Nielsen H. RNA: Methods and Protocols
Подождите немного. Документ загружается.


174 Sioud
post-transcriptional gene silencing (PTGS) had been described
years ago in plants, and now it is believed to function as a surveil-
lance system for blocking the function of har mful RNAs such
as viral RNAs (2). Remarkably, RNAi is systemic in both plants
and nematodes, spreading from cell to cell. In C. elegans,RNAi
is also heritable: silencing can be transferred to the progeny of
the worm originally injected with the trigger dsRNA. Viral infec-
tion, inverted repeat transgenes, or aberrant transcription prod-
ucts all lead to the production of dsRNA that can be converted to
siRNAs.
It should be noted that the work of Hamilton and Baulcombe
has provided the first decisive findings showing that a distinct
population of 21- to 24-nucleotide (nt)-long RNAs with anti-
sense sequence of silenced genes invariably accumulated in co-
suppressed plants (3). Their work also suggests that 21–24 nt
RNA duplexes could provide sequence specificity to the machin-
ery that degrades homologous mRNAs in RNAi/PTGS (3).
Unlike invertebrates, vertebrates react to long dsRNA by activat-
ing the interferon pathway (4). However, it has been shown that
chemically made small RNAs, known as siRNAs, with features
of Dicer cleavage products were sufficient to mediate RNAi in
human cells without activating the IFN r esponse (5). Subsequent
to this landmark finding by Tuschl and colleagues in 2001, siRNA
technology is now used as a standard tool in most laboratories for
gene function analysis and drug-target discovery and validation.
Furthermore, siRNAs have emerged as a powerful tool for ther-
apeutic gene silencing (6, 7). In principle, the mRNA encoding
any protein that is associated with a disease can be cleaved selec-
tively by siRNAs. However, recent data make it clear that siRNA
faces some major hurdles before it can used as a drug.
2. RNAi Pathway
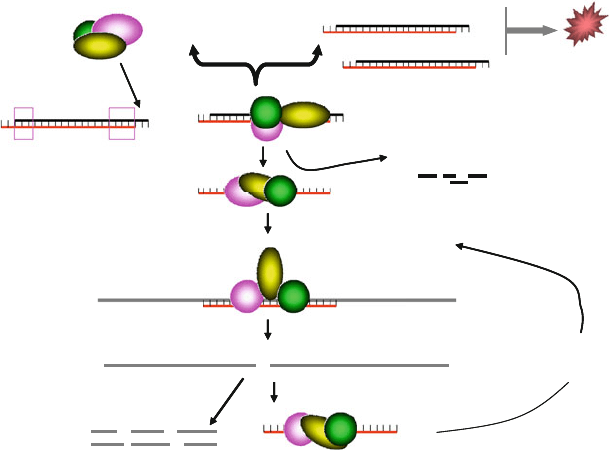
In general the RNAi pathway is initiated by the enzyme Dicer,
which cleaves long dsRNA into double-stranded siRNA. Dicer
is a dsRNA-specific RNAase III family ribonuclease, which gen-
erates siRNA duplexes containing of 20–25 nt in length. Dicer
leaves 2-nt 3
-overhangs and 5
-phosphate groups in each strand.
These siRNA duplexes are then incorporated into a multiprotein
complex, the RNA-induced silencing complex (RISC). Notably,
synthetic siRNAs enter directly into the RNAi pathway (see
Fig. 12.1). Subsequent to strand separation, the antisense (guide)
strand guides the RISC to recognize and cleave target mRNA
sequences. The catalytic activity of RISC is mediated by Arg-
onaute 2 (Ago2) protein, the only Ago family member that is
Promises and Challenges in Developing RNAi as a Research Tool and Therapy 175
Sense strand-mediated
mRNA recognition
mRNA cleavage
Synthetic
siRNA duplexes
5’p
5’p
5’p
5’p
5’p
3’
3’
3’
3’
3’
3’
5’
mRNA
5’ (A)n 3’
(A)n 3’
5’
Degradation by
cellular nucleases
Interferon
response
pathway
3’
5’
5’
5’
3’
3’
Ago2
TRBP
5’p
3’
5’p
3’
Low stability
(AU-rich)
High stability
(GC-rich)
RISC Assembly
RISC
recycling
Degradation of
the sense strand
?
