Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

72 Fluorine in Medicinal Chemistry and Chemical Biology
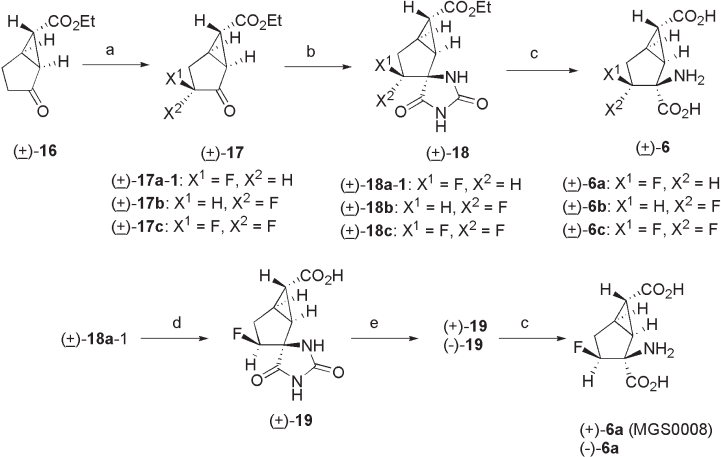
Scheme 3.1 Synthesis of (+) - 6a (MGS0008) and its congeners (1). Reagents and conditions:
(a) (i) LHMDS, TMSCl, THF, (ii) (PhSO
2
)
2
NF, CH
2
Cl
2
; (b) (NH
4
)
2
CO
3
, KCN, EtOH, H
2
O; (c)
60% aq. H
2
SO
4
, 2.5 or 3.0 M aq. NaOH; (d) 2 M aq. NaOH; (e) ( R ) - PhCH(NH
2
)Me,
acetone, H
2
O and then 1 M aq. HCl.
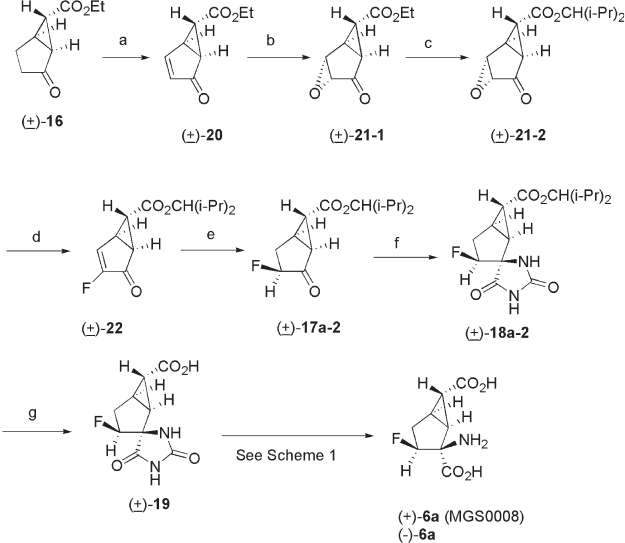
presence of Triton B to yield epoxide ( ± ) - 21 - 1 . Ethyl ester ( ± ) - 21 - 1 was transformed to
2,4 - dimethyl - 3 - pentyl ester ( ± ) - 21 - 2 in two steps to avoid hydrolysis and transesterifi ca-
tion of the ethyl ester under the fl uorination conditions. Thus, ( ± ) - 21 - 1 was hydrolyzed
under basic conditions (92% yield), followed by esterifi cation with 2,4 - dimethyl - 3 -
pentanol in the presence of dicyclohexylcarbodiimide (DCC) to give ( ± ) - 21 - 2 , which
was used in the next step without purifi cation. The nucleophilic fl uorination of epoxide
( ± ) - 21 - 2 with potassium hydrogen difl uoride [48] in ethylene glycol yielded fl uoroenone
( ± ) - 22 . The hydrogenation of ( ± ) - 22 over palladium on carbon proceeded in a highly ste-
reoselective manner to give ( ± ) - 17a - 2 (48% yield for three steps). Fluoroketone ( ± ) - 17a - 2
was converted to optically pure (+) - 6a in a four - step process: (i) formation of hydantoin
( ± ) - 18a - 2 under the Bucherer – Bergs conditions (75% yield); (ii) selective hydrolysis of
the ester moiety with 48% aqueous HBr to give ( ± ) - 19a - 2 (82% yield); (iii) optical resolu-
tion of ( ± ) - 19a - 2 with ( R ) - 1 - phenylethylamine afforded optically pure hydantoin (+) - 19a - 2
(48% yield, > 99% ee); and (iv) hydrolysis of (+) - 19a - 2 under acidic conditions to give
(+) - 6a (MGS0008) (74% yield).
