Надеина Л.В. Радиоэкология
Подождите немного. Документ загружается.

31
Atoms and Elements
Elements are the building blocks of matter. The smallest particle of an
element that still retains the identity of that element is the atom. All atoms of
a given element are identical to one another, but differ from the atoms of oth-
er elements. Ancient Greeks first predicted the existence of the atom around
500 BC. They named the predicted particle «atomos», meaning «indivisible».
In 1803, John Dalton (1766-1844) proposed a systematic set of postu-
lates to describe the atom. Dalton's work paved the way for modern day ac-
ceptance of the atom. But scientists of his day considered the atom to be
merely a subordinate player in chemical reactions, an uninteresting, homoge-
neous, positively charged «glob» that contained scattered electrons. That
premise remained unchallenged until the end of the nineteenth century, when
a series of brilliant discoveries opened the door on the atomic science of the
twentieth century. Working concurrently and often collaboratively, three pio-
neering scientists helped release the genie of the atom.
Antoine Henri Becquerel
Becquerel, a French physicist, was the son and grandson of physicists.
Becquerel was familiar with the work of Wilhelm Conrad Roentgen on De-
cember 22 1895, «photographed» his wife's hand, revealing the unmistakable
image of her skeleton, complete with wedding ring. Roentgen's wife had
placed her hand in the path of X-rays which Roentgen created by beaming an
electron ray energy source onto a cathode tube. Roentgen's discovery of these
«mysterious» rays capable of producing an image on a photographic plate
excited scientists of his day including Becquerel. Becquerel chose the study
related phenomena of fluorescence and phosphorescence. In March of 1896,
quite by accident, he made a remarkable discovery. Becquerel found that,
while the phenomena of fluorescence and phosphorescence had many simi-
larities to each other and to X-rays, they also had important differences.
While fluorescence and X-rays stopped when the initiating energy source was
halted, phosphorescence continued to emit rays some time after the initiating
energy source was removed. However, in all three cases, the energy was de-
rived initially from an outside source. In March of 1896, during a time of
overcast weather, Becquerel found he couldn't use the sun as an initiating en-
ergy source for his experiments. He put his wrapped photographic plates
away in a darkened drawer, along with some crystals containing uranium.
Much to his Becquerel's surprise, the plates were exposed during storage by
invisible emanations from the uranium. The emanations did not require the
presence of an initiating energy source– the crystals emitted rays on their
own! Although Becquerel did not pursue his discovery of radioactivity, oth-
32
ers did and, in so doing, changed the face of both modern medicine and mod-
ern science.
Ernest Rutherford and the Atom
In 1911, Rutherford conducted a series of experiments in which he
bombarded a piece of gold foil with positively charged (alpha) particles emit-
ted by radioactive material. Most of the particles passed through the foil un-
disturbed, suggesting that the foil was made up mostly of empty space rather
than of a sheet of solid atoms. Some alpha particles, however, «bounced
back», indicating the presence of solid matter. Atomic particles, Rutherford's
work showed, consisted primarily of empty space surrounding a well-defined
central core called a nucleus.
In a long and distinguished career, Rutherford laid the groundwork for
the determination of atomic structure. In addition to defining the planetary
model of the atom, he showed that radioactive elements undergo a process of
decay over time. And, in experiments which involved what newspapers of his
day called «splitting the atom», Rutherford was the first to artificially trans-
mute one element into another– unleashing the incredible power of the atom
which would eventually be harnessed for both beneficial and destructive pur-
poses. Taken together, the work of Becquerel, the Curies, Rutherford and
others, made modern medical and scientific research more than a dream.
They made it a reality with many applications. A look at the use of isotopes
reveals just some of the ways in which the pioneering work of these scientists
has been utilized. Radiation is a two edged sword: its usefulness in both med-
icine and anthropological and archaeological studies is undisputed, yet the
same materials can be used for destruction. Human curiosity drove inquiring
scientists to harness the power of the atom. Now humankind must accept the
responsibility for the appropriate and beneficial uses of this very powerful
tool.