Fig. 12.1. Schematic representation of gene silencing by siRNAs. In contrast to long double-stranded RNAs, siRNAs are
directly loaded into a multi-protein complex termed RNA-induced silencing complex (RISC), where the sense (passenger)
strand with high 5
-stability) is cleaved by the nuclease AGO2. This will lead to strand separation. Subsequently, the
RISC containing the antisense (guide) strand seeks out and binds to complementary mRNA sequences. Bound mRNA
molecules are then cleaved by AGO2 and cleaved mRNA fragments are rapidly degraded by cellular nucleases. Following
dissociation, the active RISC is able to recycle and cleave additional mRNA molecules.
cleavage competent (8, 9). The protein members of the arg-
onaute family are highly basic proteins that contain two com-
mon domains, PAZ and PIWI domains. While the PA2 domain
is responsible for RNA binding, the PIWI domain mediates the
interaction with Dicer and contains the nuclease activity that
cleaves of target mRNAs. Ago2 is also responsible for cleavage
of the passenger siRNA strand, thus facilitating the formation
of functional RISC complexes (10, 11). Analysis of the crystal
structures of a siRNA guide strand associated with Ago2 PIWI
domain revealed that nucleotides 2–8 form a seed sequence that
directs target mRNA recognition by RISC (12). While mammals
and C. elegans each have a single Dicer that makes both miR-
NAs and siRNAs, Drosophila has two Dicers (13): Dicer-1 makes
miRNAs, whereas Dicer-2 is specialized for siRNA production.
Although the discovery of RNAi has provided a new tool to study
gene function and drug target validation, recent studies have
shown that siRNA duplexes can activate innate immunity and
silence a variety of genes in addition to the intended target gene
(14–17). Therefore, the development of strategies that block
siRNA unwanted effects is crucial to their therapeutic use. Also,

176 Sioud
there remain other important obstacles for effective therapeutic
use of siRNA, including stability and delivery.
3. Design Rules
Gene silencing by siRNA varies markedly in mammalian cells,
where the gene-silencing effectiveness depends very much on the
target sequence positions (sites) selected from the target gene
(18). Since individual siRNAs Vary extremely in their effeciency
to induce down-regulation of the target, several siRNAs have to
be designed for each target gene and evaluated for their efficacy
and lack of side effects (see below). The earliest guidelines for
siRNA design were proposed by Elbashir and colleagues (5). They
suggested that synthesizing siRNA duplexes of 19 base-paired
nucleotides with 2-nt 3
-overhangs at either end (referred to as
21-nt siRNA duplexes) mediates efficient cleavage of the target
mRNA. Preferably, the 19-nt target sequence should be flanked
in the mRNA with two adenosines at the 5
-end. Regions at the
mRNA to select the target site are preferably in the coding region,
100 bp from the AUG start codon. Despite these early rules, the
siRNA efficacy is highly dependent on target sequence and siRNA
efficacy cannot be predicted from the primary sequence. There-
fore, a successful siRNA research project may require the design
of various distinct siRNAs at a high cost. More recently, some
studies showed that the 5
-end of the antisense strand might be
incorporated into the RNA-induced silencing complex and strand
incorporation may depend on weaker base pairing, indicating an
A-U terminus may lead to more strand incorporation than a G-C
terminus (19, 20). Therefore, it was concluded that the relative
thermodynamic stability of the 5
-ends of the two siRNA strands
in the duplex determines the identity of the selected strand,
either the antisense (guide) or the sense (passenger) strand. Other
factors reported to be involved in gene-silencing efficacy are GC
content, point-specific nucleotides, specific motif sequences, and
secondary structures of mRNAs ( 21). Based on the published
data, several siRNA design rules/guidelines using efficacy-related
factors have been r eported. Although there are common pre-
ferred and unpreferred nucleotides at both position 1 and 19
in some guidelines, there is the problem of inconsistencies for
nucleotide frequency of each position suggesting that some rules
from guidelines are sequence-dependent.