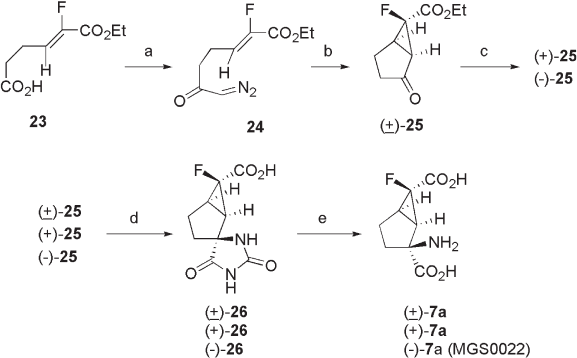
3.4.2 Synthesis of ( − ) - 7a ( MGS 0022)
Racemic ( ± ) - 7a and optically pure (+) - 7a and ( − ) - 7a (MGS0022) were originally synthe-
sized from fl uorobicyclo[3.1.0]cyclohexanecarboxylates ( ± ) - 25 , (+) - 25 , and ( − ) - 25 ,
Fluorinated Conformationally Restricted Glutamate Analogues 73
Scheme 3.2 Synthesis of (+) - 6a (MGS0008) and its congeners (2). Reagents and conditions:
(a) (i) LHMDS, TMSCl, TFA, (ii) Pd(OAc)
2
, MeCN; (b) TBHP, Triton B, PhMe; (c) (i) 2 M
aq. NaOH, (ii) (i - Pr)
2
CHOH, DCC, DMAP, CHCl
3
; (d) KF - HF, ethylene glycol, (e) H
2
, 5%
Pd/C, EtOH; (f) (NH
4
)
2
CO
3
, KCN, EtOH, H
2
O; (g) 48% HBr.
respectively, through (i) hydrolysis of the ethyl ester moieties of ( ± ) - 25 , (+) - 25 , and ( − ) - 25 ,
(ii) formation of hydantoins under Bucherer – Bergs conditions (99%, 88%, and 90% yields
(two steps), respectively), and (iii) hydrolysis of hydantoins ( ± ) - 25 (34% yield), (+) - 25
(72% yield), and ( − ) - 25 (73% yield), under acidic conditions (see Scheme 3.2 ) [15] . The
key intermediate ( ± ) - 25 was synthesized by Cu(TBS) - catalyzed (TBS = N - tert - butylsali-
cylaldimine) intramolecular cyclopropanation of diazoketo - fl uoroalkenoate 24 , which was
prepared from 23 through reaction with oxalyl chloride and then diazomethane (27% total
yield from 23 to 25 ) [49, 50] . Effective optical resolution of ( ± ) - 25 was achieved using
chiral HPLC. Enantioselective intramolecular cyclopropanation of 24 was also examined
using chiral copper catalysts. Among the catalysts examined, the Cu(II) complex with
( S ) - ( − ) - 2,2 ′ - isopropylidenebis(4 - phenyl - 4,5 - dihydro - 1,3 - oxazole) was found to be the
best, yielding ( − ) - 26 with up to 65% ee [42] .