(www: The Discovery of Radioactivity The dawn of the nuclear
Age.htm )
6.1 Answer the questions.
1. How did our conceptions of atomic properties change?
2. How has that change affected our lives and our knowledge of the world?
3. What did the predicted particle «atomos» mean?
4. Who first predicted the existence of the atom around 500 BC?
5. How many pioneering scientists working concurrently and often collabora-
tively helped release the genie of the atom?

33
6. Who chose the study related phenomena of fluorescence and phosphores-
cence?
7. When did Becquerel find that he couldn't use the sun as an initiating en-
ergy source for his experiments?
8. What did Rutherford's work show?
9. What did Rutherford show in addition to defining the planetary model of
the atom?
Grammar in Use
7. Read the texts and fill in the missing words (the verbs in Passive Voice,
see the table Passive Voice, p. 9).
A) to be observed, to be recognized, to be known, to be discovered, not to
be understood, not to be recognized, not to be appreciated, to be
awarded
Although radiation 1)……………………in late 19th century, the dan-
gers of radioactivity and of radiation 2)…………. immediately ……… .
Acute effects of radiation 3)………….. first …………….. in the use of X-
rays when the Serbo-Croatian-American electric engineer Nikola Tesla inten-
tionally subjected his fingers to X-rays in 1896. He published his observa-
tions concerning the burns that developed, though he attributed them to ozone
rather than to X-rays. His injuries healed later.
The genetic effects of radiation, including the effects on cancer risk,
4)……………….. much later. In 1927 Hermann Joseph Muller published re-
search showing genetic effects, and in 1946 5)………………… the Nobel
prize for his findings.
Before the biological effects of radiation 6)……………….., many phy-
sicians and corporations had begun marketing radioactive substances as pat-
ent medicine and radioactive quackery. Examples were radium enema treat-
ments, and radium-containing waters to be drunk as tonics. Marie Curie
spoke out against this sort of treatment, warning that the effects of radiation
on the human body 7)………… well ………….. . Curie later died of aplastic
anemia due to radiation poisoning. Eben Byers, a famous American socialite,
died in 1932 after consuming large quantities of radium over several years;
his death drew public attention to dangers of radiation. By the 1930s, after a
number of cases of bone necrosis and death in enthusiasts, radium-containing
medical products had nearly vanished from the market.
34
Nevertheless, dangers of radiation 8)……………. fully ………. by sci-
entists until later. In 1945 and 1946, two U.S. scientists died from acute ra-
diation exposure in separate criticality accidents. In both cases, victims were
working with large quantities of fissile materials without any shielding or
protection.
Atomic bombings of Hiroshima and Nagasaki resulted in a large number
of incidents of radiation poisoning, allowing for greater insight into its symp-
toms and dangers.
B) to be surprised, to be seized, to be emitted, to be established, to be
extracted, to be found, to be accompanied, to be overcast, to be known
Knowledge concerning atomic nuclei began as early as 1896 with dis-
covery of radioactivity.
In 1895, Roentgen discovered the X-rays. The French scientist Bec-
querel got interested in Roentgen’s work. Becquerel 1) ……………… of the
fact that the production of X-rays 2) …….. always ……….. by fluorescence
from the material of the X-ray tube (glass). He thought that X-rays existed
whenever there was fluorescence. To investigate this problem, Becquerel
took uranium sulphate, which fluoresces under the action of sunlight. He
found that fluorescent uranium sulphate did give out rays, which could affect
a photographic plate even when wrapped in thick black paper. Becquerel ar-
gued that the fluorescent salt had given rise to X-rays, which had penetrated
the black paper and affected the photographic plate.
But he soon saw that he was mistaken. During one such experiment the
sky happened 3) …………… and uranium salt was hardly fluorescent. On
developing the photographic plate, Becquerel 4) ……………….. to see a
dark spot on it, as before. He had obviously stumbled on some new kind of
rays (1896) which could penetrate the thick wrapper and affect the photo-
graphic plate. It 5) ……. soon ……….. that any salt of uranium emits Bec-
querel rays. Unlike the X-rays, which appear in an X-ray tube only under
special conditions, the Becquerel rays 6) ……………….. in a spontaneous
manner.
