Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

124 Fluorine in Medicinal Chemistry and Chemical Biology
Table 5.2 In vitro cytotoxicity ( IC
50
nM)
a
of C - 3 ′ - CF
2
H - taxoid ( 18 )
Taxoid R X MCF7 - S
b
(breast)
MCF7 - R
c
(breast)
R/S
d
LCC6 - WT
b
(breast)
LCC6 - MDR
e
(breast)
R/S
d
H460
f
(lung)
HT - 29
g
(colon)
Paclitaxel 1.7 300 176 3.1 346 112 4.9 3.6
Docetaxel 1.0 215 215 – – – – 1.0
SB - T - 12841 - 1 Ac MeO 0.34 4.16 12 0.26 5.57 21 0.38 0.52
SB - T - 12841 - 2 Ac F 0.44 5.33 13 0.52 10.0 19 0.20 0.35
SB - T - 12841 - 3 Ac Cl 0.40 6.48 16 0.31 5.80 19 0.49 1.94
SB - T - 12841 - 4 Ac N
3
0.32 1.68 5.3 0.22 1.57 7.1 0.48 0.57
SB - T - 12842 - 1 Et - CO MeO 1.14 4.05 3.5 0.69 4.92 7.1 0.40 0.59
SB - T - 12842 - 2 Et - CO F 0.53 7.24 14 0.88 4.63 3.5 0.41 0.86
SB - T - 12842 - 3 Et - CO Cl 0.44 5.20 12 0.52 4.71 9.1 0.30 0.43
SB - T - 12842 - 4 Et - CO N
3
0.32 0.96 3.0 0.39 1.15 2.9 0.27 0.37
SB - T - 12843 - 1 Me
2
N - CO MeO 0.45 4.51 10 0.69 7.06 10 0.40 0.43
SB - T - 12843 - 2 Me
2
N - CO F 0.52 8.13 16 0.69 10.6 15 0.20 0.35
SB - T - 12843 - 3 Me
2
N - CO Cl 0.31 2.96 9.5 0.21 3.87 18 0.36 0.58
SB - T - 12843 - 4 Me
2
N - CO N
3
0.37 1.44 3.9 0.29 1.69 5.8 0.52 0.40
SB - T - 12844 - 1 MeO - CO MeO 0.81 6.59 8.1 1.03 10.2 9.9 0.30 0.44
SB - T - 12844 - 2 MeO - CO F 0.59 11.38 19 0.86 12.6 15 0.30 0.43
SB - T - 12844 - 3 MeO - CO Cl 0.26 2.08 8.0 0.13 1.82 14 0.25 0.29
SB - T - 12844 - 4 MeO - CO N
3
1.69 2.56 1.5 0.26 2.06 7.9 0.23 0.36
a – e
See footnotes a – e of Table 5.1 .
f
Human non - small - cell lung carcinoma.
g
Human caucasian colon adenocarcinoma.