3.4.3 Synthesis of (+) - 7b ( MGS 0028)
Racemic ( ± ) - 7b as well as optically pure (+) - 7b (MGS0028) and ( − ) - 7b were originally
synthesized via hydroxy - keto compounds ( ± ) - 29 , (+) - 29 , and ( − ) - 29 , respectively (see
74 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 3.4 ) [15] . The key intermediates ( ± ) - 29 and (+) - 29 were prepared from the corre-
sponding ( ± ) - 25 and (+) - 25 in three steps: (i) reaction with TMSCl and LHMDS, followed
by dehydrosilylation catalyzed by palladium acetate (89% and 89% yields), (ii) stereose-
lective epoxidation by TBHP in the presence of Triton B (98% and 88% yields), and (iii)
regioselective reduction of α , β - epoxy ketone ( ± ) - 28 with benzeneselenol, which was
generated in situ from diphenyl diselenide (PhSe)
2
and sodium borohydride in the presence
of acetic acid (76% and 71% yields). Hydroxyketones ( ± ) - 29 and (+) - 29 were converted
to their tert - butyldimethylsilyl (TBS) ethers, followed by thioketalization (the TBS – O
bond was cleaved during the work - up) to give ( ± ) - 30 (85% yield) and (+) - 30 (94% yield),
respectively. Hydroxythioketals ( ± ) - 30 and (+) - 30 were oxidized with dimethyl sufoxide
(DMSO) and DCC in the presence of pyridine and trifl uoroacetic acid to afford the corre-
sponding ketones (85% and 76% yields, respectively). The resulting ketone ( ± ) - 31 , was
converted to ( ± ) - 7b (12% yield from ( ± ) - 32 ), in three steps via ( ± ) - 32 (79% yield) using
a protocol similar to that described above for ( ± ) - 6 (see Scheme 3.2 ). Also, optically active
(+) - 7b (MGS0028) (45% yield, from highly polar isomer 33 ) and ( − ) - 7b (28% yield, from
slightly polar isomer 33 ) were obtained by separating the diastereomers of hydantoin -
amides 33 (highly polar isomer 33 : 46% yield and slightly polar isomer 33 : 46% yield,
from ( ± ) - 32 ), which were prepared by coupling ( ± ) - 32 with ( R ) - (+) - 1 - phenylethylamine
(see Scheme 3.4 ).
Next, process chemistry for the practical synthesis of 7b (MGS0028) is discussed
(Schemes 3.5 – 3.7) [42 – 45] . First, the synthesis of key intermediate (+) - 29 from racemic
acetoxycyclopentene ( 34 ) is shown in Scheme 3.5 [43] . The key reaction in this approach
was Trost ’ s asymmetric allylic alkylation reaction of ethyl 2 - fl uoroacetoacetate with 34 ,
which afforded 35 in high yield and high enantioselectivity, especially when a bulky tetra -