Despite these design limitations, the experimental data indi-
cated four apparent features of the siRNA sequence to possibly
serve to discriminate active siRNAs from those that are non-active
(21). First, the 5
-end of the antisense strand ( AS) of active siR-

Promises and Challenges in Developing RNAi as a Research Tool and Therapy 177
NAs may always be A or U, with the counterpart of non-active
siRNAs may always be C or G. Second, the 5
-end of the sense
strand (SS) of active siRNAs are preferably G or C, with the coun-
terpart of non-active siRNAs being A or U. Third, in the case of
active siRNAs, the 5
-terminal AS are A/U-rich whereas the cor-
responding region of non-active siRNAs are G/C rich. Fourth,
position 10 of the target site should be U.
In addition to the siRNA sequence, mRNA secondary struc-
ture is also considered to be an important factor in predicting
siRNA efficacy. However, there are conflicting results concern-
ing the effects of secondary structures on siRNA functionality.
On one hand, some studies suggested that the secondary struc-
ture of the mRNA plays a role in determining the efficacy of gene
silencing. On the other hand, other studies did not find any cor-
relation between functionality of the siRNA and second structure
of the target mRNA. Therefore, this issue still requires further
investigation.
4. Detection of
Exogenous RNAs
by Innate Immune
Receptors
Notably, the immune system has evolved cellular and molecular
strategies to discriminate between foreign and self nucleic acids.
Among the cytoplasmic sensors of long dsRNA is the dsRNA-
dependent protein kinase (PKR) that phosphorylates serine and
threonine residues of target proteins (22). Most human cells con-
stitutively express a low level of PKR that remain inactive. How-
ever, upon binding to dsRNA, PKR forms a homodimer result-
ing in its autophosphorylation and activation. Activated PKR
phosphorylates various substrates including the protein synthe-
sis initiation factor elF-2α and blocks the translation of viral and
cellular proteins, an essential step in antiviral resistance. It should
be noted that PKR’s binding to dsRNAs is sequence-independent
and the pr esence of interferon upregulates its expression.
Although PKR is implicated in antiviral immunity, it is mainly
IFN effector and not absolutely required for IFN production.
More recently two additional intracellular helicases, retinoic-
acid-inducible gene I (RIG-I) and/or melanoma differentiation-
associated gene 5 (MDA-5) were identified (23). RIG-I encodes
a caspase recruitment domain (CARD) at the N terminus, in
addition to an RNA helicase domain. The RNA helicase domain
requires ATPase activity and is responsible for viral dsRNA r ecog-
nition and induction of conformational changes leading to the
interaction of the RIG-I CARD domain with another CARD-
containing adaptor protein, known as IPS-1, MAVS, Cardif, or
VISA (24). IPS-1 is an outer mitochondrial membrane binding
178 Sioud
protein, which activates IRF3 and IFR-7 through TBK1/IKKi,
resulting in the production IFN-β production. Mitochondrial
retention of IPS-1 is essential for IRF3, IRF7, and NF-κB acti-
vation by RIG-1 (24).
Although RIG-I seems to be an essential sensor of viral RNAs,
microbial nucleic acids are also recognized by toll-like recep-
tors (TLRs), especially in immune cells (25, 26). Whereas most
TLRs ar e expressed in the plasma membrane for detecting bacte-
rial components, TLR3, TLR7, TLR8, and TLR9, are expressed
in intracellular compartments (endosomes, lysosomes) (25). The
immune function of this cellular localization is to sense viral
RNAs. TLR3 is also expressed on the cell surface and it is believed
to recognize extracellular viral dsRNAs (27). TLR7 and TLR8
recognize viral ssRNA and small synthetic antiviral compounds
referred to as imidazoquinolines. TLR9 recognizes unmethylated
CpG-DNA motifs that exist in both viral and bacterial DNA, but
are suppressed or methylated in the vertebrate genomes (28, 29).
It should be noted that intracellular NOD-like receptors detect
bacteria, whereas viruses are mainly detected by TLRs and RIG-
like receptors. The virus-detecting TLRs operate mainly in plas-
macytoid dendritic cells by responding to viral nucleic acids that
enter the cell via e ndocytosis. In these cells, the major immune
response is the production of type 1 interferon (30).