Fluoro-Taxoid Anticancer Agents 125
Table 5.3 In vitro cytotoxicity ( IC
50
nM)
a
of C - 3 ′ - CF
3
- taxoids ( 19 )
Taxoid R X
MCF7 - S
b
(breast)
MCF7 - R
c
(breast)
R/S
d
LCC6 - WT
b
(breast)
LCC6 - MDR
e
(breast)
R/S
d
H460
f
(lung)
HT - 29
g
(colon)
Paclitaxel 1.7 300 176 3.1 346 112 4.9 3.6
Docetaxel 1.0 215 215 … … … 1.0
SB - T - 12821 – 1 Ac MeO 0.32 8.8 28 0.33 3.99 12 0.38 0.69
SB - T - 12821 – 2 Ac F 0.45 5.58 13 0.38 5.93 16 0.49 1.11
SB - T - 12821 – 3 Ac Cl 0.40 5.04 13 0.22 4.96 23 0.5 0.85
SB - T - 12821 – 4 Ac N
3
0.47 3.85 8.2 1.18 4.00 3.4 0.20 0.50
SB - T - 12822 – 1 Et - CO MeO 0.19 2.16 11 0.45 4.24 9 0.41 0.54
SB - T - 12822 – 2 Et - CO F 0.68 3.78 5.6 0.82 4.27 5.2 0.59 1.15
SB - T - 12822 – 3 Et - CO Cl 0.34 3.28 9.6 0.39 2.54 6.5 0.63 1.11
SB - T - 12822 – 4 Et - CO N
3
0.38 1.61 4.2 1.09 2.56 2.3 0.20 0.40
SB - T - 12823 – 1 Me
2
NCO MeO 0.57 1.84 3.2 0.28 4.48 16 0.35 0.68
SB - T - 12823 – 2 Me
2
NCO F 0.32 2.64 8.3 0.32 5.57 17 0.5 0.76
SB - T - 12823 – 3 Me
2
NCO Cl 0.12 1.02 8.5 0.27 2.55 9.4 0.42 0.45
SB - T - 12823 – 4 Me
2
NCO N
3
0.47 2.61 5.6 1.27 3.52 2.8 0.30 0.50
SB - T - 12824 – 1 MeOCO MeO 0.17 2.88 17 0.27 3.99 15 0.38 0.53
SB - T - 12824 – 2 MeOCO F 0.31 4.88 16 0.39 5.81 15 0.61 0.85
SB - T - 12824 – 3 MeOCO Cl 0.65 4.72 7.3 0.29 5.08 18 0.43 0.68
SB - T - 12824 – 4 MeOCO N
3
0.47 2.92 6.2 1.09 4.00 3.7 0.20 0.40
a – g
See footnotes of Table 5.2 .
126 Fluorine in Medicinal Chemistry and Chemical Biology
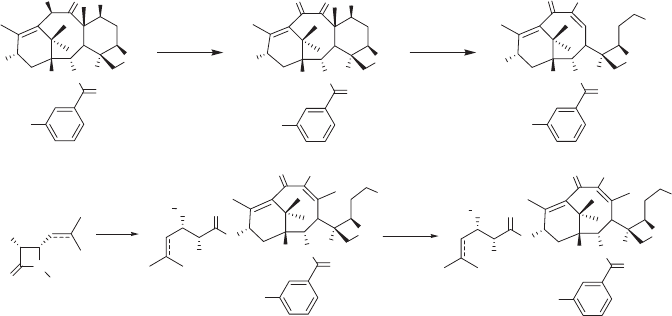
cell lines overexpressing class III isotype [29] . Accordingly, we have performed a SAR
study on IDN5390. As a part of this SAR study, we investigated two fl uorine - containing
analogues, SB - T - 10104 ( 25a ) and SB - T - 10204 ( 25b ) (see Scheme 5.4 ). Two C - seco -
fl uorotaxoids 25a and 25b were synthesized through the Ojima – Holton coupling of 7,9 -
di - TES - 2 - (3 - fl uorobenzoyl) - C - seco - baccatin ( 22 ) with β - lactams 23a [39] and 23b [40] ,
respectively, under standard conditions, followed by deprotection with HF - pyridine (see
Scheme 5.4 ). Di - TES - C - seco - baccatin 22 was prepared from 2 - (3 - fl uorobenzoyl) - 10 -
deacetylbaccatin 20 [17] using Appendino ’ s protocol [41, 42] as follows: Baccatin 20 was
oxidized with Cu(OAc)
2
and air to give the corresponding 10 - oxo - baccatin 21 , which was
then treated with L - selectride at − 78 ° C, followed by TES protection to afford di - TES - C -
seco - baccatin 22 (see Scheme 5.4 ).
