biotechnology
effectiveness ethylene oxide requires a much longer time of exposure than steam
sterilization.
It is widely used in the pharmaceutical industry for sterilizing rubber and plastic
bottles, vials, catheters and sometimes, sutures, syringes and needles and some
antibiotics and microbiological media. Residual ethylene oxide must however be
removed by allowing it to evaporate and this takes some time.
One of the main disadvantages of the sterilant is that the liquid (which form it assumes
below 107°C is highly inflammable; the gas also forms explosive mixtures with air from 3
to 80 by volume. For this reason it is mixed with inert gases such as CO
2
often in a ratio of
10% ethylene oxide and 90% CO
2
. The explosive nature of ethylene oxide is made even
worse by the fact that the pure ethylene gas has an unpleasant odor. For use it is
introduced into large containers constructed like autoclaves.
(ii) Propylene oxide: This is only about half as active as ethylene oxide. It is liquid at room
temperature. It hydrolyzes less slowly than ethylene oxide in the presence of moisture. It
is used for room fumigation, and for food because some countries discourage the use of
ethylene oxide for this purpose. Propylene oxide has been used in industry for sterilizing
culture media, powdered and flaked foods, barley seeds and dried fruits. For these dried
foods an exposure of 1,000-2,000 mg/liter of the sterilant for 2-4 hours resulted in 90–99%
kill of various microorganisms, including bacteria and fungi. Like ethylene oxide it is an
alkylating agent and should be handled carefully since it is a potential carcinogen.
(iii) b-propiolactone: b-propiolactone is a heterocyclic colorless pungent liquid. It is highly
active as an anti-bacteria agent, but it has a low penetrative power. Its probable
carcinogenicity has lowered its general use, although it has been used to fumigate
houses. It is used in the pharmaceutical industry to sterilize plasma and vaccines; when
it was used to sterilize bacterial medium all the spores introduced were killed.
Subsequently, E. coli grew indicating that no residual toxicity resulted. Indeed b-
propioplactone breaks down to the non-toxic and less carcinogenic b-hydroxypropionic
acid. Under maximum operating conditions (temperature, humidity, etc.) it has been
claimed that b-propiolactone in the vapor phase is 25 times more effective than
formaldehyde, 4000 times more than ethylene oxide and 50,000 more active than methyl
bromide. The relative humidity for maximum activity is 75%.
(iv) Formaldehyde: Formaldehyde is a gas which is highly soluble in water. Like other
gaeous sterilants relative humidity is important, but it is most active between 60-90%
humidity. It does not penetrate deeply and it should be used at 22
o
C or above to be
effective. An exposure of at least 12 hours is necessary. Formaldehyde oxidizes to formic
acid and this breakdown product could be corrosive to metals. It is used in the
pharmaceutical industries where it is used to preserve pathological specimens of
animals used for tests.
(v) Methylbromide: Methyl bromide is widely used for fumigation and disinfection in
cereal mills, warehouses, granaries, seed houses, and food processing plants. As it is
highly toxic ethylene oxide is sometimes preferred to it. Furthermore, it has been reported
to be only about one tenth as effective as ethylene oxide.
(vi) Sulfur dioxide: This is a colorless pungent gas. Due to its corrosiveness it is of limited
use, but it is used in the food industries; in wineries, it is used to partially ‘sterilize’ the
grape must before fermentation, to destroy wild yeasts and other unwanted organisms.

Sterility in Industrial Microbiology '
11.2.2.3 Other sterilants
(i) Chorine: is widely used in industry as solutions of hypochloride. It is used for washing
pipes in breweries and other establishments and in the dairy industry for sterilizing
utensils.
(ii) Phenol: Phenol and phenol-derivatives are widely used as disinfectants. Other
compounds which could find use in some aspects of industry include ozone, hydrogen
peroxide, and quaternary ammonium compounds.
11.3 ASPECTS OF STERILIZATION IN INDUSTRY
In the foregoing, principles of dealing with unwanted organisms have been stressed;
where it was possible some aspects of practice were discussed. In this section the
practical methods of dealing with contaminations and the potential for contaminations
to occur in industry will be discussed.
11.3.1 The Sterilization of the Fermentor and its Accessories
The fermentor itself, unless sterilized, is a source of contamination. Of the various
methods discussed above, steam is the most practical for fermentor sterilization. Steam is
used to sterilize the medium in situ in the fermentor but sometimes the medium may be
sterilized separately in a retort or autoclave and subsequently transferred aseptically to a
fermentor. In order to avoid microbial growth within the fermentor when not in use,
crevices and rough edges are avoided in the construction of fermentors, because these
provide pockets of media in which undesirable microorganisms can grow. These crevices
and rough edges may also protect any such organisms from the lethal effects of
sterilization. For the reasons discussed earlier, saturated steam should be used and
should remain in contact with all parts of the fermentor for at least half an hour. Pipes
which lead into the fermentor should be steam-sealed using saturated steam. The various
probes used for monitoring fermentor activities, namely probes for dissolved oxygen,
CO
2
, pH, foam, etc., should also be sterilized.
11.3.2 Media Sterilization
The following should be borne in mind when sterilizing industrial media with steam:
(i) Breakdown products may result from heating and may render the medium less
available to the microorganisms; some of the breakdown products may even be
toxic;
(ii) pH usually falls with sterilization and the usual laboratory practice of making the
pH slightly higher than the expected final pH should be followed;
(iii) Most media would have been sterilized if heat was available to all parts at a
temperature of 120-125
o
C for 15-20 minutes. Oils (sometimes used as anti-foams)
are generally more difficult to sterilize. If immiscible with water they may need to be
sterilized separately at a much higher temperature than the above and/or for a
longer period.