Although siRNAs were initially thought to bypass the IFN
response because they are too short to be recognized by dsRNA
sensors (5), we and others have shown that they could acti-
vate innate immunity in mammalian cells (14–17). Early stud-
ies indicated sequence-independent activation of PKR and TLR3
signaling pathways by siRNAs (17, 31). However, recently it
was demonstrated that PKR and TLR3 do not represent the
major pathways by which chemically synthesized siRNAs activate
immunity in immune cells (32–35). Indeed, a group of siRNA
sequences stimulated monocytes or dendritic cells to produce
proinflammatory cytokines and type I interferons. This response is
mainly mediated through TLR7 in mice and TLR7/8 in humans.
Under our experimental conditions, ss siRNAs were more effec-
tive than ds siRNAs in activating TLR7/8 in human monocytes
and peripheral blood mononuclear cells (PBMCs) (32, 35). The
extent of siRNA unwanted effects has r ecently been confirmed by
expression profiling using microarrays, which identified over 400
siRNA-affected genes in PBMCs. Genes encoding for proinflam-
matory cytokines, interferons, and Mx proteins are among the
genes that are significantly induced (36). Mx proteins are IFN-
induced GTPases that form complexes with dynamin disrupting
trafficking or activity of viral polymerases, thereby interfering with
viral replication.

Promises and Challenges in Developing RNAi as a Research Tool and Therapy 179
5. What Is the
Nature of
IFN-Inducing
Motif Present in
One Sequence But
Absent in
Another?
TLR7 and TLR8 recognize certain siRNA sequences, provided
they are delivered to the endosomes via cationic liposomes. Ini-
tial experiments indicate that some types of secondary structures
and/or specific nucleotides are responsible for the activation of
NF-κB signaling pathway by siRNAs in human monocytes (14).
Judge and colleagues found that the 5
-UGUGU motif was indis-
pensable for the immune activation by a siRNA in human blood
cells (34). However, Hornung and colleagues identified a 9-
nt motif RNA motif (5
-GUCCUUCAA) that is recognized by
TLR7 in the context of siRNA duplexes and the activity does
not depend on GU content (33). Collectively, our data indicated
that interferon induction by siRNAs can not be easily suppressed
by selecting siRNA sequences without the GU dinucleotides.
Indeed, several siRNA sequences without GU induced TNF-α
production in human PBMC and monocytes (32). Although the
precise nature of the RNA motifs responsible of innate immune
activation is not known, we showed that the ability of ssRNA or
dsRNAs to activate TNF-α production is largely depend on the
uridine content (35). Indeed, their replacement with adenosines
abrogated immune activation by either ss or ds siRNAs. A recent
study by Goodchild and colleagues (37), where they analyzed
the effects of 250 siRNA sequences, confirms the importance of
uridines for siRNA stimulation. Also, their data supported our
proposed model in which ds siRNAs are dissociated in the endo-
somes, leading to the activation of TLR7/8 by ss siRNAs (32).
6. The Molecular
Basis of RNA
Sensing by RIG-I
As indicated above, recent studies on the immune response
to chemically made siRNAs have highlighted the involvement
of the endosomes. Indeed, cytoplasmic delivery of synthetic
siRNA by electroporation into human blood cells did not induce
either inflammatory cytokines or interferons, whereas the same
sequences when delivered by lipid did (32). Thus, synthetic siR-
NAs are not detected by cytoplasmic sensors for viral RNAs in
immune cells. However, the data do not explain why ds siR-
NAs were not sensed by RIG-1, a major viral RNA sensor. It has
been demonstrated that chemically made siRNA duplexes harbor-
ing 2-nt 3
-overhangs cannot engage innate immunity activation,
whereas the same siRNA sequence with blunt ends did (38). The
authors showed that RIG-1 can bind to siRNAs with or without
180 Sioud
2-nt 3
-overhangs, but only siRNAs with blunt ends could acti-
vate RIG-1. These findings imply that endogenous shRNAs or
microRNA harboring the Dicer signature, 2-nt 3
-overhang, are
not an ideal stimulator of RIG-I. Thus, the structures of the 5
-
ends between shRNAs (substrate for Dicer) and non-self dsRNAs
such as viral RNAs are critical for self and non-self discrimination.