Novel C - seco - fl uorotaxoids, 25a and 25b , were evaluated for their cytotoxicity
against several human ovarian adenocarcinoma cell lines: A2780wt (drug - sensitive
wild - type), A2780CIS, A2780TOP, A2780ADR (resistant to cisplatin, topotecan, and
adriamycin/doxorubicin, respectively), and A2780TC1 and A2780TC3 (resistant to both
paclitaxel and cyclosporine A). The drug resistance in the A2780ADR cell line is based
on MDR, while that in the A2780TC1 and A2780TC3 cell lines is caused by the overex-
pression of class III β - tubulin subunit and other possible mutations. Thus, the activity of
these two C - seco - fl uorotaxoids is of particular interest. Results are shown in Table 5.4 .
As Table 5.4 shows, SB - CST - 10104 ( 25a ) possesses remarkable potency against
paclitaxel - resistant cell lines A2780TC1 and A2780TC3, especially the latter, that is, the
most drug - resistant cell line for paclitaxel in this series. This C - seco - fl uorotaxoid 25a is
39 times more potent than paclitaxel against cell line A2780TC3. The resistance factor
IC
50
(A2780TC3)/IC
50
(A2780 wt) for this cell line is 10 470 for paclitaxel, but it is only
41 for 25a . For comparison, IDN5390 exhibits 8.0 times higher potency than paclitaxel
O
OAc
HO
O
O
F
20
HO
OH
HO
O
N
TIPSO
O
Boc-t
OTES
OAc
O
O
O
O
OTIPS
NH
F
HO
OTES
O
O
OH
OAc
O
O
O
O
OH
NH
F
HO
OH
O
O
25a: isobutyl
25b: isobutenyl
O
OAc
HO
O
O
F
HO
OH
O
O
OTES
OAc
HO
O
O
F
HO
OTES
O
O
23a: isobutyl
23b: isobutenyl
22
21
i ii, iii
v
t-Boc
t-Boc
iv
24a: isobutyl
24b: isobutenyl
22
+
Scheme 5.4 Synthesis of 2 - (3 - fl uorobenzoyl) - C - seco - taxoids ( 25 ). (i) Cu(OAc)
2
, MeOH,
86%; (ii) L - selectride, THF, − 78
o
C 50 – 70%, (iii) methyl imidazole, TESCl, DMF, 0
o
C, 52;
(iv) LiHMDS, THF, − 40
o
C, 70 – 80%; (v) HF/pyridine, CH
3
CN/pyridine, 0
o
C – RT,
52% – 92%.

Fluoro-Taxoid Anticancer Agents 127
Table 5.4 In vitro cytotoxicity ( IC
50
nM)
a
of C - seco - fl uorotaxoids ( 24 )
C - seco - Taxoid
A2780wt
b
A2780CIS
c
A2780TOP
d
A2780ADR
e
A2780TC1
f
A2780TC3
g
Paclitaxel 1.7 2.2 7.2 1 239 10 027 17 800
IDN5390 17.4 16.8 27.5 2 617 2 060 2 237
SB - CST - 10104 (25a) 11.1 11.8 12.8 3 726 1 497 460
SB - CST - 10204 (25b) 6.1 4.9 6.9 2 218 4 454 745
a
The concentration of compound that inhibits 50% of the growth of a human tumor cell line after 72
h drug exposure.
b
Human ovarian carcinoma wild - type.
c
Cisplatin - resistant A2780.
d
Topotecan - resistant A2780.
e
Adriamycin - resistant A2780.
f,g
Clones derived from chronic exposition of A2780 to paclitaxel and cyclosporine.
128 Fluorine in Medicinal Chemistry and Chemical Biology
with a resistance factor of 129 against the same cell line. This result is quite impressive
taking into account the fact that the only structural difference between IDN5390 and 25a
is one fl uorine substitution at the meta position of the C - 2 - benzoate moiety of the C - seco -
taxoid molecule.
The C3 ′ - substituents of C - seco - fl uorotaxoids 25a (3 ′ - isobutyl) and 25b (3 ′ -
isobutenyl) also show interesting effects on the potency, which is assumed to be related
directly to their interaction with the class III β - tubulin. As Table 5.4 shows, 25b exhibits
higher potency than 25a against A2780wt, A2780CIS, A2780TOP, and A2780ADR.