n - hexyl ammonium bromide was used as a phase - transfer reagent (89% yield, 94 – 96%
Scheme 3.3 Synthesis of ( − ) - 7 (MGS0022) and its congeners. Reagents and conditions: (a)
(i) (COCl)
2
, hexane, (ii) CH
2
N
2
, Et
2
O; (b) Cu(TBS)
2
, PhH; (c) chiral HPLC; (d) (i) 1 M aq.
NaOH, (ii) KCN, (NH
4
)
2
CO
3
, EtOH, H
2
O; (e) 60% H
2
SO
4
.
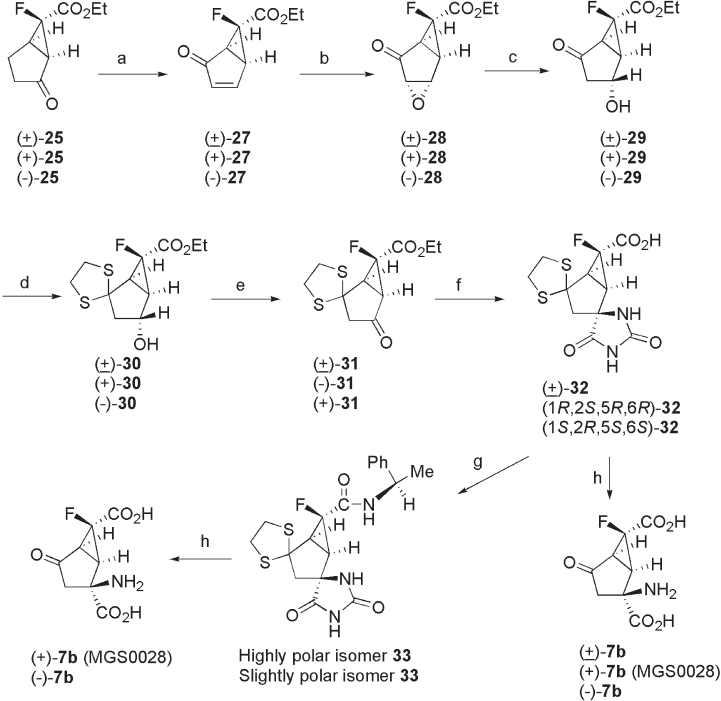
Fluorinated Conformationally Restricted Glutamate Analogues 75
Scheme 3.4 Synthesis of (+) - 7b (MGS0028) and its congeners. Reagents and conditions:
(a) (i) LHMDS, TMSCl, THF, (ii) Pd(OAc)
2
, MeCN; (b) TBHP, Triton B, PhMe; (c) (PhSe)
2
,
NaBH
4
, AcOH, EtOH; (d) (i) TBSCl, imidazole, DMF, (ii) HS(CH
2
)
2
SH, BF
3
- Et
2
O, CHCl
3
; (e)
DMSO, DCC, Py, TFA; (f) (i) 1 M aq. NaOH, (ii) KCN, (NH
4
)
2
CO
3
, EtOH, H
2
O; (g) (i) ( R ) -
(+) - PhCH(NH
2
)CH
3
, EDC - HCl, HOBt, DMF, (ii) chromatography on silica gel. (h) 60%
H
2
SO
4
.
ee). Cleavage of the acetyl group of 35 , diastereoselective epoxidation via bromohydrin
( cis : trans = 8 : 1) and intramolecular epoxide opening, followed by oxidation with 1 -
hydroxy - 1,2 - benziodoxal - 3(1 H ) - one - 1 - oxide (IBX) gave intermediate (+) - 25 [43] . Ketone
(+) - 25 was converted to enone (+) - 27 in fi ve steps: (i) bromination of (+) - 25 (100% yield),
(ii) azeotropic ketalization (92% yield), (iii) dehydrobromination, (iv) deketalization (92%
yield for two steps), and (v) treatment with diazomethane (90% yield). Finally, enone
(+) - 27 was transformed to β - hydroxy ketone 29 through epoxidation, followed by reduc-
tion with benzeneselenol generated in situ .
76 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 3.5 Synthesis of the key intermediate (+) - 29 for (+) - 7b (MGS0028) (1). Reagents
and conditions: (a) ( R,R ) - Trost ligand, allylpalladium chloride dimer, ( n - hex)
4
NBr, NaH,
AcCHFCO
2
Et, CH
2
Cl
2
; (b) 0.1 M, EtONa, EtOH; (c) (i) NBS, acetone, H
2
O, (ii) DBU, CH
2
Cl
2
;
(d) LHMDS, Et
3
Al, THF; (e) IBX, DMSO, PhMe; (f) (i) Br
2
, CH
2
Cl
2
, (ii) ethylene glycol, p -
TsOH - H
2
O, PhMe, (iii) t - BuOK, H
2
O (1 eq.) THF, (iv) 1 M HCl; (v) CH
2
N
2
, (g) (i) TBHP,
Triton B, PhMe, (ii) (PhSe)
2
, NaBH
4
, AcOH, EtOH.
Scheme 3.6 Synthesis of key intermediate (+) - 29 for (+) - 7b (MGS0028) (2). Reagents and
conditions: (a) LDA, NFSI, THF; (b) TBHP, VO(acac)
2
, PhMe; (c) TBSCl, imidazole, DMF;
(d) Et
3
Al, LHMDS; (e) (i) NaClO, RuCl
3
(1 mol%), MeCN, (ii) 1 M HCl.