It should be noted that the predominant form of naturally
occurring dsRNAs in mammalian cells is derived from endoge-
nously expressed miRNAs that constitute a large class of noncod-
ing small RNAs involved in gene regulation in a variety of organ-
isms ranging from plants to mammalians (39). Presently, more
than 1,000 potential human miRNAs have been identified and
numerous have been experimentally validated. Usually miRNAs
are transcribed from endogenous genes by RNA polymerase II
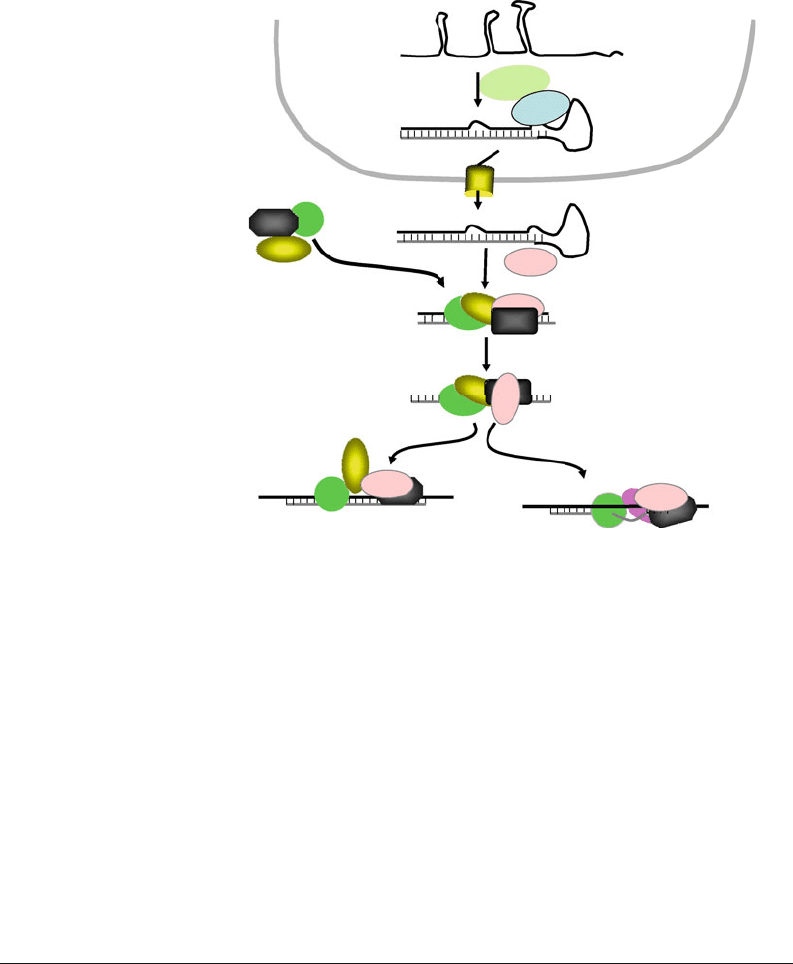
as long RNA precursor called a primary miRNA (pri-miRNA),
containing one or more distinct miRNAs. In the nucleus the
RNA pr e cursors are processed by Drosha to 60–80 nt RNA hair-
pin intermediate, bearing 2-nt 3
-overhang, called a pre-miRNA.
Interestingly, the Drosha cleavage site was shown to be 11 base
pairs from the stem single-stranded RNA junction (40). Pro-
cessed pre-miRNAs are then transported from the nucleus to
the cytoplasm by exportin-5, where its 2-nt 3
-overhang is rec-
ognized by Dicer, which generates the mature miRNA that can
evade the detection by innate immune receptors such as RIG-I
(see Fig. 12.2).
During our studies, we have also found that synthetic ss
siRNAs (21 nt) do not activate innate immunity when delivered
to the cytoplasm via electroporation (32). These used RNAs do
not contain 2-nt 3
-overhang because they are single-stranded.