However, the reversal of this SAR is observed against A2780TC1 and A2780TC3, in
which the class III β - tubulin is overexpressed. Overall, it has been shown that the intro-
duction of one fl uorine to the C - 2 - benzoate moiety of C - seco - taxoid molecule substantially
increases the potency against both paclitaxel - sensitive and paclitaxel - resistant human
ovarian cancer cell lines.
5.5 Synthesis and Biological Evaluation of C - 3 ′ - Difl uorovinyl - Taxoids
As described above, the introduction of isobutyl, isobutenyl, CF
2
H, and CF
3
groups to the
C3 ′ - position of taxoids, replacing the phenyl group of paclitaxel and docetaxel, has led
to the development of highly potent second - generation taxoids, especially against drug -
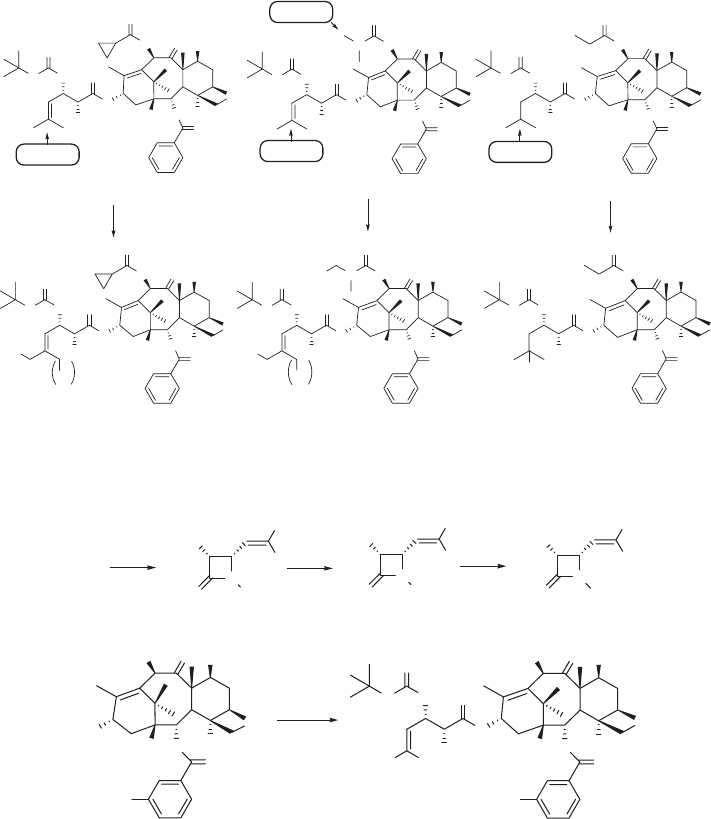
resistant cancer cell lines expressing MDR phenotype. Our recent metabolism studies
on 3 ′ - isobutyl - and 3 ′ - isobutenyl - taxoids has disclosed that the metabolism of second -
generation taxoids (SB - T - 1214, SB - T - 1216, and SB - T - 1103) is markedly different from
that of docetaxel and paclitaxel [43] . These taxoids are metabolized (via hydroxylation)
by CYP 3A4 of the cytochrome P450 family enzymes, primarily at the two allylic methyl
groups of the C - 3 ′ - isobutenyl group and the methyne moiety of the 3 ′ - isobutyl group (see
Figure 5.2 ). This forms a sharp contrast with the known result that the tert - butyl group of
the C - 3 ′ N - t - Boc moiety is the single predominant metabolic site for docetaxel [44] . These
unique metabolic profi les prompted us to design and synthesize 3 ′ - difl uorovinyl - taxoids,
in order to block the allylic oxidation by CYP 3A4, which should enhance the metabolic
stability and activity in vivo .