Next, the most effi cient route to (+) - 7b (MGS0028), to date, via monoprotected fl uo-
rinated diol 43 and ketal 45 as key intermediates is discussed (see Schemes 3.6 and 3.7)
[45] . Key intermediate 43 was synthesized from hydroxycyclopentenylacetate 39 in four
steps: (i) fl uorination of the dianion of 39 (85% yield) to form 40 , (ii) vanadium - mediated
epoxidation of 40 to give epoxide 41 (93% yield), (iii) protection of the hydroxyl moiety
of 41 to form TBS - ether 42 (95% yield), and (iv) cyclopropanation of 42 through ring -
opening of the epoxide moiety to afford key intermediate 43 (96% yield). It should be
noted that the epoxidation of 40 proceeded with excellent stereoselectivity to give the
Fluorinated Conformationally Restricted Glutamate Analogues 77
desired trans - epoxide 41 by exploiting the effi cient directing effect of the free hydroxyl
group. RuCl
3
- mediated oxidation of 43 , followed by desilylation gave (+) - 29 in 95% yield
(see Scheme 3.6 ).
The key intermediate (+) - 29 was converted to the second key intermediate 45 through
ketalization with the bis - O - TMS ester of ( S , S ) - hydrobenzoin to form 44 (quantitative
yield), and the subsequent RuCl
3
- mediated oxidation (93% yield). Strecker reaction of 45
gave aminonitrile 46 with a very good diastereomer ratio (13.1 : 1 by HPLC analysis) as
highly crystalline product. Thus, the desirable diastereomer of 46 was isolated as single
product from the reaction mixture in 80 – 85% yield. Hydrolysis of ketal - aminonitrile 46
proceeded under much milder conditions than those for dithioketal - hydantoin 32 (see
Scheme 4), and (+) - 7b was isolated without utilizing ion - exchange column chromatogra-
phy (94% yield) (see Scheme 3.7 ) [45] . Thus, this optimized process was able to avoid
cumbersome handling, resolution using chiral HPLC and ion - exchange column chroma-
tography, which were included in the original synthesis of (+) - 7b (see Scheme 3.5 ).
3.4.4 Pharmacology and Pharmacokinetics of (+) - 6a ( MGS 0008),
( − ) - 7a ( MGS 0022) and (+) - 7b ( MGS 0028)
3.4.4.1 In vitro Pharmacology of Compounds 6 and 7
The in vitro pharmacological data of fl uorine - containing conformationally restricted
glutamate analogues 6 and 7 are summarized in Table 3.1 [15] . Optically active (+) - 6a
(MGS0008), bearing a fl uorine atom at the C - 3 position of 4 (LY354740), is a potent and
selective agonist for mGluR2/3. Compound (+) - 6a exhibited a high agonist activity for
mGluR2 (EC
50
= 29.4 nM) and mGluR3 (EC
50
= 45.4 nM) as well as a high binding affi nity
to mGluR2 ( K
i
= 47.7 nM) and mGluR3 ( K
i
= 65.9 nM), but did not exhibit signifi cant
Scheme 3.7 Synthesis of (+) - 7b (MGS0028) from key intermediate (+) - 29 . Reagents and
conditions: (a) ( S,S ) - PhCH(OTMS) - CH(OTMS)Ph, TfOH, CH
2
Cl
2
; (b) NaClO, RuCl
3
(0.5 mol%), MeCN; (c) NH
3
/MeOH, Ti(O - Pr - i )
4
, TMSCN; (d) (i) HCl (8 M), AcOH, (ii) H
2
O.