Is uranium the only substance emitting Becquerel rays? Marie Curie
found the pitchblende, the ore from which uranium 7) ……………….. emits
Becquerel rays with a much stronger intensity than what its uranium content
would. After a long and laborious process of chemical separation, Marie Cu-
rie and her husband Pierre Curie discovered two new elements, polonium and
radium, which emitted Becquerel rays.
35
They gave the name «radioactive» to all substances capable of emitting
Becquerel rays and the phenomenon itself came to 8) ……………….. as it
9) ……………. To be about a million times more radioactive than uranium.
This power of radium radiation made it possible to study radioactivity sys-
tematically.
8. Mind the meaning of the following words and use them to read the text
bellow.
English Russian
achieve добиваться, достигать
afford приносить, доставлять
astonishing ошеломительный, изумительный
artificial искусственный
breakdown распад, разрушение
cancer рак (болезнь)
clue ключ (к разгадке чего-л.)
cumulative совокупный, накопленный
current поток, течение
distinguished выдающийся, знаменитый
emit испускать, выделять
establish устанавливать
exposure внешнее воздействие
excitation возбуждение, воздействие
fame известность, слава
fund запас, резерв
genius гений
impact сильное воздействие, влияние
intensity мощность, энергия
investigation исследование, изучение
isolate изолировать, выделять
lack нехватка, потеря, недостаток
outward наружный, внешний
piezoelectricity пьезоэлектричество
pitchblende уранинит, урановая смолка
polonium полоний
radium радий
radiotherapy радиотерапия, рентгенотерапия
regard внимание, забота

36
scintillation вспышка люминесценции
spinthariscope спинтарископ
subsequent последующий, следующий
substance вещество
stirring побуждение, стимул
thallium таллий
8.1 Read the text «The Curies: Lives Devoted to Research»
Wedding photo of Pierre and Marie Curie, 1895.
Marie Curie, her husband Pierre and their daughter Irène were responsi-
ble for the discovery of radioactivity and subsequent research work. Polis
French chemist and physicist famous for discovering radioactivity Marie
Sklodowska was born in Warsaw in 1867. She was a brilliant student and
dreamed of studying at the Sorbonne in Paris but it took eight years of saving
before she could afford to go. Despite very poor living conditions, and a lack
of French, she graduated in physics in 1893 and mathematics in 1894. While
looking for a laboratory in Paris to continue with her experiments she was in-
troduced to Pierre Curie, a highly regarded professor at the School of