For the synthesis of a series of C - 3 ′ - difl uorovinyl - taxoids 29 , novel (3 R ,4 S ) - 1 - t - Boc -
3 - TIPSO - 4 - difl uorovinyl - β - lactam 28 (+) is the key component for the coupling with
baccatins 15 (see Scheme 5.5 ). We prepared this β - lactam 28 (+) in three steps from 4 -
formyl - β - lactam 5 (+) (see Scheme 5.1 ) using the Wittig reaction of the formyl moiety with
difl uoromethylphosphorus ylide generated in situ from (Me
2
N)
3
P/CF
2
Br
2
/Zn (see Scheme
5.5 ). The Ojima – Holton coupling reaction [45 – 47] of β - lactam 28 (+) with C - 2 - modifi ed,
C - 10 - modifi ed or C - 2,10 - modifi ed baccatins 15 (X = H, MeO, N
3
) [17] and the subsequent
removal of the silyl protecting groups gave the corresponding C - 3 ′ - difl uorovinyl - taxoids
29 in good to excellent yields.
The cytotoxicities of the 3 ′ - difl uorovinyl - taxoids 29 were evaluated in vitro against
MCF7 - S, MCF7 - R, HT - 29 (human colon carcinoma), and PANC - 1 (human pancreatic
carcinoma) cell lines [37] . The results are summarized in Table 5.5 .
As Table 5.5 shows, all difl uorovinyl - taxoids 29 are exceedingly potent compared
with paclitaxel. A clear effect of C - 2 - benzoate modifi cation at the meta position (X = H
Fluoro-Taxoid Anticancer Agents 129
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
SB-T-1214
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
SB-T-1216
CYP 3A4
O
O
N
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
SB-T-1103
O
CYP 3A4
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
O
HO
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
O
NHO
HO
OO
OH
O
OAc
HO
O
O
O
O
OH
NH
O
O
O
OH
CYP 3A4
CYP 3A4
OH OH
Figure 5.2 Primary sites of hydroxylation on the second - generation taxoids by P450 family
enzymes.
N
TIPSO
O PMP
F
F
N
TIPSO
O H
F
F
N
TIPSO
O t-Boc
F
F
27(+) 28(+)
5(+)
26(+)
i
ii
iii
ORO
OTES
O
OAc
HO
HO
O
O
ORO
OH
O
OAc
HO
O
O
O
O
OH
NHO
O
F F
X
X
R = Ac, EtCO, c-PrCO, Me
2
NCO, MeOCO; X = H, MeO, N
3
,
iv, v
29
15
28(+)
vs. X = MeO or N
3
) is observed on the increase in potency against drug - sensitive and
drug - resistant MCF7 cell lines (entries 2 – 5 vs. entries 6 – 11). Difl uorovinyl - taxoids with
2,10 - modifi cations (entries 6 – 11) have impressive potency, exhibiting IC
50
values in the
< 100 pM range (78 – 92 pM), except for one case against MCF7 - S (entry 7), and in the
subnanomolar range (0.34 – 0.57 nM) against MCF7 - R, which is 3 orders of magnitude
Scheme 5.5 Synthesis of C - 3 ¢ - difl uorovinyl-taxoids ( 28 ). (i) CBr
2
F
2
, HMPT, Zn, THF, 84%;
(ii) CAN, H
2
O/CH3CN, − 15 ° C, 92%; (iii) Boc
2
O, Et
3
N, DMAP, CH
2
Cl
2
, 96%; (iv) LiHMDS,
THF, − 40 ° C; (v) HF/Py, Py/CH
3
CN, overnight, 0 ° C – RT, 57 – 91% (for two steps).