78 Fluorine in Medicinal Chemistry and Chemical Biology
Table 3.1 In vitro and in vivo pharmacological data of m G lu R 2/3 ligands 4 , 6 , 7 and 12
Y
CO
2
R
2
X
3
H
H
CO
2
H
X
2
NH
2
X
1
Compound X
1
X
2
X
3
Y
Agonist activity
a
Antagonist activity
b
Binding affi nity
c
EC
50
± SEM (nM) IC
50
± SEM (nM) K i ± SEM (nM)
c
mGluR2 mGluR3 mGluR2 mGluR3 mGluR2 mGluR3
4 (LY354740) H H H CH
2
18.3 ± 1.6 62.8 ± 12
– –
23.4 ± 7.1 53.5 ± 13
( ± ) - 6a
F H H CH
2
67.7 ± 9.3
– – – – –
(+) - 6a (MGS0008) F H H CH
2
29.4 ± 3.3 45.4 ± 8.4 > 100 000 > 100 000 47.7 ± 17 65.9 ± 7.1
( − ) - 6a
F H H CH
2
2 640 ± 290
– 36 200 – – –
( ± ) - 6b
H F H CH
2
> 100 000
– 17 100 – – –
( ± ) - 6c
F F H CH
2
> 100 000
– 36 200 – – –
( ± ) - 7a
H H F CH
2
34.2 ± 6.3
– – – – –
(+) - 7a H H F CH
2
1 120 ± 200
– – – – –
( − ) - 7a (MGS0022)
H H F CH
2
16.6 ± 5.6 80.9 ± 31 > 100 000 > 100 000 22.5 ± 7.3 41.7 ± 7.1
( ± ) - 7b
H H F CO
1.26 ± 0.2
– – – – –
(+) - 7b (MGS0028) H H F CO
0.570 ± 0.10 2.07 ± 0.40 > 100 000 > 100 000 3.30 ± 0.31 3.62 ± 1.6
( − ) - 7b
H H F CO
94.7 ± 5.6
– – – – –
( ± ) - 7c
H H Me CH
2
> 100 000
–
> 100 000
– – –
(+) - 12a H OH H CH
2
– – – –
52
d
89
d
( ± ) - 12b
H Me H CH
2
– –
1750 ± 620
e
9830 ± 370
e
983 ± 35
f
146 ± 56
f
– : not determined.
EC
50
: 50% effective concentration.
IC
50
: 50 inhibitory concentration.
K
i
: inhibition constant.
a
Compounds (+) - 6a , ( − ) - 7a and (+) - 7b exhibited no signifi cant agonist activities for rat mGluR1a, mGluR4, mGluR6, and mGluR7 expressed in CHO cells (ED
50
> 100 000 nM).
b
Compounds (+) - 6a , ( − ) - 7a and (+) - 7b exhibited no signifi cant antagonist activities for rat mGluR1a, mGluR4, mGluR6 and mGluR7 expressed in CHO cells (ED
50
> 100 000 nM).
c
Binding affi nities for mGluR2 and mGluR3 were determined by binding study utilizing [
3
H] - MGS0008 in rat mGluR - expressing cells.
d
Displacement of [
3
H]LY354740 binding in rat brain [36] .
e
cAMP responses in human mGluR - expressing cells [37] .
f
Displacement of [
3
H]LY341495 binding to human mGluR - expressing cells [37] .
Fluorinated Conformationally Restricted Glutamate Analogues 79
agonist or antagonist activities for mGluR1a, mGluR4, mGluR5, mGluR6 or mGluR7,
which was similar to compound 4 (LY354740). The agonist activity of (+) - 6a was found
to be highly stereospecifi c, since its enantiomer ( − ) - 6a showed approximately 90 - fold
lower agonist activity (EC
50
= 2,640 nM) than (+) - 6a for mGluR2. Racemic ( ± ) - 6b , dia-
stereomer of ( ± ) - 6a at the C - 3 position, did not exhibit signifi cant agonist (EC
50
> 100 000 nM)
or antagonist (EC
50
= 17 100 nM) activities for mGluR2. The conspicuous difference in
activities between ( ± ) - 6a and ( ± ) - 6b does not seem to be ascribable to the steric effect of
fl uorine incorporation, because mGluR2/3 antagonists 12a (Ro 65 – 3479) or 12b , bearing
a hydroxyl group or a methyl group at the C - 3 α position of 4 , exhibited high or moderate
binding affi nities to mGluR2 ( K
i
= 52 nM or 983 nM) and mGluR3 ( K
i
= 89 nM or 146 nM)
[36, 37] . These results suggest that the decrease in the activity of ( ± ) - 6b for mGluR2 is
due to the large electronegativity of the incorporated fl uorine atom, which infl uences the
electron density of the amino and/or carboxyl groups at the C - 2 position, depending on
its stereochemistry. Similarly, the difl uoro analogue ( ± ) - 6c did not exhibit appreciable
agonist (EC
50
= > 100 000 nM) or antagonist (EC
50
= 36 200 nM) activities for mGluR2.
This lack of activity may be due to the α - fl uorine atom of ( ± ) - 6c , since ( ± ) - 6b with a fl uo-
rine atom at the C - 3 α position exhibited neither agonist nor antagonist activities for
mGluR2, as mentioned above.