To further examine the contribution of RIG-I in sensing exoge-
nous RNAs, we have transfected monocytes with either T7 RNA
polymerase (RNAP) transcribed siRNAs or chemically made
siRNAs. The inhibition of endosome maturation by chloroquine
abrogated the immuostimulatory activity of chemically made
siRNAs, but not the T7 RNAP-made siRNAs (41). In addition,
the immunostimulatory effect of the T7 RNAP-made siRNAs was
not inhibited with 2-aminopurine, a specific inhibitor of PKR.
Therefore, which cytoplasmic factors are able to sense in vitro
transcribed RNA? Additional studies from other investigators
showed that RIG-I senses ssRNA-bearing 5
-triphosphate, a
specific signature of viral and in vitro transcribed RNAs (42).
Interestingly, artificial capping or base modifications of the
5
-triphosphate bearing RNA abolished immune response. In
general, self-RNA undergo several modifications to eliminate or
mask the 5
-triphosphate group. However, the reported data do
not explain why certain endogenous RNA with 5
-triphosphates
(e.g., 7SL RNA, an abundant cytoplasmic RNA) escape RIG-I
recognition. Natural 2
ribose-modifications might protect
Promises and Challenges in Developing RNAi as a Research Tool and Therapy 181
Dicer
Pre-miRNA
RNPs
miRNA-mediated
target recognition
mRNA cleavage
p
5’
p
p
3’
3’
3’
3’
3’
5’
(A)n 3’
5’
3’
Drosha
Nucleus
Translation arrest
Cytoplasm
AAAAA..3’
5’
Pri-miRNAs
Pre-miRNA
Exportin 5
5’
5’
5’
5’
3’
5’
(60-80 nt)
DGCR8
miRNA:miRNA*
duplex
Ago-2
Fig. 12.2. Gene regulation by miRNAs. MiRNAs are derived from genome transcribed
primary transcripts (pri-miRNAs) that are predicted to form multiple stems and hairpin
structures. Pri-miRNAs are processed by the ribonuclease Drosha to 70–80 nucleotide
pre-miRNAs that are transported to the cytoplasm by exportin 5. Subsequently, they are
processed by Dicer into mature 22–24 nucleotide miRNAs, which are then incorporated
into a RNP complex that can direct either RNA cleavage (perfect complementarity with
mRNA) or translation arrest (mismatches with target mRNA).
endogenous RNA-bearing 5
-triphosphate from being detected
by RIG-I and other RNA sensors. MDA5, the most closely
related protein of RIG-I, is also an IFN-inducible protein.
However, the exact mechanisms of RNA sensing by MDA5 has
yet to be defined but seems to sense dsRNA structures from
certain viruses.
7. Separation of
siRNA Unwanted
Effects from Gene
Silencing
Much of the recent interest in the mechanisms involved in RNA
sensing or tolerance by the immune system was generated by
the observation that siRNA can activate innate immunity (14,
17). Considering the high frequency of uridines in messen-
ger RNAs it is more likely that a high proportion of self and
non-self chemically made siRNA sequences will activate innate
182 Sioud
immunity. Therefore, it would be desirable to develop strategies
that evade immune activation. At least three distinct ways to avoid
immune activation by siRNAs can be used. The first would be to
use delivery agents that avoid the delivery and/or retention of
siRNA within the endosomes. The second way relies on the use
of modified nucleotides. In this respect, Morrissey and colleagues
showed that the incorporation of various 2
-modified nucleotides
in siRNA sequence abrogated their immunostimulatory potency
(43). However, the chemical modifications that block immune
activation must be chosen carefully so as not to inhibit siRNA
silencing potency. Thus, finding the appropriate chemical mod-
ifications for interfering with siRNA immune activation will be
important for exploring their therapeutic applications.