130 Fluorine in Medicinal Chemistry and Chemical Biology
Table 5.5 In vitro cytotoxicity ( IC
50
nM)
a
of 3 ′ - difl uorovinyl - taxoids( 29 )
Entry Taxoid R X
MCF7 - S
b
(breast)
MCF7 - R
c
(breast)
R/S
HT - 29
d
(colon)
PANC - 1
e
(pancreatic)
1 Paclitaxel 1.2 300 250 3.6 25.7
2 SB - T - 12851 Ac H 0.099 0.95 9.6 0.41 1.19
3 SB - T - 12852 c - Pr - CO H 0.12 6.0 50 0.85 5.85
4 SB - T - 12853 Et - CO H 0.12 1.2 10 0.34 0.65
5 SB - T - 12854 Me
2
N - CO H 0.13 4.3 33 0.46 1.58
6 SB - T - 12852 - 1 c - Pr - CO MeO 0.092 0.48 5.2 – –
7 SB - T - 12853 - 1 Et - CO MeO 0.34 0.57 1.7 – –
8 SB - T - 12855 - 1 MeO - CO MeO 0.078 0.50 6.4 – –
9 SB - T - 12851 - 3 Ac N
3
0.092 0.34 3.7 – –
10 SB - T - 12852 - 3 c - Pr - CO N
3
0.092 0.45 4.9 – –
11 SB - T - 12855 - 3 MeO - CO N
3
0.078 0.40 5.3 – –
a – d
See footnotes of Table 5.2 .
e
Human pancreatic carcinoma.
Fluoro-Taxoid Anticancer Agents 131
more potent than paclitaxel. The resistance factor for these taxoids is 1.7 – 6.4, while that
for paclitaxel is 250. Difl uorovinyl - taxoids with unmodifi ed C - 2 - benzoate moiety (entries
2 – 5) also show highly enhanced potency against MCF7 - S and MCF7 - R as compared to
paclitaxel. These taxoids exhibit impressive potency against HT - 29 (human colon) and
PANC - 1 (human pancreatic) cancer cell lines as well. SB - T - 12853 appears particularly
promising against these gastrointestinal (GI) cancer cell lines. Although difl uorovinyl -
taxoids with 2,10 - modifi cations (entries 6 – 11) have not yet been evaluated against HT - 29
and PANC - 1 cell lines, it is anticipated that these taxoids will exhibit remarkable potency
against these GI cancer cell lines in a similar manner to that for MCF7 - R.
5.6 Possible Bioactive Conformations of Fluoro - Taxoids
19
F NMR combined with advanced 2D spectroscopic methods provides a powerful tool
for the study of dynamic conformational equilibria of fl uorine - containing bioactive mole-
cules. The wide dispersion of fl uorine chemical shifts is particularly useful for the observa-
tion of conformers at low temperatures. We have successfully used fl uorine - containing
taxoids as probes for NMR analysis of the conformational dynamics of paclitaxel in con-
junction with molecular modeling [48] . We have further applied the fl uorine - probe proto-
col to solid - state magic angle spinning (SSMAS)
19
F NMR analysis with the radiofrequency
driven dipolar recoupling (RFDR) method to measure the F – F distance in the microtubule -
bound conformation of F
2
- 10 - Ac - docetaxel (see Figure 5.3 ) [47] . Schaefer and co - workers
used rotational echo double - resonance (REDOR) to investigate the structure of the micro-
tubule - bound paclitaxel by determining the
19
F –
13
C distances of a fl uorine probe of pacli-
taxel (see Figure 5.3 ) [49] . These solid - state NMR studies have provided critical information
on the bioactive conformation of paclitaxel and docetaxel.