Racemic ( ± ) - 7a , bearing a fl uorine at the C - 6 position of ( ± ) - 4 , exhibited a strong
mGluR2 agonist activity (EC
50
= 34.2 nM), which was also found to be highly stereospe-
cifi c. The optically active ( − ) - 7a (MGS0022) exhibited an approximately 67 - fold higher
agonist activity (EC
50
= 16.6 nM) than its enantiomer (+) - 7a (EC
50
= 1 120 nM). Further-
more, compound ( − ) - 7a exhibited a high agonist activity for mGluR3 (EC
50
= 80.9 nM),
but did not for mGluR4, mGluR6, mGluR7, mGluR1a, or mGluR5. No signifi cant antago-
nist activity of ( − ) - 7a was observed for mGluR1a and mGluRs2 – 7. In contrast, ( ± ) - 7c ,
bearing a methyl group at the C - 6 position of ( ± ) - 4 , exhibited neither agonist nor antagonist
activities for mGluR2 (EC
50
> 100 000 nM). The dramatic difference in activity between
( ± ) - 7a and ( ± ) - 7c may be ascribed to the difference in stereoelectronic properties between
fl uorine and a methyl group.
Interestingly, the replacement of the methylene group at the C - 4 position of 7a with
a carbonyl group has substantially enhanced the agonist activity, that is, the resulting
(+) - 7b (MGS0028) is one of the best known agonists for mGluR2/3 to date. Compound
(+) - 7b demonstrated a potent and stereospecifi c agonist activity for mGluR2 (EC
50
= 0.57 nM)
and mGluR3 (EC
50
= 2.07 nM), but neither showed agonistic effect on mGluR1a or
mGluR4 – mGluR7 or antagonistic effect on mGluR1a or mGluR2 – mGluR7. The agonist
activity of (+) - 7b for mGluR2 was approximately 165 - fold higher than that of its enan-
tiomer ( − ) - 7b (EC
50
= 94.7 nM). The greatly enhanced agonist activity of (+) - 7b as com-
pared with ( − ) - 7a may be ascribed to a conformational change caused by the replacement
of the C - 4 methylene moiety to a carbonyl group, especially the relative positions of the
three key functional groups (i.e., one amino group and two carboxylic acids), as well as
the stereoelectronic effects of the carbonyl group introduced.
3.4.4.2 Behavioral Pharmacology of (+) - 6a ( MGS 0008) and (+) - 7b ( MGS 0028)
The antipsychotic - like effects of (+) - 6a (MGS0008) and (+) - 7b (MGS0028) on laboratory
animals are shown in Table 3.2 [15, 51] . It was recently found that phencyclidine
80 Fluorine in Medicinal Chemistry and Chemical Biology
Table 3.2 Antipsychotic - like effects of (+) - 6a ( MGS 0008) and (+) - 7b ( MGS 0028) on
laboratory animals
Y
CO
2
R
2
X
3
H
H
CO
2
H
X
2
NH
2
X
1
Compound X
1
X
2
X
3
Y PCP - induced
hyperactivity
PCP - induced
head - weaving
behavior
Conditioned
avoidance
ED
50
(mg/kg)
ED
50
( µ g/kg)
ED
50
(mg/kg)
4 (LY354740) H H H CH
2
> 100
3000
> 30
(+) - 6a (MGS0008) F H H CH
2
5.1 260 6.55
(+) - 7b (MGS0028) H H F CO 0.30 0.090 1.67
ED
50
: 50% effective dose.
(PCP) - induced head - weaving behavior in rats was inhibited by the intraperitoneal admin-
istration of 4 (LY354740) [17] . The oral administration of 4 (LY354740) also inhibited
PCP - induced head - weaving behavior (ED
50
= 3.0 mg/kg), but did not affect PCP - induced
hyperactivity (ED
50
> 100 mg/kg) or conditioned avoidance responses (ED
50
> 30 mg/kg)
in rats. In contrast, the oral administration of (+) - 6a (MGS0008) (ED
50
= 0.26 mg/kg)
inhibited PCP - induced head - weaving behavior in rats more effectively (11 - fold) than 4 .