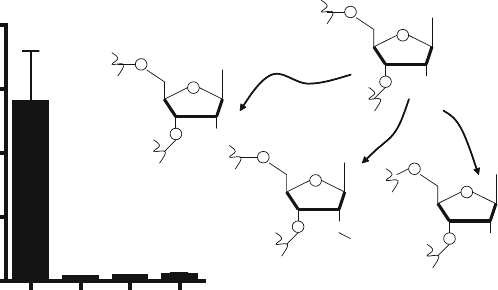
Fortunately, we have shown that replacement of only uridines
with their 2
-fluoro, 2
-deoxy,or2
-O-methyl modified coun-
terparts can abrogate immune recognition of ss siRNA or ds
siRNAs by TLRs without reducing their silencing potency (35,
see Fig. 12.3). In accordance with our data, Judge and col-
leagues demonstrated that the incorporation of 2
-O-methyl-
uridine or 2
-O-mehtyl guanosine residues into siRNAs abrogates
their immunostimulatory potency (44). Collectively, the pub-
lished data offer the possibility of choosing the appropriate mod-
ifications that evade immune activation without reducing siRNA-
silencing. I recommend the use of 2
-deoxy uridine or thymidine
R
O
R
CH3
U-2'-OH
U-2'-F
U-2'-O-methyl
U-2'-H
0
2500
5000
7500
10000
R
F
R
H
TNF-α (pg/ml)
5’-UGCUAUUGGUGAUUGCCUCTT-3’
OH
Fig. 12.3. A representative example of TNF-α production in human monocytes in response to unmodified or 2
-uridine
modified RNAs. Cells were transfected with either unmodified or 2
-uridine modified single-stranded siRNA. Subsequent
to 18 h transfection time, secreted TNF-α in culture supernat ants was measured by ELISA.
Promises and Challenges in Developing RNAi as a Research Tool and Therapy 183
TLR7 TLR8 TLR7 TLR8
TLR7 TLR8
TLR7 TLR8
2’-hydroxy
uridines
2’-O-methyl
uridines
2’-fluoro
uridines
2’-deoxy
Uridines or
Thymidines
Recognition
Gene
silencing
Gene
silencing
Gene
silencing
Gene
silencing
Immune
Activation
No activation No activation
No activation
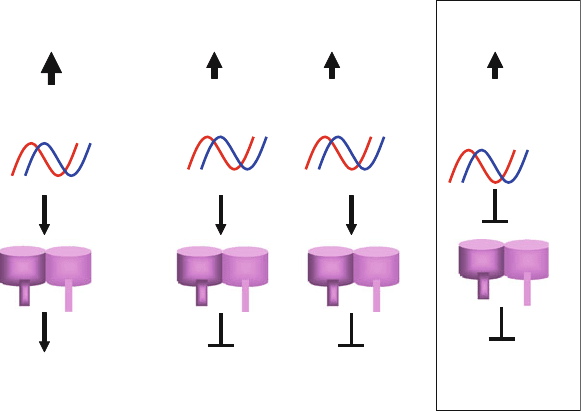
Fig. 12.4. An overview of the effects of chemical modifications on gene silencing and activation of endosomal TLR7/8.
For details see the text.
modified siRNAs because they showed no significant immunos-
timulatory effects and binding to TLR7/8 ( see Fig. 12.4).
The finding that 2
-modified RNAs can evade immune acti-
vation suggests that naturally modified RNAs are not sensed by
TLR7/8. Support of this view has been provided by Karikó and
colleagues, who demonstrated that modifications that are fre-
quently found in mammalian RNA (such as pseudouridine, 2
-O-
methyl) can interfere with the capacity of RNA to activate TLR-7
in dendritic cells (45). Thus, unmodified RNA corresponding to
mammalian sequences would be expected to activate TLR7 more
effectively than native RNAs provided they are delivered to the
endosomes (32).
The finding that unmodified, but not 2
-modified RNA, are
potent triggers of innate immunity also raised questions about the
differences in their structures that might be relevant to binding
to TLR7/8. Which step is affected by 2
-modifications, and why
can’t 2
-modified RNAs trigger immune activation?. One way
to address the first question is to assess whether 2
-modified
RNA antagonize with immunostimulatory RNAs to trigger
TLR7/8 signaling. Studies of transfected human monocytes
show that 2
-O-methyl modified RNAs abrogates the activation
of TLR7 by immunostimulatory RNAs (46). Of considerable
interest, is that 2
-O-methyl modified RNAs suppressed immune
activation at very low concentrations (47). In addition, we have
shown that they can effectively inhibit immune activation by a
variety of immunostimulatory sequences including bacterial and
mitochondrial RNAs. Also, chemically modified RNA can