Recently, we proposed a new bioactive conformation of paclitaxel, “ REDOR - Taxol ”
[50] , based on (i) the
19
F –
13
C distances obtained by the REDOR experiment [49] , (ii) the
photoaffi nity labeling of microtubules [51] , (iii) the crystal structure (PDB code: 1TUB)
of the Zn
2+
- stabilized α β - tubulin dimer model determined by cryo - electron microscopy
(cryo - EM) [52] , and (iv) molecular modeling (Monte Carlo; Macromodel) [50] . In this
computational biology analysis, we fi rst docked a paclitaxel - photoaffi nity label molecule
to the position identifi ed by our photoaffi nity labeling study and then optimized the
O
OH
H
O
OH
O
AcO
O
O
O
O
OH
NH
O
d
1
=7.8 Å
d
2
=6.3 Å
R
2
REDOR (
19
F-
2
H)
R
1
O
CD
3
d
3
>8 Å
R
1
/R
2
=
19
F or
2
H
O
OH
H
O
OH
O
AcO
O
OAc
O
O
OH
NH
O
d
1
=9.8 Å
d
2
=10.3 Å
19
F
REDOR (
19
F-
13
C)
O
OH
H
O
OH
O
AcO
O
OAc
O
O
OH
NH
O
O
d=6.5 Å
19
F
19
F
RFDR
(Radio Frequency Driven Recoupling)
(Rotational Echo Double Resonance)
Figure 5.3 Solid - state NMR studies on microtubule - bound fl uoro - taxoid probes.
132 Fluorine in Medicinal Chemistry and Chemical Biology
position with a free paclitaxel molecule in the binding space using the REDOR distances
as fi lters [50] .
More recently, three additional intramolecular distances of the key atoms in the
microtubule - bound
19
F/
2
H - labeled paclitaxel were determined by the REDOR method (see
Figure 5.3 ) [53] . It has also been shown that the optimized cryo - EM crystal structure of
tubulin - bound paclitaxel (PDB code: 1JFF) [54] serves better for the computational struc-
ture analysis. Accordingly, we have optimized our REDOR - Taxol structure, using the 1JFF
coordinates as the starting point, by means of molecular dynamics simulations (Macro-
model, MMFF94) and energy minimization (InsightII 2000, CVFF) [55] .
We applied the same computational protocol to investigate the microtubule - bound
structures of the 3 ′ - CF
2
H - , 3 ′ - CF
3
- , and 3 ′ - CF
2
C = CH - taxoids, using the updated REDOR -
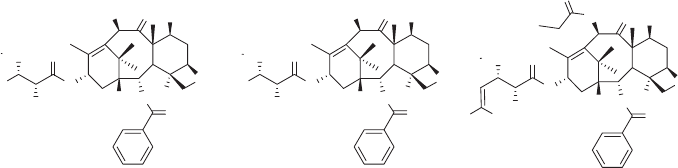
Taxol [55] as the starting structure. Three fl uoro - taxoids, SB - T - 1284, SB - T - 1282, and
SB - T - 12853 (see Figure 5.4 ), were docked into the binding pocket of paclitaxel in the β -
tubulin subunit by superimposing the baccatin moiety with that of the REDOR - Taxol, and
their energies were minimized (InsightII 2000, CVFF). The resulting computer - generated
binding structures of three fl uoro - taxoids are shown in Figure 5.5 (a, b, c).
As Figure 5.5 (a, b, c) shows, the baccatin moiety occupies virtually the same space
in all cases, as expected. Each fl uoro - taxoid fi ts comfortably in the binding pocket without
any high - energy contacts with the protein. There is a very strong hydrogen bond between
the C - 2 ′ - OH of a fl uoro - taxoid and His227 of β - tubulin in all cases, which shares the same
key feature with the REDOR - Taxol structure [50] . [ Note: Our preliminary study on the
tubulin - bound structures of these three fl uoro - taxoids using the 1TUB coordinates [52] led
to different structures in which the C - 2 ′ - OH had a hydrogen bond to Arg359 of β - tubulin
[56] . However, the use of the updated REDOR - Taxol structure based on the 1JFF coordi-
nates [54] unambiguously led to fl uoro - taxoid structures bearing a strong hydrogen bond
between the C - 2 ′ - OH and His227.]