Furthermore, (+) - 6a antagonized PCP - induced hyperactivity (ED
50
= 5.1 mg/kg) and
impaired conditioned avoidance responses (ED
50
= 6.55 mg/kg) in rats. These results indi-
cate that the PCP - induced head - weaving behavior is a sensitive method for screening
mGluR2/3 agonists and the introduction of fl uorine to 4 has clearly increased the oral activ-
ity. It has been reported that the oral bioavailability of 4 in rats is low ( F = 10%), apparently
because of ineffi cient drug transfer across the intestinal epithelial membrane [52] . The
enhanced oral activity of (+) - 6 can be attributed to the increase in oral bioavailability and
BBB penetration as a result of the introduction of fl uorine to the drug molecule, which
would enhance lipophilicity as well as bring in unique properties associated with fl uorine.
The oral administration of (+) - 7b (MGS0028) very strongly inhibited PCP - induced
head - weaving behavior in rats (ED
50
= 0.090 µ g/kg), that is, (+) - 7b was much more effec-
tive than 4 (LY354740) and (+) - 6a (MGS0008). Furthermore, the oral administration of
(+) - 7b strongly antagonized PCP - induced hyperactivity in rats (ED
50
= 0.30 mg/kg) and
impaired the conditioned avoidance responses (ED
50
= 1.67 mg/kg) in rats. Excellent anti-
psychotic - like effects of (+) - 6a (MGS0008) and (+) - 7b (MGS0028) as mGluR2/3 agonists
are very encouraging for their potential use in the treatment of schizophrenia.
3.4.4.3 Pharmacokinetics of (+) - 7b ( MGS 0028)
The metabolism and disposition of (+) - 7b (MGS0028) in three preclinical species (rats,
dogs, and monkeys) are summarized in Table 3.3 [53] . In rats, (+) - 7b (MGS0028) was
Fluorinated Conformationally Restricted Glutamate Analogues 81
Table 3.3 Pharmacokinetic parameters of (+) - 7b ( MGS 0028) in Sprague - Dawley rats, rhesus monkeys, and beagle dogs
Parameter
a
Sprague - Dawley rats Rhesus monkeys Beagle dogs
3 mg/kg i.v. 1 mg/kg p.o. 10 mg/kg p.o. 1 mg/kg i.v. 5 mg/kg p.o. 0.3 mg/kg i.v. 0.1 mg/kg p.o. 0.3 mg/kg p.o.
C
max
( µ M)
NA
b
1.0 ± 0.2 6.0 ± 1.9
NA
b
1.0 ± 0.5
NA
b
1.5 ± 0.8 4.0 ± 1.0
t
max
(h) NA
b
0.8 ± 0.2 3.0 ± 1.4
NA
b
2.3 ± 1.5
NA
b
0.4 ± 0.1 0.5 ± 0.0
AUC
0 – 24 h
( µ M h) 14.3 ± 0.8 3.4 ± 0.5 35.8 ± 3.8 10.2 ± 2.6 10.4 ± 5.0 8.2 ± 1.1 1.7 ± 0.9 5.7 ± 0.3
Effective t
1/2
(h)
1.1
NA
b
NA
b
1.1
NA
b
0.9
NA
b
NA
b
CL
p
(ml/min/kg)
16.2 ± 0.9
NA
b
NA
b
8.0 ± 1.8
NA
b
2.8 ± 0.4
NA
b
NA
b
V
d
(L/kg)
0.5 ± 0.2
NA
b
NA
b
0.6 ± 0.1
NA
b
0.1 ± 0.01
NA
b
NA
b
F (%) NA
b
70.7 75.3
NA
b
20.3
c
NA
b
63.4 69
AUC
Metabolite
/AUC
parent
0.8 0.4 0.3 0.3 0.9 0.0 0.0 0.0
a
C
max
: maximum plasma or tissue concentration, t
max
: time to reach maximum plasma concentration, AUC
0 – 24
: area under the concentration – time curve from time 0 to 24 hours,
Effective t
1/2
: effective elimination half - life, CL
p
: plasma clearance, V
d
: volume of distribution, F : bioavailability.
b
Not applicable.
c
Some monkeys experienced emesis, and this may be partially responsible for low bioavailability.