Physics. They married and joined forces in the laboratory to astonishing ef-
fect – they soon made the fantastic discovery of radium and radioactivity.
In 1903 Marie and Pierre Curie were awarded half the Nobel Prize in
Physics «in recognition of the extraordinary services they have rendered by

37
their joint researches on the radiation phenomena discovered by Professor
Henri Becquerel». Pierre was tragically killed in 1906, leaving Marie with
two daughters: Irène aged 9 and Eve aged 2. Marie was determined to con-
tinue their work. She became the first ever woman professor at the Sorbonne
and as well as teaching, she discovered how to isolate radium in metallic
form
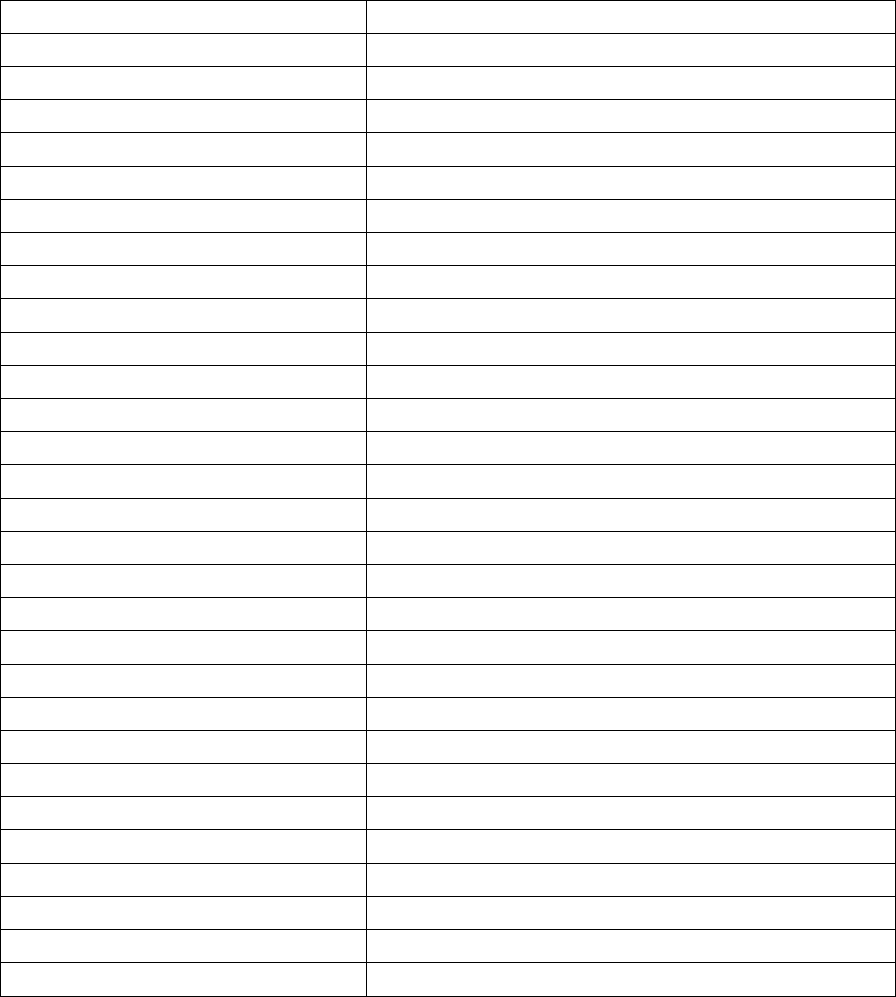
Marie Curie and her daughter Irène in the laboratory at the Radium In-
stitute in Paris, France, 1921.
38
In 1911 she was awarded the Nobel Prize for Chemistry for the discov-
ery of the elements radium and polonium. This she achieved by the isolation
of radium and also from her study of the nature and compounds of this re-
markable element. During World War One she established a front-line X-ray
service in the battlefields of Belgium and France, tirelessly fundraising, train-
ing staff and driving the X-ray vans. After the war, along with her research,
Marie continued to fund raise, this time for her Institutes and for a hospital
and laboratory devoted to radiology. She eventually died in 1934 from the
cumulative effects of radiation exposure.
In April 1895 Marie and Pierre Curie’s remains were enshrined in the
Pantheon in Paris. Marie Curie is the first woman to be honoured in such a
way for the achievements she made in Physics. Marie met Albert Einstein at
the first Solvay Conference of the world’s leading physicists. They became
friends and in 1913 went on a walking holiday together with their children.
Einstein said of Marie «[she was] the only person not corrupted by fame».
Marie Curie and her daughter Irène at the Hoogstade Hospital in Bel-
gium, 1915. Radiographic equipment is installed.
Pierre Curie met Marie Sklodowska when he was 35 years old and al-
ready an internationally recognized physicist. With his brother Paul-Jacques,
he discovered piezo-electricity: the fact that crystals under pressure produce
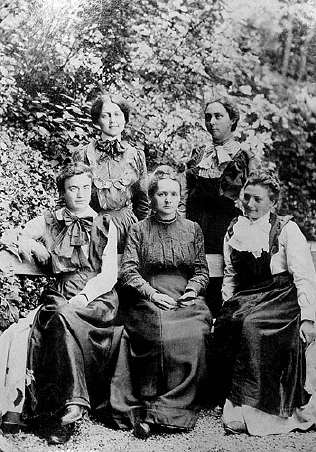
39
electric currents. He also studied crystal symmetries and the magnetic proper-
ties of bodies at different temperatures. His papers had been well received by
distinguished colleagues but he was still an outsider in the French academic
community. Like Marie he did not care for outward distinctions or a career.