The CF
2
H and CF
3
moieties fi ll essentially the same space, as anticipated. However,
the CF
2
C = CH moiety occupies more extended hydrophobic space than the CF
2
H and CF
3
moieties. It is likely that this additional hydrophobic interaction contributes substantially
to the exceptional cytotoxicity of difl uorovinyl - taxoids 29 . The overlay of SB - T - 12853
with a representative second - generation taxoid, SB - T - 1213 shows excellent fi t, which may
demonstrate that the difl uorovinyl group mimics the isobutenyl group (see Figure 5.5 , d).
However, the difl uorovinyl group is in between vinyl and isobutenyl groups in size,
and two fl uorine atoms may mimic two hydroxyl groups rather than two methyl groups
SB-T-1284
O
OAc
O
O
O
HF
2
C
O
HO
NH
HO
OH
AcO
O
t-Boc
SB-T-1282
O
OAc
O
O
O
F
3
C
O
HO
NH
HO
OH
AcO
O
t-Boc
O
OAc
O
O
O
O
OH
NH
HO
OH
O
O
t-Boc
F
F
SB-T-12853
O
Figure 5.4 Structures of fl uoro - taxoids analyzed by molecular modeling.
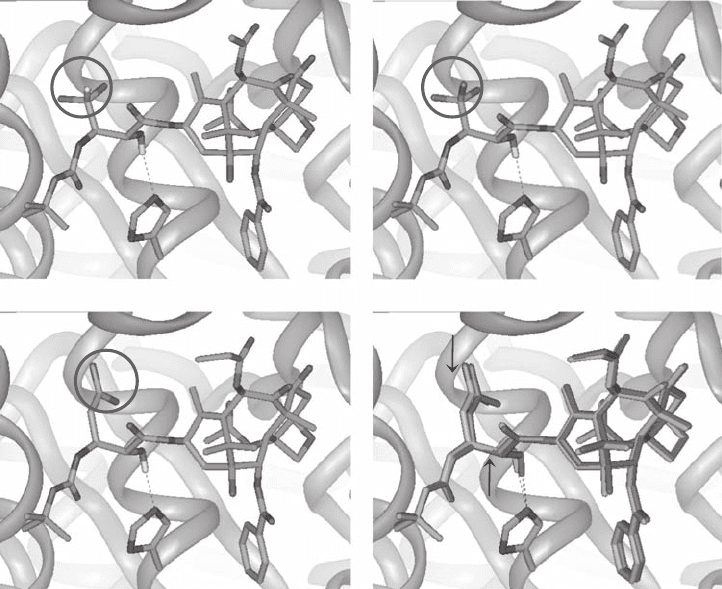
Fluoro-Taxoid Anticancer Agents 133
His227
(a) (b)
(c) (d)
SB-T-1213
SB-T-12853
His227
His227
electronically. Accordingly, the difl uorovinyl group can be regarded as “ magic vinyl ” in
drug design, similarly to “ magic methyl ” for the trifl uoromethyl group, including its
anticipated metabolic stability against P - 450 family enzymes.
5.7 Use of Fluorine in Tumor - Targeting Anticancer Agents
Although current cancer chemotherapy is based on the premise that rapidly proliferating
tumor cells are more likely to be killed by cytotoxic drugs, the difference in activity of
cytotoxic drugs against tumor tissues and against primary tissues is relatively small. Con-
sequently, the amount of an anticancer drug required to achieve clinically effective level
of activity against the targeted tumor cells often causes severe damage to actively propa-
gating non - malignant cells such as cells of the gastrointestinal tract and bone marrow,
resulting in a variety of undesirable side - effects. Accordingly, it is very important to
develop new chemotherapeutic agents with improved tumor specifi city.
Figure 5.5 Computer - generated binding structures of fl uoro - taxoids to β - tubulin: (a) SB - T -
1284 (3 ′ - CF
2
H); (b) SB - T - 1282 (3 ′ - CF
3
); (c) SB - T - 12853 (3 ′ - CF
2
= CH); (d) Overlay of SB - T -
12853 and SB - T - 1213 (C3 ′ - isobutenyl). See color plate 5.5.