They married in July 1895.
Pierre followed his wife’s work closely and he and his brother made her
an electrometer which measured weak electrical currents, based on the piezo-
electric effect. After the exciting results of Marie’s early experiments Pierre
abandoned his passionate study of crystals to join her in her search for new
substances.
He continued to construct pieces of laboratory equipment such as the io-
nisation chamber. Together they laboured Marie carrying out the chemical
separations and Pierre taking the measurements. Together they discovered
polonium and radium and used the word «radioactivity» for the first time.
This sensitive device was developed by Pierre Curie for investigations
into radioactivity. The chamber consists of a positive and negative plate con-
nected
by an electrometer. Radiation ionises the air in the chamber. The
breakdown of air molecules into positive and negative ion pairs allows them
to act as carriers of electric current. The negative ions migrate to the positive
plate and the positive ions to the negative plate. This causes a weak electric
current to flow which can be measured on the electrometer. The level of ra-
dioactivity will determine the strength of the current.
Marie Curie and four of her students. (Photo taken between 1910
and 1915.)
40
At a Royal Institution Lecture in London in 1903, Pierre described the
amazing properties of radium and the medical tests he had been carrying out
on himself. He had tied a piece of radium to his arm for ten hours and then
studied the burn-like wound that left a permanent scar. Because of this, Pierre
observed the potential of radium in treating cancer. In 1903 Marie and Pierre
Curie were awarded half the Nobel Prize.
Irene Joliot-Curie … was born in the stirring days of radioactivity when
her parents [Marie and Pierre Curie] were making great discoveries, she grew
up with radioactivity, and all her life was devoted to its study.
In 1926 she married Frédéric Joliot … and there began a collaboration
of husband and wife in scientific work rivalling in productive genius even
that of her parents. The most outstanding of their joint papers were published
in the years 1932-1934.
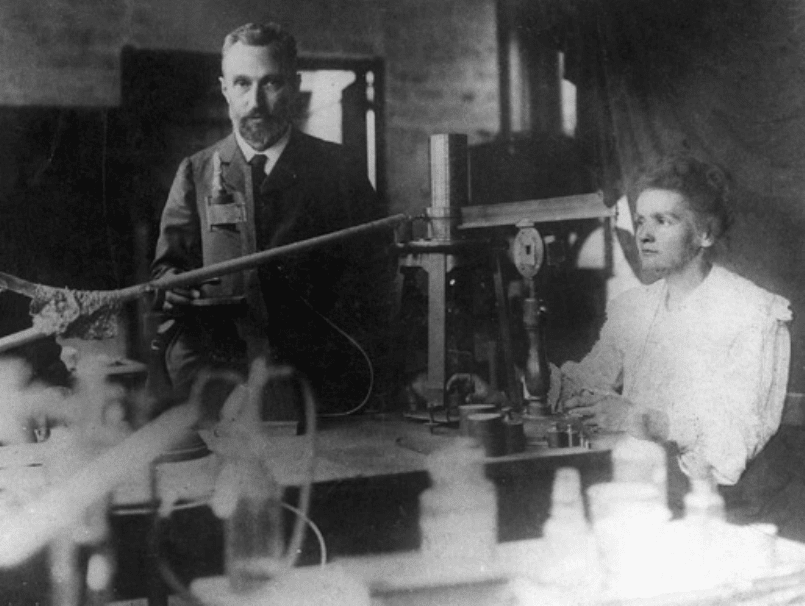
Pierre and Marie Curie in the «hangar» at l’Ecole de physique et
chimie industrielles in Paris, France, where they made their discovery.
(Photo taken 1898.)
This was followed by a systematic study of the radiations emitted from
the lighter chemical elements under the impact of alpha-particles, which
through the light of intuition – and good technique – led them, in early 1934,
to their beautiful discovery of artificial radioactivity. Then, after studying the
conditions of excitation of neutrons by the impact of alpha-particles on vari-
